Taste sensing and sugar detection mechanisms in Drosophila larval primary taste center
Figures
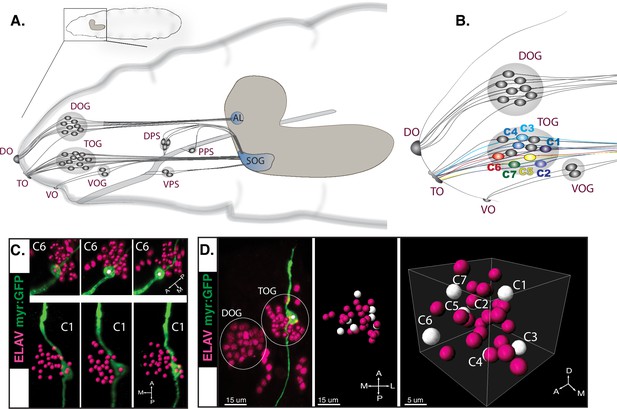
Peripheral taste neurons of the fruit fly larva.
(A) The chemosensory system of the larva. External sense organs extend dendrites to the periphery and have main olfactory function—the DOG (dorsal organ ganglion), or gustatory function—the TOG (terminal organ ganglion) and the VOG (ventral organ ganglion). Dendrites of the internal dorsal, ventral, and posterior pharyngeal sense organs (DPS, VPS, and PPS) innervate the pharynx, thus involved in taste sensing during food ingestion. All chemosensory neurons project axons to the brain in the subesophageal ganglion (SOG)—first central taste integration relay—or to the antennal lobe (AL) for central olfactory processing. (A) Has been adapted from (C) from Gerber and Stocker, 2007. (B) External chemosensory organs. Seven GSNs previously identified and named C1–C7 are represented here by the color code they were first described with (van Giesen et al., 2016a; Kwon et al., 2011). Used Gal4 lines: Gr22e-Gal4 (C1), Gr94a-Gal4 (C2), Gr66a-Gal4 (C1, C2, C3, and C4), Gr59e-Gal4 (C5), Gr21a-Gal4 (C6), and GMR57BO4-Gal4 (C7). (C) UAS-myrGFP reporter was expressed in individual GSNs using corresponding Gal4 lines (B). We observed a relatively stereotypic position of specific neurons within the organ across animals (n≥3)—exemplified on C6 (Gr21a-Gal4) and C1 (Gr22e-Gal4). (D) Illustrative 3D map of TOG segmented cells. Position of neurons with known identities (white dots) is approximated based on separate immunostainings.
© 2007, Gerber and Stocker. Figure 1A has been adapted from Figure 1C from Gerber and Stocker, 2007.
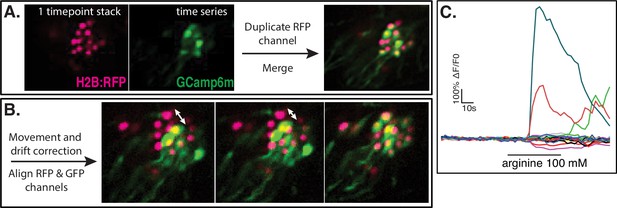
Whole-organ calcium imaging—data processing.
(A) The RFP nuclear signal acquired as a single stack was duplicated to all time points of the GCaMP time-series using an in-house written plugin. Step performed in Fiji/ImageJ. (B) On the recording comprising both fluorescence channels, any misalignment between the two signals due to animal movement or slight drift was corrected through a manually assisted macro. Step performed in Fiji/ImageJ. The resulting stabilized and aligned recording was transferred to Imaris v9.6.0 for cell segmentation. (C) Fluorescence traces extracted from all GRNs of one preparation stimulated with arginine 100 mM.
-
Figure 1—figure supplement 1—source data 1
Whole organ calcium imaging—data processing.
CaImg_analysis_pipeline contains ImageJ scripts for macro/plugin and instructions.
- https://cdn.elifesciences.org/articles/67844/elife-67844-fig1-figsupp1-data1-v2.zip
Whole-organ recording and cell segmentation.
A recording of the TOG (larval main peripheral taste organ) using genetically encoded GCaMP reporter in all neurons. Nuclear RFP expression is used for cell segmentation, allowing the creation of spots corresponding to each individual neuron. GCaMP fluorescence intensity over time for each spot is represented by spot color, ranging from blue (low intensity) to red (high intensity).
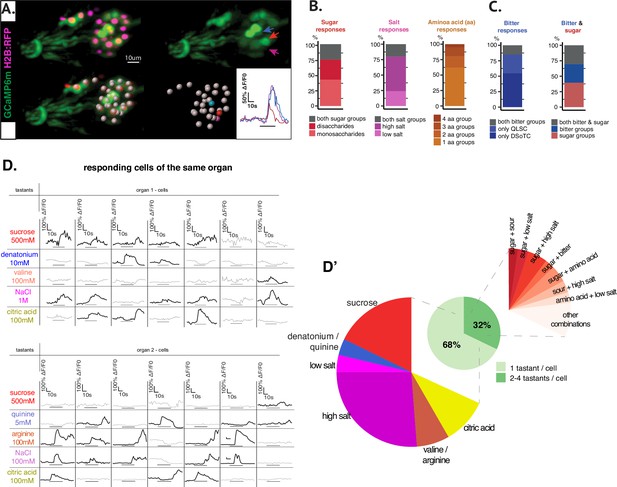
Whole-organ calcium imaging shows an overview of response tuning of GRNs.
(A) Representative recording of the larval primary taste organ stimulated with citric acid 100 mM. Cytoplasmic expression of GCaMP6m and nuclear expression of RFP in all neurons using nsyb-Gal4 driver line (upper left panel) and cell segmentation (white spots, lower left panel). Responding neurons are indicated by blue, red, and magenta arrows (upper right panel) and corresponding dots and fluorescence traces in the same colors (lower right panels). (B) Stimulation with groups of tastants by taste modality, in order: two sugar categories (mono and disaccharides), low and high concentration salts, and four amino acid groups. A response intensity threshold of 20% DF/F was considered and neuronal responses across different preparations were pooled together. Among all neurons responding to sugar group stimulation, a comparable ratio of cells showed activation to either one or the other category and 25.4% to both groups of mono and disaccharides. For salt-responding neurons, a higher proportion of neurons showed activation to high salt than to low salt concentration, and 20% neurons responded to both categories. Amino acids split into four groups gave a rounded 62% of uni-group responses and the rest of 38% neurons responded to two or more amino acid groups per animal. (C) Responses in animals stimulated with two groups of bitter compounds or with both sugar and bitter test groups. Within the total number of bitter-responsive neurons, 55% were uniquely activated by the DSoTC group and respectively 30% by QLSC group. In animals stimulated with both modality groups, integration between sweet and bitter taste has been calculated and we observed similar proportions of taste cells responding only to bitter groups (30.3%), cells responding only to sugar (39.4%) and cells responding to groups of both modalities (30.3%). (D) GRN responses to series of individual tastants. Taste stimulation was done using a series of five tastants per animal, each belonging to one of the five canonical taste modalities represented by sucrose 500 mM (sweet/red color), denatonium 10 mM or quinine 5 mM (bitter/blue), NaCl 1 M for high salt or NaCl 100 mM for low salt (salt/purple), valine 100 mM or arginine 100 mM (amino acids/brown) and citric acid (CiA) 100 mM (sour/yellow). Seven and respectively eight separate organs were recorded within the two series of stimulation (N=15) and a total of 197 neurons showed responses above 20% DF/F0 and were further analyzed. The table shows illustrative traces for cells responding to one or more tastants in two separate representations, each for one of the two stimulation sets. (D’) Total percentages of taste integration in pooled data are represented on the pie chart: 68% of total responding neurons were activated by only one tastant per organ and up to 32% of neurons responded to more than one tastant. Most unimodal responses (uni-taste/cell, left extended pie) were recorded to sucrose and to high salt. Conversely, numerous neurons were activated by different combinations of taste categories, with different frequency of occurrence (multi-taste/cell, right extended pie). Among the most frequent combinations of taste modalities activating the same cell we noted sucrose (sweet)+ any other taste category. Some of these co-modalities involve a presumed positive valence taste (sucrose/sweet) together with a negative valence taste (bitter or high salt) sensed by the same neuron.
-
Figure 2—source data 1
Referring to graphs B & C.
Excel files comprising neuronal calcium imaging data: sheet 1 represents a summary of cell and response numbers to different modalities, sheet 2 contains the list of neurons activated by taste groups of sugar, salts, and amino acids, and sheet 3 contains the list of neurons activated by taste groups of sugar and bitter in Figure 2C. Each cell in the list received a cell name (cell_name_in_prep) as identifier for the given biological replicate, not valid for cell mapping.
- https://cdn.elifesciences.org/articles/67844/elife-67844-fig2-data1-v2.xlsx
-
Figure 2—source data 2
Excel sheet one refers to data in Figure 2D’, comprising the list of neuronal responses above 20% DF/F0.
Signals below this threshold have not been used for figure generation; cell_name_in_prep is the cell identifier in a given biological preparation; map_cell_name refers to cell response mapping presented in Figure 3 and Figure 3—figure supplement 1: all activation signals were considered comprising both ON and OFF responses and map_cell_name is the id for cell mapping across different biological preparations.
- https://cdn.elifesciences.org/articles/67844/elife-67844-fig2-data2-v2.xlsx
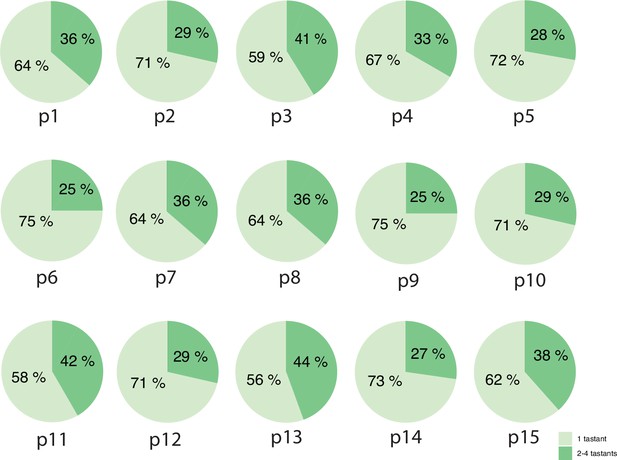
Whole-organ calcium imaging—an overview of response tuning of GRNs per recorded organ.
Percentages of uni/multimodal taste integration per each recorded organ. The total average is 67% unimodality versus 33% multimodality, numbers very closely mirroring the 68%/32% fraction from the alternative representation of pooled data in Figure 2D’.
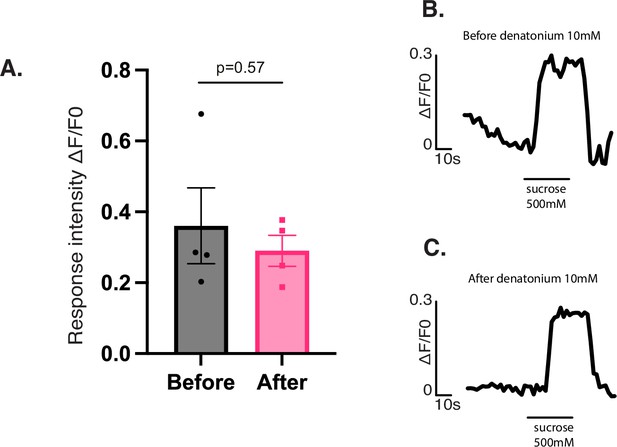
Single neuron sugar responses based on order.
(A) Single neuron calcium imaging responses to sugar before and after bitter tastant (denatonium 10 mM) presentation. Response intensity of the C7 neuron to sucrose before denatonium presentation was not significantly different from responses after denatonium presentation. N=4 for both trials, one-way ANOVA test, plot as mean with SEM. (B, C) Representative calcium imaging traces for responses of the C7 neuron to sucrose before and after denatonium presentation, respectively.
-
Figure 2—figure supplement 2—source data 1
Referring to Figure 2—figure supplement 2.
Recorded responses and p-values.
- https://cdn.elifesciences.org/articles/67844/elife-67844-fig2-figsupp2-data1-v2.xlsx
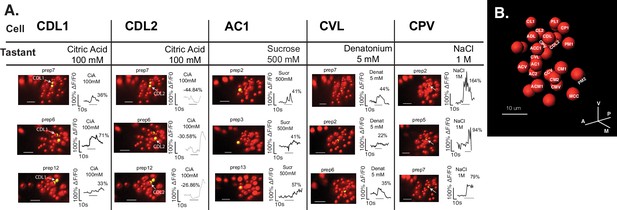
Taste-elicited responses mapped to neurons across animals.
(A) Illustrative traces for a given tastant stimulation from three different organs are shown for manually mapped neurons. (B) Representative map used for matching neuronal identities across different recorded organs. Names were chosen as reference for difference in neighboring cell positions, suggested with respect to the sagittal plane, anterior-posterior, and ventral-dorsal axis (C=central/center, V=ventral, D=dorsal, P=posterior, A=anterior, M=medial/n, L=lateral).
-
Figure 3—source data 1
Referring to Figure 3 and Figure 3—figure supplement 1C; excel file comprising responses associated to mapped cells.
Data were considered for responses mapping to a given cell (map_cell_name) at least in four different preparations, N≥4 (exception for bitter stimulation, N≥3). CDL2 neuron associates deactivation responses as represented in Figure 3—figure supplement 1B.
- https://cdn.elifesciences.org/articles/67844/elife-67844-fig3-data1-v2.xlsx
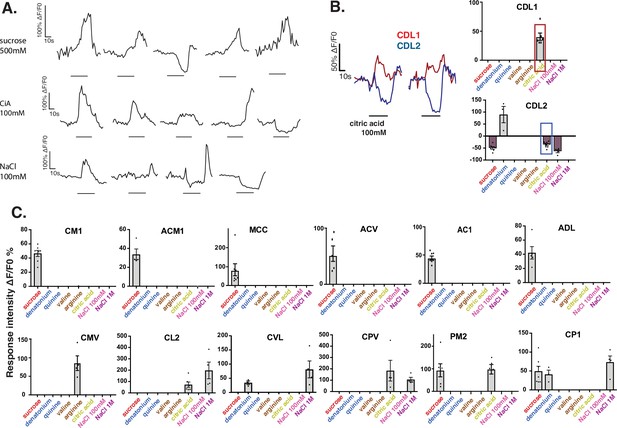
Responses across animals show broad tunning of GRNs and distinct temporal dynamics.
(A) Types of responses elicited by a given taste substance in different neurons of the same organ. Illustrated are traces of response to sucrose 500 mM, citric acid (CiA) 100 mM, and NaCl 100 mM in different neurons belonging to the same organ. Black horizontal line under each trace corresponds to the onset and duration of the stimulus. (B) Illustrative traces elicited by citric acid in neighboring neurons CDL1 and CDL2 (cell position illustrated in examples from Figure 3A) show distinct response dynamics: fluorescence rise in CDL1 and concomitant fluorescence decrease in CDL2. CDL2 associates deactivation responses also to other tastants as seen in the right panel graph, but most robustly to citric acid. (C) Responses identified in organs stimulated with series of individual tastants (Figure 2D) were assigned to respective physical locations for mapping and assigning neuronal names (See Figure 2—source data 2, sheet 2). Some cellular locations (neuron identities) showed more consistent responses to single tastants (one modality), predominantly to sucrose, while several others showed multimodal tuning. Bar charts represent average fluorescence intensity with SEM and the dots show values from separate recorded organs.
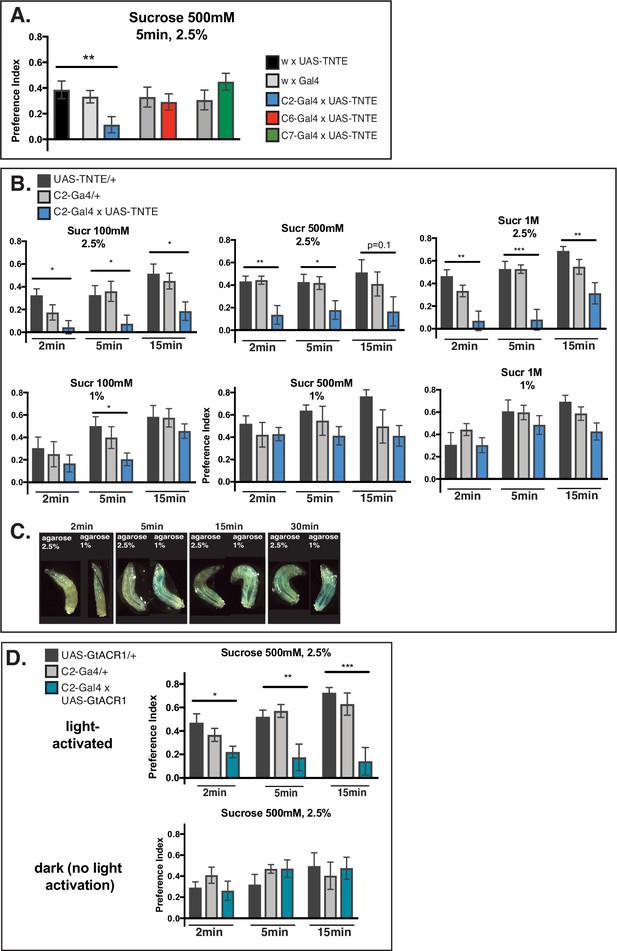
Mechanism of larval sugar taste at the periphery.
(A) Behavioral tests on C2, C6, or C7 neuron silenced larvae. Although all three neurons have previously been associated with physiological responses to sucrose 500 mM (van Giesen et al., 2016a), a defect in preference index to Sucrose 500 mM compared to control larvae is found only for C2 (p-value=0.038), indicating a direct contribution of this neuron in sucrose sensing. Driver lines: Gr94a-Gal4 (C2), Gr63a-Gal4 (C6), and GMR57BO4-Gal4 (C7). N=10 for all genotypes; one-way ANOVA; *p<0.05, **p<0.005, ***p<0.001; plot as mean with SEM. (B) The role of the C2 neuron in preference to a range of sucrose concentrations on 1% versus 2.5% agarose. Larvae with C2 silenced show a greater defect for sugar preference on 2.5% agarose compared to 1% for all concentrations. This suggests larvae rely at least partly on C2 in sucrose sensing on a denser substrate. At 1%, C2 silencing has a lesser effect on sugar preference, suggesting that other mechanisms come into play for behavior on a softer substrate. N=10 for all genotypes; one-way ANOVA, *p<0.05, **p<0.005, ***p<0.001; plot as mean with SEM. (C) Food ingestion measurement using blue dye. Larvae were tested at different timepoints to compare eating on agarose 1% versus agarose 2.5%, both containing Sucrose 500 mM. Blue coloring of the intestine was observed as early as 2 min on 1% concentration, while on 2.5% animals didn’t seem to be able to eat as easily as indicated by the lack of tint of their abdomen at 2 min and 5 min testing. For longer time points, larvae also start showing food ingestion on 2.5% agarose, but the intestinal blue dye is less prominent than on 1% concentration. This suggests that animals easily eat the softer 1% food substrate but that on denser 2.5% concentration ingestion is slower and evidently impaired especially for early test time points. (D) Temporally restricted inhibition of C2 in sucrose 500 mM sensing. Alternative silencing of C2 using G. theta anion channelrhodopsin 1 (GtACR1) confirms the observed defect for sugar preference on 2.5% agarose across the timelapse. In dark conditions (no light activation), a defect is not noted. N=7–8; one-way ANOVA, *p<0.05, **p<0.005, ***p<0.001; plot as mean with SEM.
-
Figure 4—source data 1
Referring to Figure 4.
The excel file contains larval preference indexes and p-values Mann-Whitney test comparisons shown in the figure.
- https://cdn.elifesciences.org/articles/67844/elife-67844-fig4-data1-v2.xlsx
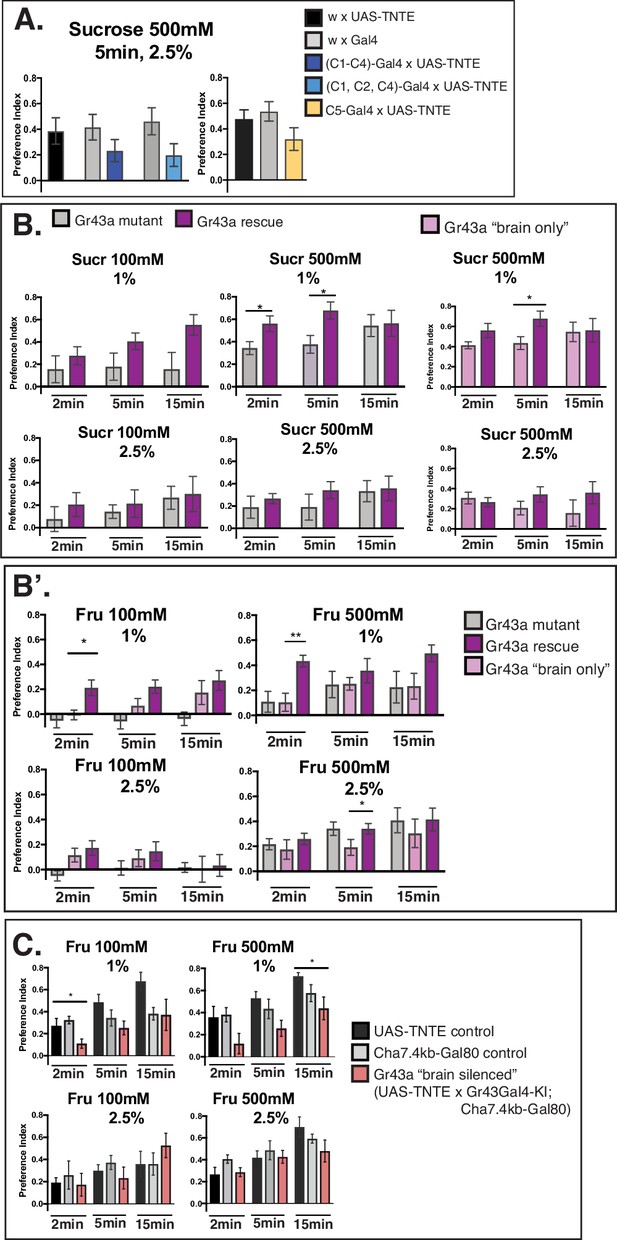
Mechanism of larval sugar taste at the periphery.
(A) Behavioral tests on C1–C5 neuron silenced larvae. Several neurons expressing Gr33a/Gr66a bitter receptors were silenced (driver line Gr66a-Gal4 for neurons C1–C4—preference index in dark blue and driver line Gr59d-Gal4 for neurons C1, C2, and C4—preference index in light blue), showing a reduced preference to sucrose 500 mM similar to the observed defect in C2 silenced larvae in Figure 4A. Preference index to sucrose in larvae with C5 neuron (Gr59e-Gal4) silenced is smaller than control, indicating a potential participation of C5 in larval sucrose taste. N=10 for all genotypes except for Gr59d-Gal4 (N=5–7); one-way ANOVA; plot as mean with SEM. (B) Role of the Gr43a in preference to sucrose on 1% versus 2.5% agarose: Sucrose 100 mM and 500 mM preference in Gr43a mutant against rescue, sucrose 500 mM preference in rescue against Gr43a-‘brain only’ expression. N=7–10; Mann-Whitney test, *p<0.05, **p<0.01, ***p<0.001; plot as mean with SEM; comparisons between mutant against rescue and rescue against Gr43a-‘brain only.’ (B’) Role of the Gr43a in preference to fructose on 1% versus 2.5% agarose: Fructose 100 mM and 500 mM preferences in Gr43a mutant, Gr43a-‘brain only’ and rescue. Preference to fructose 100 mM on 1% reproduces previous observations (Mishra et al., 2013) that a Gr43a null mutant comports a lasting alteration of preference, whereas the ‘brain-only’ expression gradually rescues the phenotype after immediate preference—here at 5 min. Similar preference trends have been recorded for the higher fructose concentration at 500 mM, except that the mutant and the ‘brain-only’ expression become indistinguishable suggesting that Gr43a only partially accounts for preference at higher fructose concentration, perhaps due to compensation of sensing through external neurons TOG. Overall, pharyngeal Gr43a guides immediate preference for fructose at both low and high concentration on 1% agarose (upper panels) but this effect is dampened at 2.5% agarose when feeding is decelerated (lower panels). Also, the role of Gr43a in sucrose preference (B) seems to be less prominent than for fructose. N=8–15; one-way ANOVA, *p<0.05, **p<0.01, ***p<0.001; plot as mean with SEM. (B, B’) As described before (Mishra et al., 2013) genotypes were: Gr43a-Gal4-ki/Gr43a-Gal4-ki; Cha7.4kb-Gal80/+ (Gr43a mutant), Gr43a-Gal4-ki/Gr43a-Gal4-ki; Cha7.4kb-Gal80/UAS-Gr43a (brain-only), Gr43a-Gal4-ki/Gr43a-Gal4-ki; UAS-Gr43a/+ (rescue). (C) Role of the silencing brain Gr43a in preference on 1% versus 2.5% agarose. Silencing brain Gr43a neurons shows overall similar preferences to fructose as control larvae, although altered behavior for this genotype was observed for both fructose concentrations on 1% but not on 2.5% agarose. This could be explained by an interference of brain Gr43a deficiency on internal signaling, seemingly on our hands more marked in effect than reported previously (Mishra et al., 2013). Genotypes used as described by Mishra et al., 2013: UAS-TNTE × w (UAS-TNTE control), Gr43a-Gal4-ki; Cha7.4kb-Gal80 × w (Cha7.4kb-Gal80 control), Gr43a-Gal4-ki; Cha7.4kb-Gal80 × UAS-TNTE (Gr43a brain receptor silenced). N=5–7; one-way ANOVA, *p<0.05, **p<0.01, ***p<0.001; plot as mean with SEM.
-
Figure 4—figure supplement 1—source data 1
File containing larval preference indexes presented in Figure 4—figure supplement 1, as well as corresponding statistics and p-values.
- https://cdn.elifesciences.org/articles/67844/elife-67844-fig4-figsupp1-data1-v2.xlsx
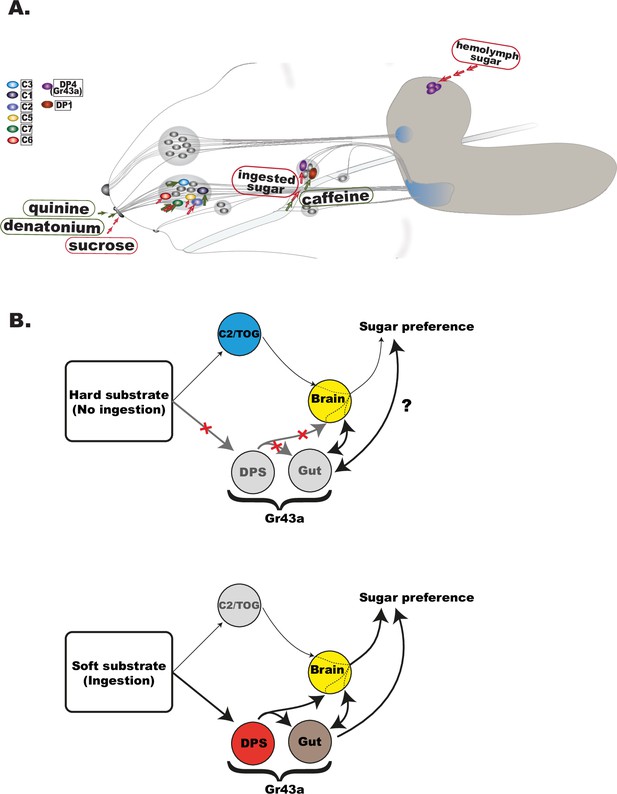
Peripheral taste sensing in Drosophila larvae—contribution to sugar and bitter taste of GRNs at different anatomical levels.
(A) Schematic representation of the larval head and the chemosensory system. Bitter tastants detected at the level of external sense neurons are quinine in C3 neuron (Apostolopoulou et al., 2014) and denatonium in C7 and C1 (van Giesen et al., 2016a; Choi et al., 2020) but not caffeine, the latter being sensed through DP1 pharyngeal neuron (Choi et al., 2016; Apostolopoulou et al., 2016). Similarly, sugar taste seems to be also segregated at different levels of detection—brain Gr43a receptor neurons detect hemolymph sugar (Mishra et al., 2013), Gr43a expression in the pharyngeal organs (DP4 neuron) is central in sensing ingested sugar, while neurons like C2 and C5 contribute to sucrose preference at external level especially when food ingestion is less accessible (Figure 4 and Figure 4—figure supplement 1). As the first gate for food evaluation, it is important to note the capacity of TOG neurons for integration of opposed valence taste wither within the same neuron as in C7 (van Giesen et al., 2016a), or by co-activation of a sugar sensing with a bitter sensing neuron as suggested for C2 and C1 (Hernandez-Nunez et al., 2015). (B) Schematic representation of the proposed model in larval sugar sensing. Upper panel: When unable to ingest as readily, larvae utilize C2 and/or other TOG neurons to guide sugar preference via a C2/TOG pathway. Gr43a is the main internal nutrient sensor, expressed in the pharyngeal taste neurons, proventriculus (gut), and brain. While the lack of ingestion prevents pharyngeal-expressing Gr43a neurons from contributing to immediate sugar preference, internal metabolic state signals may alter behavioral output. Lower panel: upon ingestion, internal sensing mechanisms take over sugar-associated preference most specifically for fructose, with a diminished role of external neurons (C2/TOG).
Tables
Chemicals used for taste stimulation in calcium imaging.
| Tastant or taste group | Compound | Associated taste (humans) | Concentration | Group final Concentration |
|---|---|---|---|---|
| Tastant series 1 | Sucrose | Sweet | 500 mM | – |
| Denatonium benzoate | Bitter | 10 mM | – | |
| Valine | Amino acid/Bitter | 100 mM | – | |
| NaCl | Salty | 1 M | – | |
| Citric acid | Sour | 100 mM | – | |
| Tastant series 2 | Sucrose | Sweet | 500 mM | – |
| Quinine | Bitter | 5 mM | – | |
| Arginine | Amino acid/Bitter | 100 mM | – | |
| NaCl | Salty | 100 mM | – | |
| Citric acid | Sour | 100 mM | – | |
| Group sugars Monosaccharides | Fructose | Sweet | 100 mM | 500 mM |
| Glucose | Sweet | 100 mM | ||
| Arabinose | Sweet | 100 mM | ||
| Mannose | Sweet | 100 mM | ||
| Galactose | Sweet | 100 mM | ||
| Group sugars Disaccharides | Sucrose | Sweet | 100 mM | 500 mM |
| Trehalose | Sweet | 100 mM | ||
| Maltose | Sweet | 100 mM | ||
| Lactose | Sweet | 100 mM | ||
| Cellobiose | Sweet | 100 mM | ||
| Group bitter DSoTC | Denatonium benz. | Bitter | 1 mM | 22 mM |
| Sucrose octaacetate | Bitter | 1 mM | ||
| Theophylline | Bitter | 10 mM | ||
| Coumarin | Bitter | 10 mM | ||
| Group bitter QLSC | Quinine | Bitter | 1 mM | 13 mM |
| Lobeline | Bitter | 1 mM | ||
| Strychnine | Bitter | 1 mM | ||
| Caffeine | Bitter | 10 mM | ||
| Group Amino acids A | Valine | Bitter | 10 mM | 60 mM |
| Leucine | Bitter | 10 mM | ||
| Isoleucine | Bitter | 10 mM | ||
| Methionine | Bitter | 10 mM | ||
| Tryptophan | Bitter | 10 mM | ||
| Cysteine | Bitter | 10 mM | ||
| Group Amino acids B | Alanine | Sweet | 10 mM | 41 mM |
| Phenylalanine | Bitter | 10 mM | ||
| Glycine | Sweet | 10 mM | ||
| Proline | Sweet | 10 mM | ||
| Tyrosine | Bitter | 1 mM | ||
| Group Amino acids C | Arginine | Bitter | 10 mM | 50 mM |
| Lysine | Salty-Bitter | 10 mM | ||
| Aspartic acid | Umami | 10 mM | ||
| Glutamic acid | Umami | 10 mM | ||
| Histidine | Bitter | 10 mM | ||
| Group Amino acids D | Serine | Sweet | 10 mM | 40 mM |
| Threonine | Sweet | 10 mM | ||
| Asparagine | Neutral | 10 mM | ||
| Glutamine | Sweet | 10 mM | ||
| Group high salt | NaCl | Salty | 500 mM | 1M |
| KCl | Salty | 500 mM | ||
| Group low salt | NaCl | Salty | 25 mM | 50 mM |
| KCl | Salty | 25 mM |





