Perceptual restoration fails to recover unconscious processing for smooth eye movements after occipital stroke
Figures
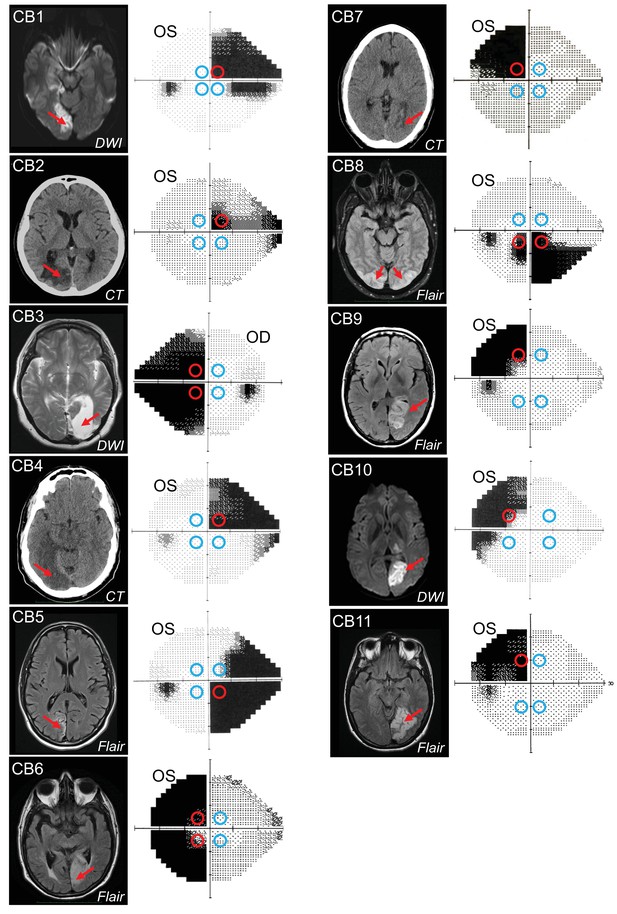
Occipital lesions, Humphrey visual field maps, and post-saccadic following response (PFR) testing locations.
Single radiographic images through the brains of each V1-stroke participant (N=11), illustrating region(s) of occipital damage (red arrows), shown with right brain hemispheres on image right. The location, size, and shape of visual stimuli presented during the PFR testing protocol are indicated by colored circles superimposed on initial 24-2 Humphrey visual field maps acquired for the tested eye in each case. Red circles: blind field testing locations; blue circles: intact field locations; OS: left eye; OD: right eye; DWI: diffusion-weighted imaging; FLAIR: T2-weighted fluid-attenuated inversion recovery; CT: computed tomography.
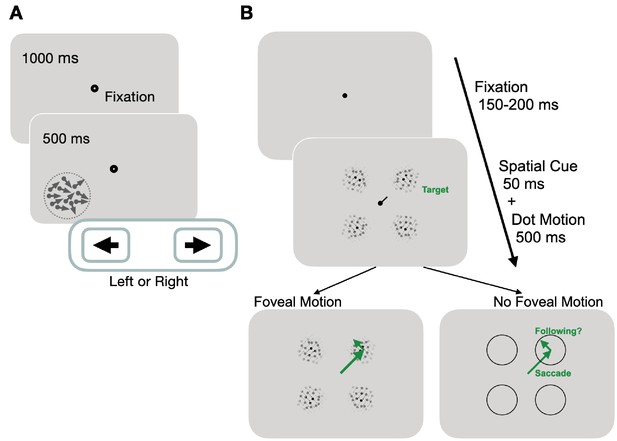
Experimental paradigms for measuring motion perception and oculomotor functions.
(A) Trial sequence for assessing global motion perception: trials started with a fixation period of 1000 ms, followed by appearance of a random dot stimulus either in the blind or intact field for 500 ms. Dots moved globally to the right or left, with a range of directions defined an adaptive staircase. On each trial, subjects were asked to report the stimulus’ global direction of motion by pressing the left or right arrow keys on a keyboard. They received auditory feedback on the correctness of each response. (B) Trial sequence for assessing oculomotor behavior: each trial started with a variable fixation period after which, participants were presented with four equi-eccentric motion apertures and a spatial cue at fixation. Dot motion apertures were Gaussian-enveloped and contained 100% coherent motion along a randomly assigned direction (clockwise or counter-clockwise) in each aperture, that was tangential to the center-out saccade to the aperture. The spatial cue (50 ms) indicated a peripheral target aperture toward which the participant was instructed to initiate a saccade as fast as possible. In half the trials, the dot motion stimuli persisted for 500 ms, and were thus present upon saccade offset. In remaining trials, stimuli disappeared during saccade flight, such that no stimulus motion was present at the fovea upon saccade landing.
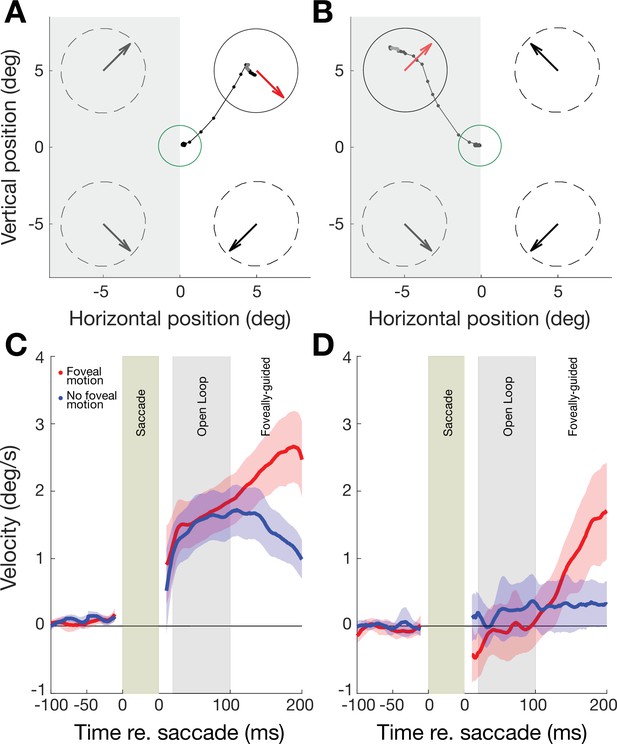
Oculomotor behavior in perceptually trained, 11 V1-stroke participants.
(A) Eye movement traces to a cued target in the intact field (white background) of a single V1-stroke patient in a stimulus absent condition (i.e. with no foveal motion upon saccade landing at the cued target). Small, connected black dots denote the raw eye movement sampled from our eye tracker; the green circle represents the electronic window around the fixation spot; random dot stimuli were presented inside the four dashed circles and their global motion direction is indicated by large arrows inside each circle. Note the accurate saccade to the target center, and how the eye follows the pre-saccadic target motion direction (red arrow) upon saccade landing. (B) Raw eye movement traces to a cued target in the blind field (gray background) of a single V1-stroke patient in a stimulus absent condition. Note successful saccade landing onto the cued target but how the eye fails to follow the pre-saccadic target motion direction. Labelling conventions as in A. (C) Eye velocity traces for saccades to intact portions of the visual field, averaged across intact field locations per patient then averaged across all 11 stroke patients. In half the trials, stimuli were present upon saccade landing, resulting in foveal motion (red trace). In the remaining trials, stimuli were absent – that is, there was no foveal motion upon saccade landing on the target (blue trace). Error bars represent ± 2 SEM across subjects. Average eye velocities were projected along the target motion direction time-locked prior to the saccade onset (−100 to 0 ms) and offset (0 to 200 ms), such that positive values reflected motion consistent with the stimulus, while negative values reflected motion opposite. (D) Eye velocity traces for saccades to blind portions of the visual field, averaged across blind field locations per patient and then averaged across all 11 stroke patients (same conventions as in A). Error bars represent ± 2 SEM across subjects. Note the near-zero eye movement velocity during the ‘open-loop’ period, reflecting the lack of post-saccadic following response (PFR).
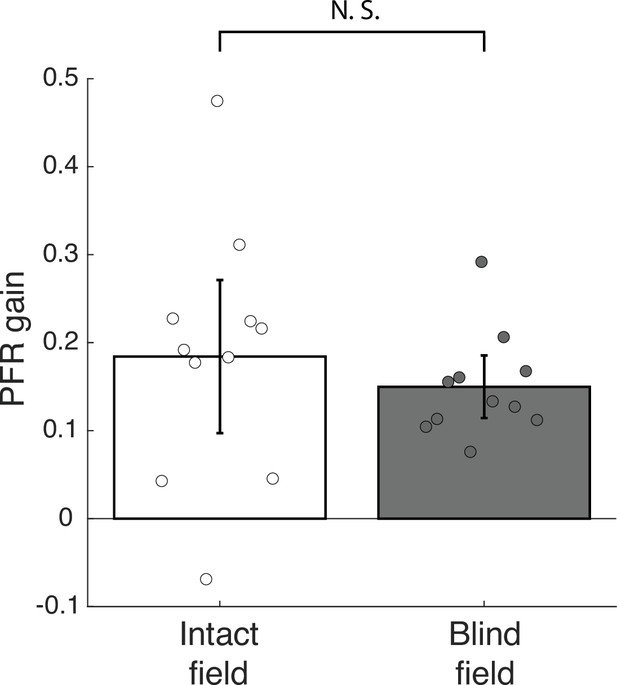
Post-saccadic following response (PFR) gain in the intact and blind field of V1-stroke patients during the foveally-guided period.
When stimulus motion was presented foveally after the saccade on trials where the stimulus remained present, patients exhibited following movements for both intact and blind visual fields. Following was evidenced by a positive PFR gain that emerged after a visuomotor delay of about 100 ms (Figure 3D, red curves). To quantify the smooth following, PFR gain was computed for each patient measured during the ‘foveally guided period’, which was defined as 100–200 ms after the saccade offset for motion targets presented in cortically blind (CB) patients’ intact and blind fields (N=11 patients). Individual dots represent the mean PFR gains for each participant. Error bars represent ±2 SEM across subjects. The PFR gain is positive and significant for both the intact (18.6% gain, CI95 = [18.5%, 18.6%], t10=4.2314, p=0.0017, BF = 25.4627) and the blind fields (15.0% gain, CI95 = [14.9%, 15.0%], t10=8.4130, p<0.0001, BF > 1000) but no difference between intact and blind fields is found in this later period (t10=1.2041, p=0.2563, BF = 0.5355). Thus, CB patients generate smooth, following eye movements for stimulus motion at the fovea after a visual response delay. The data suggests that occipital stroke patients retain functional, smooth following eye movements at the fovea.
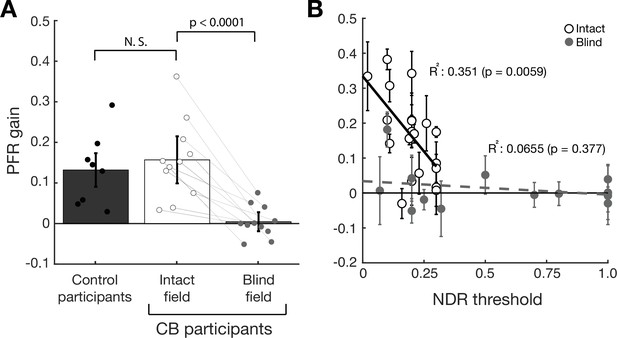
Post-saccadic following response (PFR) gain in the intact and blind field of V1-stroke participants.
(A) Plot of mean PFR gain in eight visually intact controls (Kwon et al., 2019), and in 11 V1-stroke patients’ intact and blind fields during the open-loop period. The PFR gain is represented as a proportion of pursuit gain along the target motion direction (1=perfect following, 0=no following, –1=following in the opposite direction). Individual dots represent the mean PFR for each participant. There was no significant difference in PFR gain between the intact fields of stroke patients and visually intact controls. PFR gain approximated 0 for saccades to targets in stroke patients’ blind fields. Individual gray lines represent PFR gains in intact and blind fields belonging to the same patients. Each error bars represent ± 2 SEM across subjects. (B) PFR gain for individual intact field locations (open circles, N=20) and (C) blind field locations (gray circles, N=14) as a function of the normalized direction range (NDR) threshold measured at each location in stroke participants. The solid line and dashed line represent the line of best fit for intact and blind field PFR gain as a function of NDR threshold. Within intact regions of the visual field, there was a negative correlation between this global motion threshold and PFR gain: the lower the threshold (i.e. the better the motion perception), the higher the PFR gain. This relationship was lost in the blind field of stroke patients, with no significant correlation between NDR thresholds and PFR gain – the latter remaining abnormally low. Error bars represent 2 standard deviations per visual field locations.
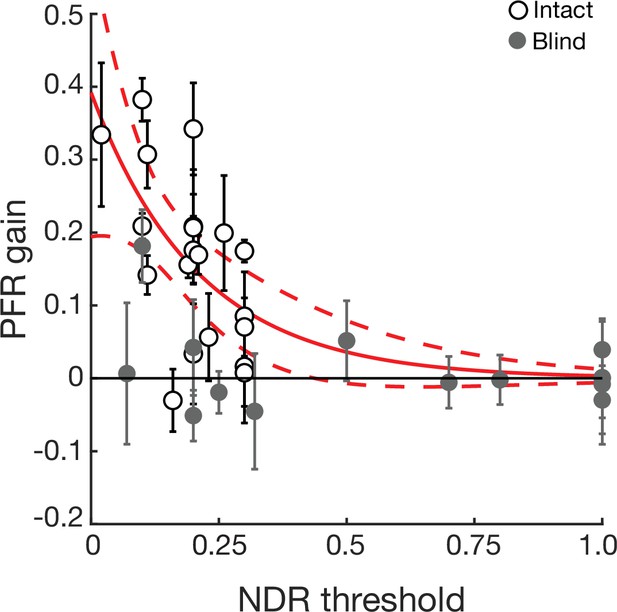
Post-saccadic following response (PFR) gain as a function of normalized direction range (NDR) thresholds.
An exponential fit to the intact visual fields was computed to describe the dependence of PFR on the NDR threshold performance (goodness of fit: SSE = 0.1717, R2=0.3502, and RMSE = 0.0977). The plot shows PFR gains for individual intact field locations (open circles, N=20) and blind field locations (gray circles, N=14) as a function of the NDR threshold measured at each location in stroke patients. The bold red line represents the model fit based on the intact visual field data. The dashed red lines represent the confidence interval boundaries at 95% and the error bars represent ± 2 SD. Out of a total of six blind field data points, five lie outside these confidence intervals, and also demonstrate respective confidence intervals on their PFR gain that are overlapping or below zero. The probability that five of six points would lie outside the 95% confidence intervals, assuming they came from the same distribution as intact fields is highly improbable (p ≤ 1.79 × 10–6, binomial test). A single point, which fell within the range of the intact visual fields, was unusual in other ways. Unlike other blind field locations in the study, this point (Figure 1, CB6, lower left quadrant) did exhibit significant detection performance in Humphrey visual field tests (spared Humphrey visual field performance – i.e. luminance detection), even though global motion discrimination at that location was impaired and thus selected for training (see Materials and methods). However, this point was included to be consistent and more conservative in the analyses. Nonetheless, even when this potential outlier was included, there exists a quantitative evidence to support a distinction in PFR gain between intact and blind fields within the range where normal motion discrimination performance (NDR <0.35) has recovered.
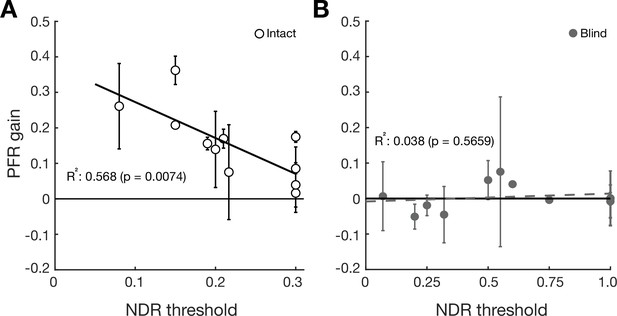
Post-saccadic following response (PFR) gain as a function of normalized direction range (NDR) thresholds pooled per V1-stroke patients.
The plots show PFR gain for individual intact field locations (Panel A, open circles) and blind field locations (Panel B, gray circles) as a function of the NDR threshold measured per each stroke patient (N=11). The error bars represent ± 2 SEM. The solid line and dashed line represent the line of best fit for intact and blind field PFR gain as a function of NDR threshold. After pooling all the fields per subject, the previous the trend in which the lower NDR is correlated with higher PFR gain in the intact fields (R2=0.568, F9=11.8334, p=0.0074, BF = 8.0855) persists and there is no significant correlation between NDR and PFR gain in the blind fields (R2=0.038, F9=0.3552, p=0.5659, BF = 2.650). Further, for NDR <0.35, the correlation between PFR and performance remains significant for intact fields (R2=0.568, F9=11.8334, p=0.0074, BF = 8.0855) but not for blind fields (R2=0.5406, F2=2.3538, p=0.2647, BF = 0.7678). When the slopes from each linear regression with respect to intact fields and blind fields under NDR <0.35 were compared, the slopes (–1.012 for intact and –0.1845 for blind) were significantly different from each other (t11=−2.6039, p=0.0245). Thus, the main findings hold when visual fields are pooled per participant to insure independence.
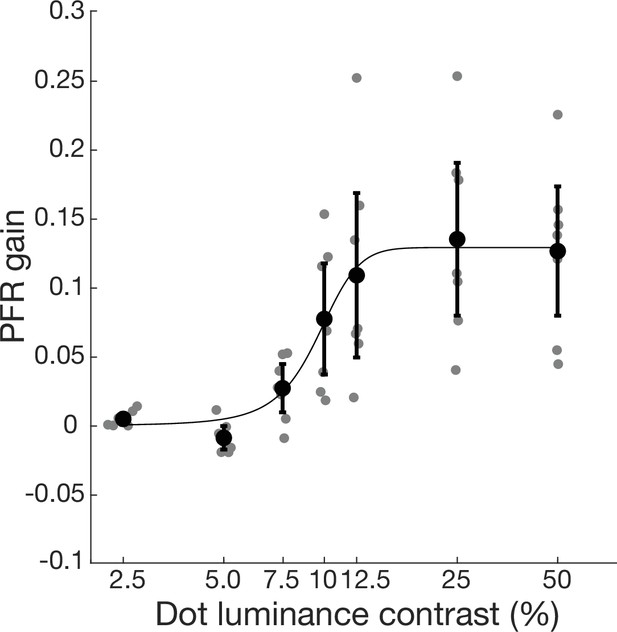
Luminance contrast-dependence of the post-saccadic following response (PFR) gain in visually intact controls.
A logistic function (sigmoid fit) was used to represent the best fit to the average PFR data across contrasts. Visually intact participants (N=7) performed the same task as described in the main PFR experiment except that in each session, the target aperture was presented with a fixed stimulus luminance contrast between 2.5% and 50%. Large black symbols and error bars represent ± 2 SEM of PFR gain across participants. Small gray dots represent individual participant PFR gains at each luminance level. PFR gain increased steeply with stimulus contrast, starting around 5–7.5% contrast and reaching saturation quickly between 12.5% and 25% contrast (R2=0.9862, SSE = 0.000293, RMSE = 0.0086). This is comparable to neuronal responses in cortical area middle temporal (MT) which are sensitive to low stimulus contrasts and saturate in response at roughly 10% or higher contrasts (Heuer and Britten, 2002; Kohn and Movshon, 2003; Sclar et al., 1990). Next, to what extent behavior of cortically blind (CB) patients in the blind field might reflect a response to a stimulus of effectively reduced contrast was considered. For comparison, when CB patients performed the same test in their intact field with 100% contrast, the observed mean PFR was 0.1448, a value that matched the performance of intact controls for contrasts >25% (Figure 4—figure supplement 4). The lowest contrast for which the PFR was significantly different from zero in controls was 7.5% (t6=0.3150, p=0.0202). Thus, if recovered motion perception in the blind field resembles a lower-contrast stimulus representation, then we would estimate its contrast to be less than 7.5%.
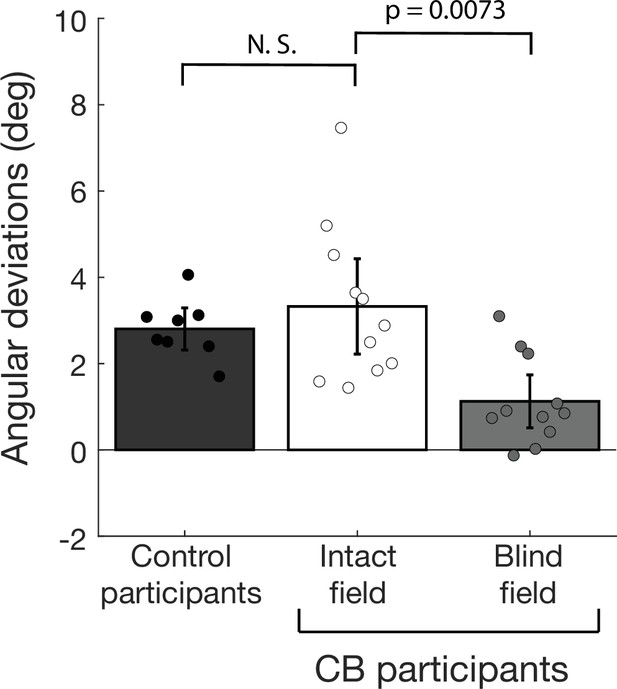
Saccade angular deviations in the intact and blind field of cortically blind (CB) participants.
Previous studies showed that location of an aperture is perceived as shifted along the direction of target motion contained in the aperture (De Valois and De Valois, 1991; Kwon et al., 2015; Ramachandran and Anstis, 1990). This reflects a perceptual mis-localization error along the target motion direction that also influences saccade programming by causing saccade end-points to be shifted along the target motion (Kosovicheva et al., 2014). Consistent with previous studies, another earlier study in visually intact controls found the position of saccade end-points to be displaced along the direction of dot motion contained in a peripheral target aperture (Kwon et al., 2019). Thus, like the post-saccadic following response (PFR), saccade end-points are also influenced by pre-saccadic selection of target motion, providing another measure of the predictive influence of target motion. A previous study also found that saccade end-points provide a measure that correlates well with perception (Kosovicheva et al., 2014). Thus, the deviations of saccade end-points were analyzed to investigate if they were biased by the direction of motion in both the blind and intact fields of our CB participants, and whether restoration of motion perception in the blind field influenced these saccade parameters. For each saccade, the angle of the line from fixation to the saccade end-point relative to the line from fixation to the center of the target aperture was computed. Positive angular deviations were interpreted to reflect a bias along the target motion. The mean saccade angular deviations in 8 visually intact controls (from Kwon et al., 2019), and for motion targets presented in 11 CB patients’ intact and blind fields were computed as shown in the figure. Individual dots represent the mean saccade angular deviations for each participant. Error bars represent ± 2 SEM across subjects.The visually intact controls (from Kwon et al., 2019) showed a net positive saccade angular deviations that differed significantly from 0 (t7=11.53, p<0.001 – leftmost gray bar). This was also observed in CB patients, in intact portions of their visual fields (3.33°, CI95 = [3.32°, 3.34°], t10=6.0170, p<0.0001, BF > 100 – white bar). In fact, there were no significant differences between saccade end-point deviations between the two groups (t17=−0.7607, p=0.4573, BF = 0.4982). In the blind field of CB participants, saccade angular deviations were smaller than in their intact fields (t10=3.3586, p=0.0073, BF = 7.8454 – light gray bar), but unlike the PFRs, they were greater than 0 (1.12°, CI95 = [1.11°, 1.13°], t10=3.6538, p=0.044, BF = 11.7368), providing positive evidence of pre-saccadic motion integration within the blind field. Thus, occipital stroke does not abolish motion-induced perceptual shifts reflected by saccade targeting.
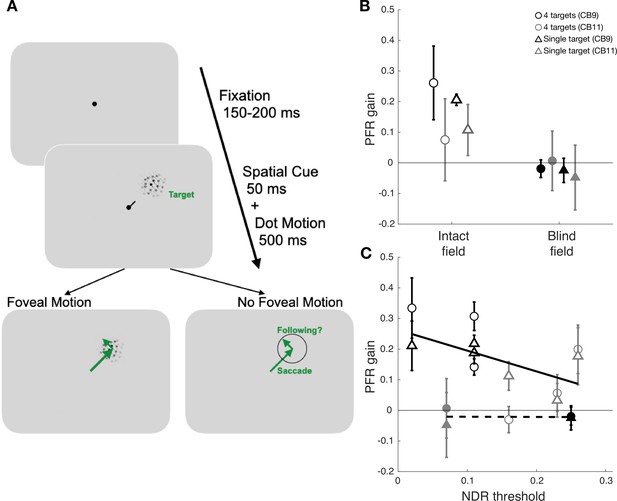
Experimental paradigm for measuring oculomotor functions with a single saccade target.
To test for deficits in attention allocation into the blind field, a control experiment was conducted in two new participants. This experiment tested whether post-saccadic following response (PFR) gain might be recovered when competing distractors in the visual field were removed. This control was designed to rule out the possibility that attention impairments with respect to the blind field might emerge because attention is preferentially pulled/allocated toward stimuli that appear within intact fields, due to their higher saliency. (A) Trial sequence of assessing oculomotor behavior for a single saccade target without distractors. The paradigm followed the main experimental sequence as defined in Figure 2B except only a single target that matched the spatial cue was presented at every trial. A central line cue directed patients for where to saccade, and as in the main experiment, their saccades remained accurate for both intact (98.60% ± 1.15%) and blind field (98.72% ± 0.24%) locations. (B) PFR gain across two patients’ intact and blind fields for the four aperture and single target experiments. The open symbols represent the intact field data while filled symbols represent the blind field data. The circles represent the four apertures experiment and the triangles represent the single aperture experiment. The color (gray or black) represents each participant. For intact visual fields, each data point represents the average across the three intact fields. The error bars denote the mean ± 2 SEM. There was no measurable PFR in the blind fields (t3=−1.8996, p=0.1537, BF = 1.1120), while there was robust PFR in the intact fields (t3=3.7698, p=0.0327, BF = 3.1780). PFR in intact fields was significantly larger than in their blind fields (t3=3.9793, p=0.0284, BF = 6.8888), and the blind fields still had PFR that was not significantly different from zero (t3=-1.8996, p=0.1537, BF = 1.1120). (C) PFR gain as a function of NDR thresholds in each individual visual field location. The solid line and dashed line represent the line of best fit for intact and blind field PFR gain as a function of NDR threshold. Note, there are more data points because individual intact fields are shown across NDR rather than averaged as a single point. There remained a weak correlation between PFR and normalized direction range (NDR) threshold for intact fields (R2=0.2818, F10=3.9243, p=0.0757, BF = 1.0577) when compared with the blind fields (R2=0.0010, F2=0.0019, p=0.9690, BF = 0.3368). Thus, removing competing distractors in the other three aperture locations had little impact on the PFR suggesting the deficit was sensory in nature rather than involving a misallocation of attention, indicating that attention allocation appears to be intact in V1-stroke patients.
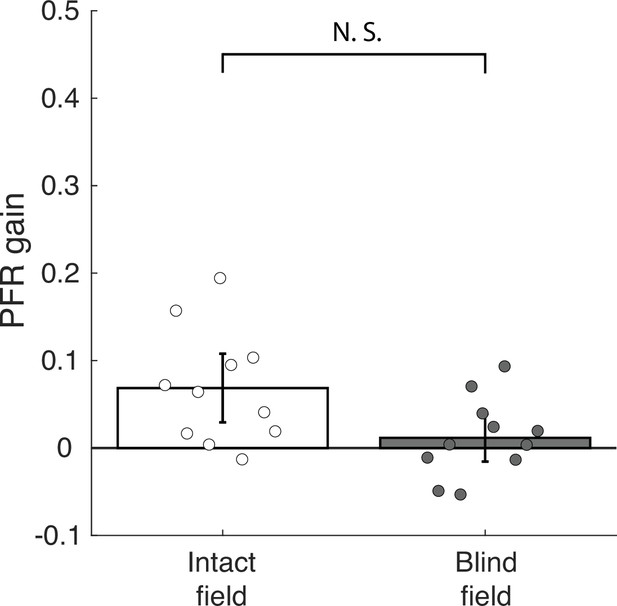
Post-saccadic following response (PFR) gain in the intact and blind field of V1-stroke patients driven by motion stimuli in the distractor apertures.
As a final control analysis, the control analyses was performed to test what extent PFR was driven by the motion in distractor apertures. If the motion in the other three apertures produced any measurable influence on the PFR, then one might expect that influence to be stronger when saccades were made into the blind field because attention could not be properly allocated to its location, and instead would be allocated to intact field distractor locations with more salient motion targets. To test for this we repeated our PFR analyses but instead projected it onto each of the distractor aperture’s motion directions, averaging across all three of the distractor apertures. Plot of mean PFR gains driven by motion in distractor apertures when cortically blind (CB) patients made a saccade either into an intact or blind field. PFR gains driven on average by the other three distractor apertures. Error bars represent ± 2 SEM across subjects. Motion in distractor apertures contributed to a weak PFR gain when saccades were performed to a target in an intact visual field (6.85%, CI95 = [6.81%, 6.88%], t10=3.4926, p=0.0058, BF = 9.4228), but for saccades made into the blind field there was no measurable PFR gain for distractor locations (1.16%, CI95 = [1.14%, 1.19%], t10=0.8594, p=0.4102, BF = 0.4054), which does not support the hypothesis that greater attention was allocated to distractors for saccades made into the blind field. Although the PFR gains were not significantly different when saccades were made into the intact or blind fields (t10=2.0156, p=0.0715, BF = 1.3046). Thus, analysis of PFR for distractor apertures does not support that attention was mis-allocated for saccades made into the blind field. It seems unlikely that the lack of PFR in the blind field reflects an impairment to engage pre-saccadic attention.
Tables
Demographics and global motion integration thresholds in retrained, V1-stroke participants.
M, male; F, female; NDR, normalized direction range (low value = good performance, 1 = bad performance/no measurable threshold). Each NDR threshold denotes performance measured at a single blind or intact field location in each patient (see Figure 1 for positioning of these locations relative to the pre-training Humphrey visual field). Pre-training NDR thresholds were always 1 in blind fields.
| Subject | Sex | Age (years) | Time post-stroke (months) | Blind field NDR thresholds | Intact field NDR thresholds |
|---|---|---|---|---|---|
| CB1 | F | 27 | 65.0 | 1 | 0.3 |
| CB2 | F | 68 | 24.8 | 0.2 | 0.2 |
| CB3 | F | 57 | 48.6 | 0.7, 0.8 | 0.3 |
| CB4 | M | 66 | 32.2 | 0.5 | 0.2, 0.2, 0.2 |
| CB5 | M | 54 | 52.2 | 1 | 0.3, 0.3 |
| CB6 | M | 79 | 22.7 | 1, 0.1 | 0.1, 0.2 |
| CB7 | M | 53 | 36.8 | 1 | 0.3 |
| CB8 | M | 52 | 65.5 | 1, 0.2 | 0.1, 0.2 |
| CB9 | F | 65 | 56.5 | 0.3 | 0.1, 0.1, 0.02 |
| CB10 | F | 31 | 66.8 | 0.3 | 0.2 |
| CB11 | F | 43 | 6.0 | 0.1 | 0.3, 0.2, 0.2 |





