An octopamine receptor confers selective toxicity of amitraz on honeybees and Varroa mites
Figures
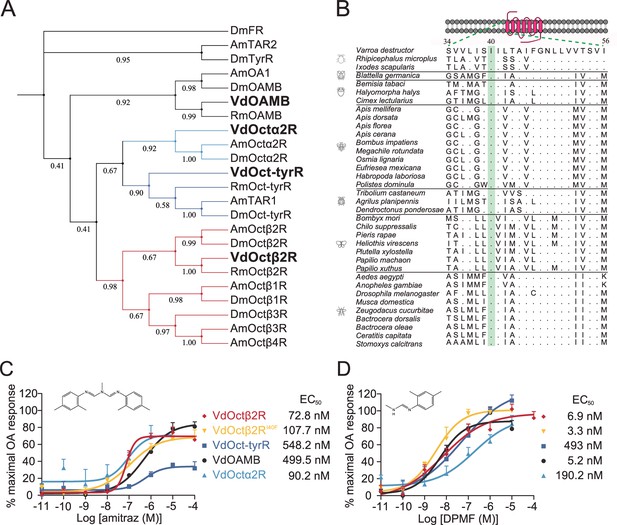
Amitraz and its main metabolite DPMF can activate Varroa multiple octopamine (OA) receptors in vitro.
(A) Phylogenetic tree of OA receptors from Varroa destructor, Apis mellifera, Rhipicephalus microplus, and Drosophila melanogaster. The values on the branches represent the bootstrap support. The candidate Varroa receptors are in bold. (B) The Isoleucine40 in the TM1 of Octβ2R, which is associated with amitraz resistance in the cattle tick Rhipicephalus microplus, is highly conserved in Arachnida and Insecta. (C, D) Dose-response curves of amitraz (C) and DPMF (D) against the Varroa OA receptors. EC50 values were calculated using log(agonist) versus response nonlinear fit, mean ± SEM, n = 3 trials, three replicates per trial.
-
Figure 1—source data 1
Source data for Figure 1 and Figure 1—figure supplement 1 and Figure 1—figure supplement 2.
- https://cdn.elifesciences.org/articles/68268/elife-68268-fig1-data1-v2.xlsx
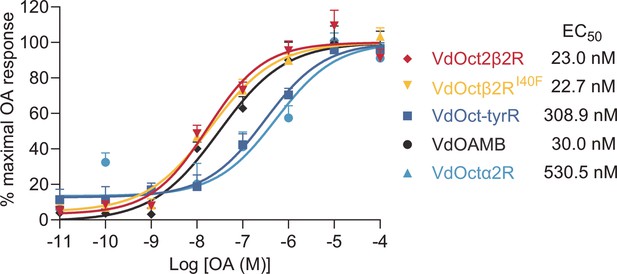
Dose-response curves of octopamine (OA) against the indicated OA receptors.
EC50 was calculated using log(agonist) versus response nonlinear fit, mean ± SEM, n = 3 trials, three replicates per trial.
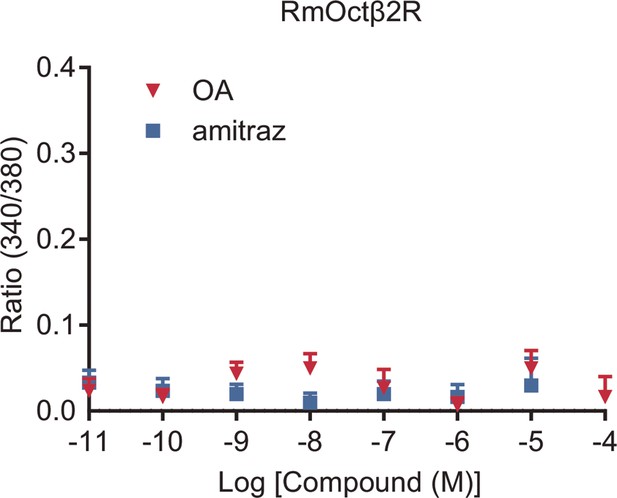
Effects of octopamine (OA) and amitraz on the Rhipicephalus microplus Octβ2R expressed in HEK293 cells.
Mean ± SEM, n = 3 trials.
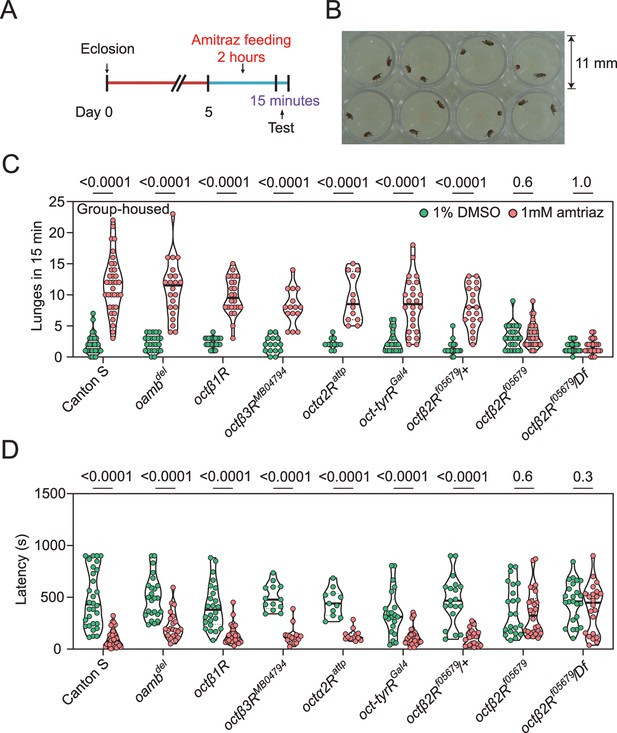
Amitraz affects Drosophila aggression through Octβ2R.
(A) Preparation of test flies. In brief, group-housed male flies (~10–15) were fed 1% DMSO plus 5% sucrose (control) or 1 mM amitraz plus 5% sucrose for 2 hr (see Materials and methods). (B) The 8-well aggression arena used in this behavioral test. (C, D) Effects of 1 mM amitraz on the number of lunges (C) and latency to initiate fighting (D) in different octopamine (OA) receptor mutants and control flies. p values, Mann–Whitney U tests were performed to analyze statistically significant differences between treatment with 1% DMSO versus 1 mM amitraz in the indicated genotypes, mean ± SEM, n = 12–34.
-
Figure 2—source data 1
Source data for Figure 2 and Figure 2—figure supplement 1.
- https://cdn.elifesciences.org/articles/68268/elife-68268-fig2-data1-v2.xlsx
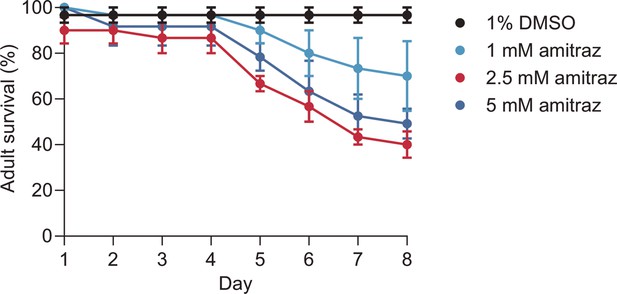
Adult survival of flies reared on diets containing 1% DMSO or a range of amitraz concentrations.
In brief, 10 wild-type female flies were allowed to feed on diets with 1% DMSO or a series of amitraz concentrations ranging from 1 mM to 5 mM. Data are shown as mean ± SEM. n = 3 biological replicates.
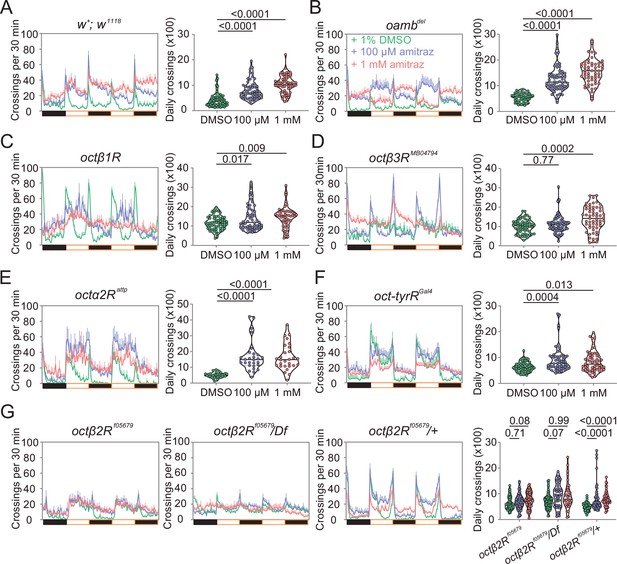
Amitraz affects Drosophila locomotion through Octβ2R.
(A–G) Effects of amitraz on midline crossing activity in flies of the indicated genotypes. One 5–7-day-old female fly was gently introduced into each tube, which contained 1% DMSO (control), 100 µM amitraz or 1 mM amitraz added to the agarose-sucrose medium (2% agarose and 5% sucrose) at one end. The other end was sealed with a cotton plug. The tubes were placed in a Drosophila Activity Monitor System (see Materials and methods). Black and white bars represent the night and day periods of the 12:12 LD cycle. Yellow boxes indicate the 2-day window of daily crossing activity test. p values, one-way ANOVA and post hoc Bonferroni correction, mean ± SEM, n = 16–32.
-
Figure 3—source data 1
Source data for Figure 3.
- https://cdn.elifesciences.org/articles/68268/elife-68268-fig3-data1-v2.xlsx
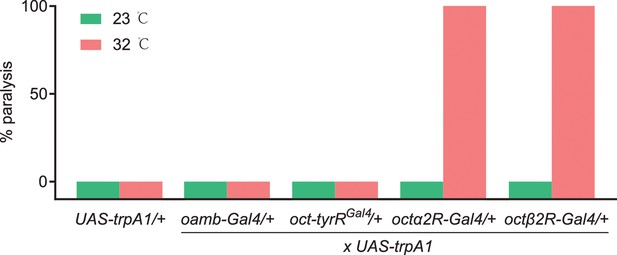
The percentage of paralysis behavior, in which four octopamine (OA) receptor-expressing neurons were thermally hyperactivated with UAS-trpA1.
The following transgenes were used: oamb-Gal4> UAS-trpA1; oct-tyrRGal4> UAS-trpA1; octα2R-Gal4> UAS-trpA1; octβ2R-Gal4> UAS-trpA1. n = 50–100.
-
Figure 4—source data 1
Source data for Figure 4.
- https://cdn.elifesciences.org/articles/68268/elife-68268-fig4-data1-v2.xlsx
Thermogenetic activation of four octopamine (OA) receptor-expressing neurons using UAS-trpA1 induces paralysis behavior (related to Figure 4).
The following transgenes were used: oamb-Gal4>UAS-trpA1; oct-tyrRG4>UAS-trpA1; octα2R-Gal4>UAS-trpA1; octβ2R-Gal4>UAS-trpA1. The movie was speeded up 2×.
Thermogenetic activation of octβ2R-Gal4 neurons using UAS-trpA1 induces paralysis behavior (related to Figure 4).
The following transgenes were used: octβ2R-Gal4>UAS-trpA1.
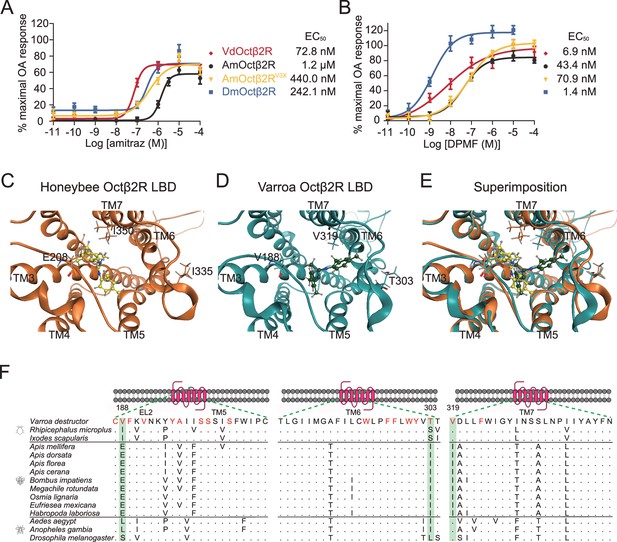
Triple amino acids differences determine amitraz sensitivity in Octβ2R in vitro.
(A, B) The predicted ligand-binding domain of amitraz in the Varroa (A) and the honeybee (B) Octβ2R. Amitraz and three amino acids mutated in this study are shown. (C) Superposition of the predicted ligand-binding domain of the honeybee (golden cartoon) and the Varroa (blue cartoon) Octβ2R structures. (D) Amino acid substitution in the ligand-binding domain (TM5-TM7) of Octβ2R in representative species from Arachnida and Hymenoptera. The predicted amino acids involved in the binding of amitraz are indicated in red. Three amino acids (E208, I335, I350) highlighted in green are conserved among species of bees. EL: extracellular loop; TM: transmembrane domain. (E, F) Dose-response curve of amitraz (E) and DPMF (F) against the indicated octopamine (OA) receptors. EC50 was calculated using log(agonist) versus response nonlinear fit, mean ± SEM, n = 3–4 trials, three replicates per trial.
-
Figure 5—source data 1
Source data for Figure 5 and Figure 5—figure supplements 1–4.
- https://cdn.elifesciences.org/articles/68268/elife-68268-fig5-data1-v2.xlsx
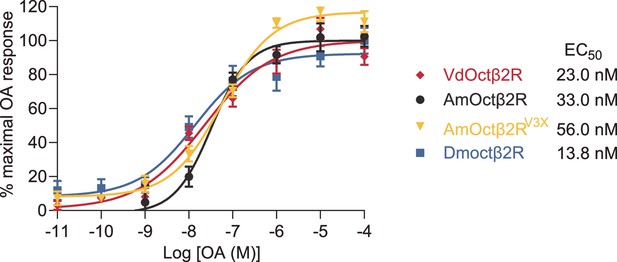
Dose-response curves of octopamine (OA) against the indicated OA receptors.
EC50 values were calculated using log (agonist) versus response nonlinear fit, mean ± SEM, n = 3 trials, three replicates per trial.
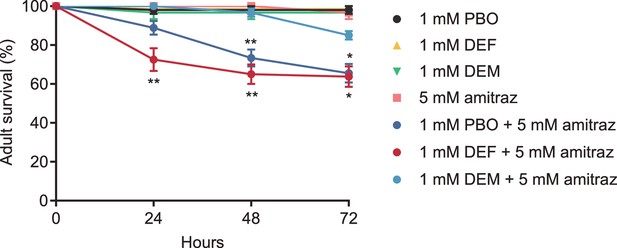
Adult survival when reared on sucrose solution containing 5 mM amitraz and 1 mM detoxicative enzyme inhibitor.
Abbreviations: PBO: piperonyl butoxide; DEF: S,S,S-tributylphosphorotrithioate; DEM: diethyl maleate. One way ANOVA and post hoc Mann-Whitney U tests, mean ± SEM, n = 3-9 biological replicates. * p<0.05; ** p<0.01.
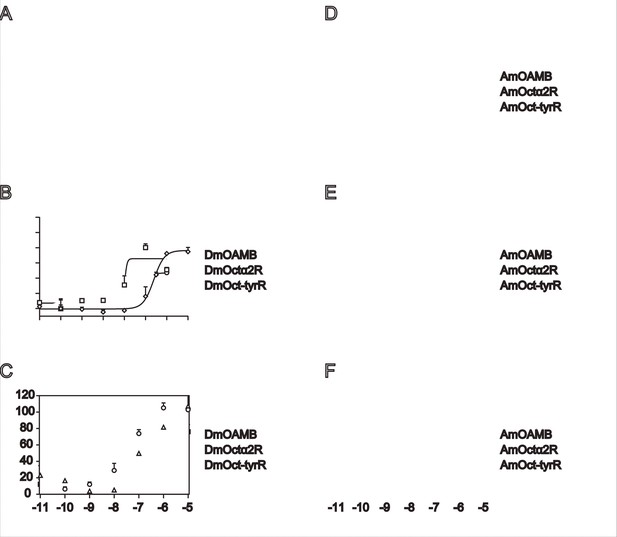
Dose-response curves of OA, amitraz, and DPMF against the fly (A-C) and honeybee (D-F) OA receptors.
EC50 values were calculated using log (agonist) versus response nonlinear fit, mean ± SEM, n = 1-3 trials, 3 replicates per trial.
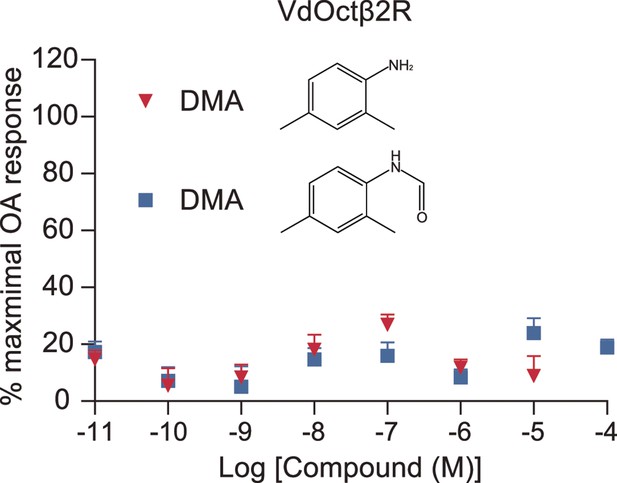
Effects of 2,4-dimethylaniline (DMA) and 2,4-dimethylformanilide (DMF) on VdOctβ2R in vitro.
Mean ± SEM, n = 5 trials.
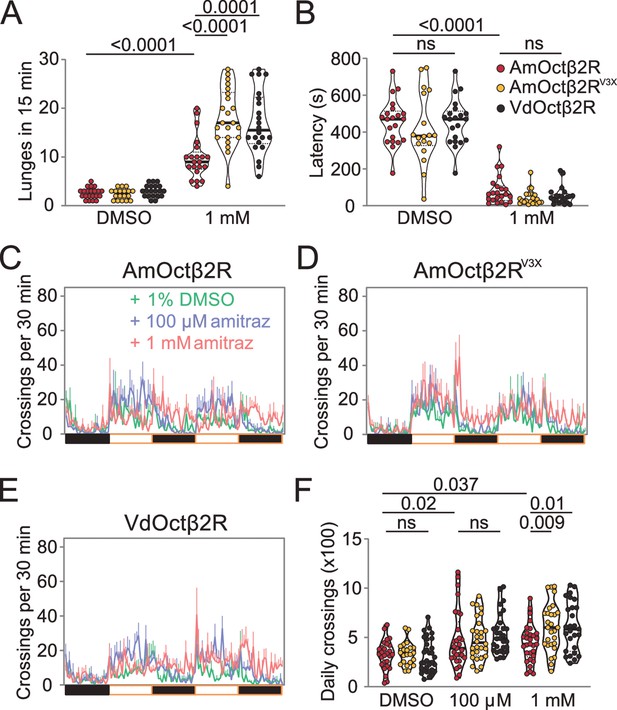
Transgenic flies expressing Octβ2R variants show different sensitivities to amitraz.
(A, B) Number of lunges (A) and latencies before initiating fighting (B) in the Octβ2R null mutant expressing VdOctβ2R, AmOctβ2R, or AmOctβ2RV3X. Changes were compared to the AmOctβ2R flies. AmOctβ2RE208V, I335T, I350V, abbreviated to AmOctβ2RV3X. Genotype: elav-gal4/UAS-XXOctβ2R; octβ2Rf05679/octβ2Rf05679. p values, Kruskal–Wallis and post hoc Mann–Whitney U tests, mean ± SEM, n = 18–24. (C–E) Midline crossing activity in Octβ2R null mutants expressing VdOctβ2R, AmOctβ2R, or the AmOctβ2RV3X. (F) Daily crossing activities exhibited by Octβ2R null mutants expressing VdOctβ2R, AmOctβ2R, or the AmOctβ2RV3X. Changes were compared to the AmOctβ2R flies. Genotype: elav-gal4/UAS-XXOctβ2R; octβ2Rf05679/octβ2Rf05679. p values, two-way ANOVA and post hoc Bonferroni correction, mean ± SEM, n = 16.
-
Figure 6—source data 1
Source data for Figure 6.
- https://cdn.elifesciences.org/articles/68268/elife-68268-fig6-data1-v2.xlsx
Videos
Silencing of octα2R-Gal4 neurons using UAS-Shibirets decreases activity.
The following transgenes were used: octα2R-Gal4> UAS-Shibirets. The movie was speeded up 2×.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (Drosophila melanogaster) | Canton-S | Shanghai Institute of Biochemistry and Cell Biology | Cat#BCF47 | |
| Genetic reagent (Drosophila melanogaster) | w1118 | Bloomington Drosophila Stock Center | Cat#5905RRID:BDSC_5905 | |
| Genetic reagent (Drosophila melanogaster) | elav-Gal4 | Bloomington Drosophila Stock Center | Cat#8765RRID:BDSC_8765 | |
| Genetic reagent (Drosophila melanogaster) | octβ2R-Df | Bloomington Drosophila Stock Center | Cat#56254RRID:BDSC_56254 | |
| Genetic reagent (Drosophila melanogaster) | octβ3RMB04794 | Bloomington Drosophila Stock Center | Cat#24819RRID:BDSC_24819 | |
| Genetic reagent (Drosophila melanogaster) | octβ1R | Koon and Budnik, 2012 | ||
| Genetic reagent (Drosophila melanogaster) | octβ2Rf05679 | Lim et al., 2014 | Cat#18896RRID:BDSC_18896 | |
| Genetic reagent (Drosophila melanogaster) | oambdel | Deng et al., 2019 | ||
| Genetic reagent (Drosophila melanogaster) | oamb-Gal4 | Zhou et al., 2012 | ||
| Genetic reagent (Drosophila melanogaster) | octα2Rattp | Deng et al., 2019 | ||
| Genetic reagent (Drosophila melanogaster) | octα2R-Gal4 | Deng et al., 2019 | ||
| Genetic reagent (Drosophila melanogaster) | octβ2R-Gal4 | Deng et al., 2019 | ||
| Genetic reagent (Drosophila melanogaster) | UAS-trpA1 | Hamada et al., 2008 | ||
| Genetic reagent (Drosophila melanogaster) | UAS-Shibirets | Kitamoto, 2001 | ||
| Genetic reagent (Drosophila melanogaster) | oct-tyrRGal4 | This paper | Mutant allele; Materials and methods, ‘Fly strains’ | |
| Genetic reagent (Drosophila melanogaster) | UAS-VdOctβ2R | This paper | Mutant allele; Materials and methods, ‘Fly strains’ | |
| Genetic reagent (Drosophila melanogaster) | UAS-AmOctβ2R | This paper | Mutant allele; Materials and methods, ‘Fly strains’ | |
| Genetic reagent (Drosophila melanogaster) | UAS-AmOctβ2RV3X | This paper | Mutant allele; Materials and methods, ‘Fly strains’ | |
| Chemical compound, drug | Amitraz | Sigma | Cat#45323 | |
| Chemical compound, drug | N2-(2,4-Dimethylphenyl)-N1-methyformamidine | Sigma | Cat#BP641 | |
| Chemical compound, drug | 2,4-Dimethylaniline | Sigma | Cat#301493 | |
| Chemical compound, drug | (±)-Octopamine hydrochloride | Sigma | Cat#68631 | |
| Chemical compound, drug | 2,4-Dimethylformanilide | AccuStandard | Cat#P-1100S-CN | |
| Chemical compound, drug | Piperonyl butoxide | Aladdin | Cat#P113864 | |
| Chemical compound, drug | S,S,S-Tributylphosphorotrithioate | Aladdin | Cat#T114221 | |
| Chemical compound, drug | Diethyl maleate | Aladdin | Cat#D104017 | |
| Chemical compound, drug | Poly-D-lysine | Sigma | Cat#P0296 | |
| Chemical compound, drug | Sucrose | Sinopharm | Cat#10021418 | |
| Chemical compound, drug | Agarose | Sinopharm | Cat#63005518 | |
| Cell lines | HEK 293 | The Cell Bank of Type Culture Collection of Chinese Academy of Sciences | Cat#GNHu43 | https://www.cellbank.org.cn/ |
| Recombinant DNA reagent | Plasmid: pcDNA3.1-VdOAMB | This paper | See ‘Construction of expression plasmids’ | |
| Recombinant DNA reagent | Plasmid: pcDNA3.1-VdOctβ2R | This paper | See ‘Construction of expression plasmids’ | |
| Recombinant DNA reagent | Plasmid: pcDNA3.1-VdOctβ2RI40F | This paper | See ‘Construction of expression plasmids’ | |
| Recombinant DNA reagent | Plasmid: pcDNA3.1-VdOctα2R | This paper | See ‘Construction of expression plasmids’ | |
| Recombinant DNA reagent | Plasmid: pcDNA3.1-VdOct-tyrR | This paper | See ‘Construction of expression plasmids’ | |
| Recombinant DNA reagent | Plasmid: pcDNA3.1-AmOAMB | This paper | See ‘Construction of expression plasmids’ | |
| Recombinant DNA reagent | Plasmid: pcDNA3.1-AmOctβ2R | This paper | See ‘Construction of expression plasmids’ | |
| Recombinant DNA reagent | Plasmid: pcDNA3.1-AmOctβ2RV3X | This paper | See ‘Construction of expression plasmids’ | |
| Recombinant DNA reagent | Plasmid: pcDNA3.1-AmOctα2R | This paper | See ‘Construction of expression plasmids’ | |
| Recombinant DNA reagent | Plasmid: pcDNA3.1-AmOct-tyrR | This paper | See ‘Construction of expression plasmids’ | |
| Recombinant DNA reagent | Plasmid: pcDNA3.1-DmOAMB | This paper | See ‘Construction of expression plasmids’ | |
| Recombinant DNA reagent | Plasmid: pcDNA3.1-DmOctβ2R | This paper | See ‘Construction of expression plasmids’ | |
| Recombinant DNA reagent | Plasmid: pcDNA3.1-DmOctα2R | This paper | See ‘Construction of expression plasmids’ | |
| Recombinant DNA reagent | Plasmid: pcDNA3.1-DmOct-tyrR | This paper | See ‘Construction of expression plasmids’ | |
| Recombinant DNA reagent | Plasmid: pcDNA3.1-RmOctβ2R | This paper | See ‘Construction of expression plasmids’ | |
| Recombinant DNA reagent | Plasmid: pcDNA3.1-RmOctβ2RI61F | This paper | See ‘Construction of expression plasmids’ | |
| Software | SoftMax Pro software (v. 7.1.2.0) | Molecular Devices | https://www.moleculardevices.com/ | |
| Software | Molecular Operating Environments (MOE, 2015.10) | Chemical Computing Group | https://www.chemcomp.com/ | |
| Software | Prism 7.0 | GraphPad | GraphPad Prism, RRID:SCR_002798 | |
| Other | DMEM media | ThermoFisher Scientific | Cat#10566016 | |
| Other | Lipofectamine 2000 | ThermoFisher Scientific | Cat#11668019 | |
| Other | 96 well polystyrene microplates | ThermoFisher Scientific | Cat#165305 | |
| Other | 0.25% Trypsin-EDTA | ThermoFisher Scientific | Cat#25200072 | |
| Other | Fura 2-AM and Pluronic F-127 | Dojindo Molecular Technologies | Cat#F025 |





