Chitinase 3-like-1 contributes to acetaminophen-induced liver injury by promoting hepatic platelet recruitment
Figures
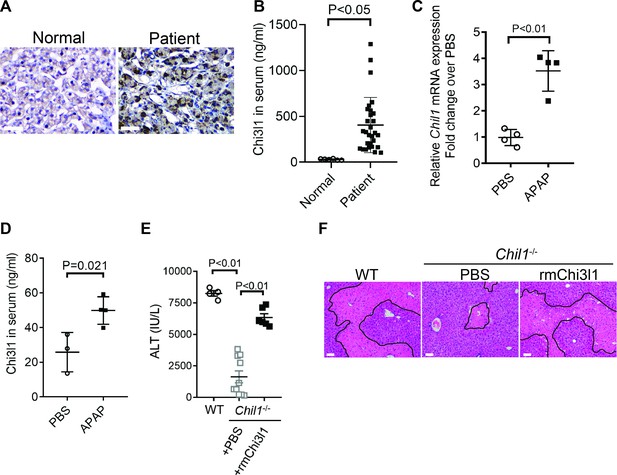
Chitinase 3-like-1 (Chi3l1) is upregulated and plays a critical role in acetaminophen-induced liver injury (AILI).
(A) Immunohistochemical (IHC) staining for Chi3l1 in normal liver biopsies (Normal) and those from patients with AILI (Patient). Images shown are representative of 10 samples/group. Scale bar, 250 μm. (B) Enzyme-linked immunosorbent assay (ELISA) analysis of Chi3l1 in serum of healthy individuals (Normal, n = 6) and those from patients with AILI (Patient, n = 29). Data were presented as median+interquartile range. (C, D) Male C57B/6 mice treated with PBS or acetaminophen (APAP). (C) Chil1 mRNA in liver homogenates and (D) Chi3l1 protein levels in serum were measured by quantitative reverse transcription polymerase chain reaction (qRT-PCR) and ELISA at 3 and 24 hr, respectively (n = 4 mice/group). (E, F) Male C57B/6 (wild-typr [WT]) and Chil1-/- mice were treated with APAP. Additionally, Chil1-/- mice were divided into two groups treated with either PBS or recombinant mouse Chi3l1 (rmChi3l1) simultaneously with APAP (n = 4–10 mice/group). (E) Serum levels of ALT and (F) liver histology with necrotic areas outlined were evaluated 24 hr after APAP treatment. Scale bar, 250 μm. Mann-Whitney test was performed in B. Two-tailed, unpaired Student’s t-test was performed in C, D. One-way ANOVA were performed in E.
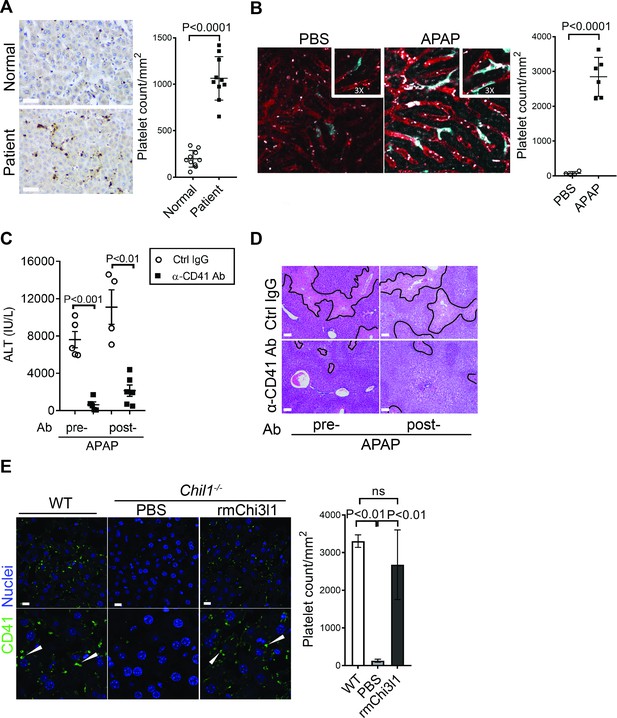
Chitinase 3-like-1 (Chi3l1) contributes to acetaminophen-induced liver injury (AILI) by promoting hepatic platelet recruitment.
(A) Immunohistochemical (IHC) staining to detect platelets (CD41+) in healthy liver biopsies (Normal) and those from patients with AILI (Patient). Scale bar, 250 μm (n = 10/group). (B) Male C57B/6 mice treated with PBS or acetaminophen (APAP). Intravital microscopy analyses were performed around 3 hr post-APAP. Mɸs (cyan) and platelets (white) in liver sinusoids (red) are indicated. Representative images were chosen from intravital microscopy videos: https://bcm.box.com/s/15hmtryyrdl302mihrsm034ure87x4ea (Supplementary video 1, PBS treatment) and https://bcm.box.com/s/tuljfmstvv4lvoksx16fkxkpirkekynz (Supplementary video 2; n = 6–7 mice/group, 4–15 videos/mouse). (C–E) Male C57B/6 (wild-type [WT]) mice were treated with control IgG (Ctrl IgG) or an anti-CD41 antibody (α-CD41 Ab) either 3 hr before or 3 hr after APAP administration. (C) Serum levels of ALT and (D) liver histology with necrotic areas outlined were evaluated 24 hr after APAP treatment (n = 5 mice/group in C, D). Scale bar, 250 μm. (E) Male C57B/6 (WT) and Chil1-/- mice were treated with APAP. Additionally, Chil1-/- mice were divided into two groups treated with either PBS or recombinant mouse Chi3l1 (rmChi3l1) simultaneously with APAP. Immunofluorescence (IF) staining was performed to detect intrahepatic platelets (CD41+) 3 hr after APAP treatment (n = 3 mice/group). Scale bar, 25 μm. Two-tailed, unpaired Student’s t-test was performed in A–C. One-way ANOVA were performed in E.
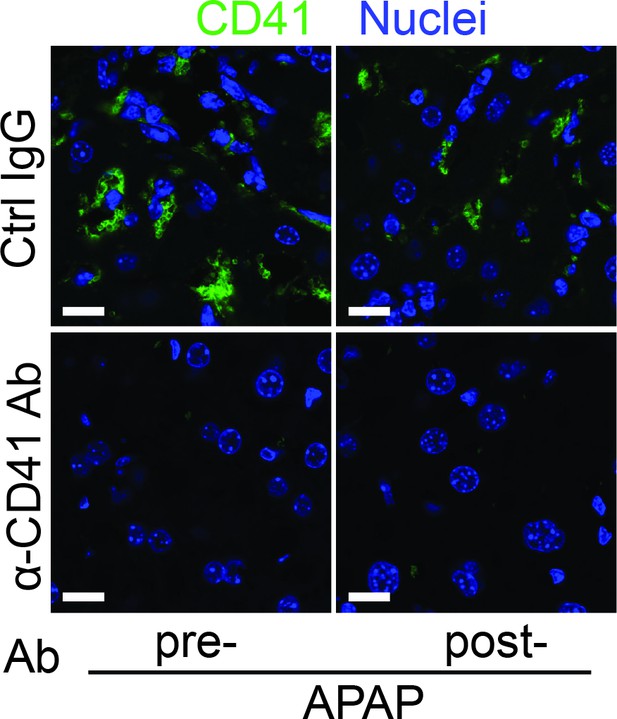
Depletion of platelets by anti-CD41 antibody reduces hepatic platelets recruitment.
Male C57B/6 mice were treated with control IgG (Ctrl IgG) or an anti-CD41 antibody (α-CD41 Ab) either 3 hr before (pre-) or 3 hr after (post-) APAP administration. Immunofluorescence (IF) staining was performed to identify intrahepatic platelets (CD41+) (n = 5 mice/group). Scale bar, 25 μm.
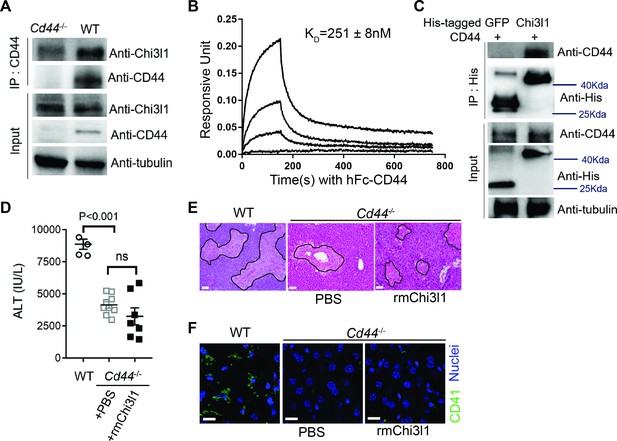
Chitinase 3-like-1 (Chi3l1) functions through its receptor CD44.
(A) Immunoprecipitation with anti-CD44 antibody was performed using liver homogenates obtained from wild-type (WT) and Cd44-/- mice treated with acetaminophen (APAP) for 2 hr. Input proteins and immune-precipitated proteins were blotted with the indicated antibodies. (B) Interferometry measurement of the binding kinetics of human His-Chi3l1 with human Fc-CD44. (C) His-tagged control GFP and human Chi3l1 were incubated with recombinant human CD44. Proteins bound to Chi3l1 were immune-precipitated with an anti-His antibody. Input proteins and immune-precipitated proteins were blotted with indicated antibodies. (D–F) Male WT mice were treated with APAP and Cd44-/- mice were treated with PBS or recombinant mouse Chi3l1 (rmChi3l1) plus APAP. (D) Serum levels of ALT and (E) liver histology with necrotic areas outlined were evaluated 24 hr after APAP treatment (n = 4–9 mice/group in A, B). Scale bar, 250 μm. (F) Immunofluorescence (IF) staining was performed to detect intrahepatic platelets (CD41+) 3 hr after APAP treatment (n = 3 mice/group). Scale bar, 25 μm. One-way ANOVA were performed in D.
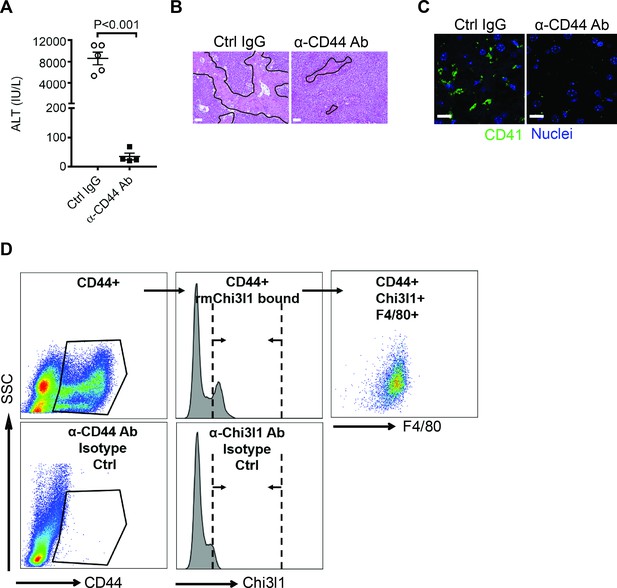
Chitinase 3-like-1 (Chi3l1) promotes hepatic platelet recruitment and acetaminophen-induced liver injury (AILI) through CD44 expressing on Mɸs.
(A–C) Chil1-/- mice reconstituted with recombinant mouse Chi3l1 (rmChi3l1) were treated with either Ctrl IgG or α-CD44 Ab 30 min prior to acetaminophen (APAP) challenge. (A) Serum levels of ALT and (B) liver histology with necrotic areas outlined were evaluated 24 hr after APAP treatment (n = 4–5 mice/group). Scale bar, 250 μm. (C) Immunofluorescence (IF) staining was performed to detect intrahepatic platelets (CD41+) 3 hr after APAP treatment (n = 3 mice/group). Scale bar, 25 μm. (D) Flow cytometry analysis was performed to identify Chi3l1-binding cells among liver non-parenchymal cells (NPCs) isolated from wild-type (WT) mice treated with APAP for 2 hr. CD44+ cells were gated from single live cells. CD44+ cells that bind to rmChi3l1 were further gated. The Chi3l1+CD44+ cells were then identified by markers for various cell types, including CD45+ CD146-F4/80+ (Mɸs), CD45-CD146+ (liver sinusoidal endothelial cells [LSECs]), and Ly6G+ (neutrophils). Two-tailed, unpaired Student’s t-test was performed in A.
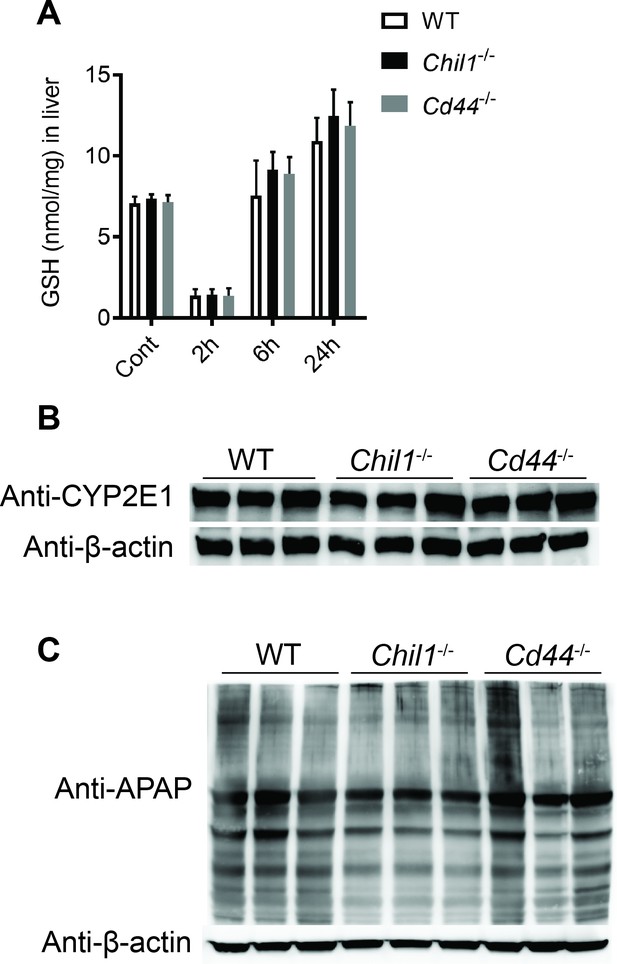
Deletion of neither chitinase 3-like-1 (Chi3l1) nor CD44 affects acetaminophen (APAP) bio-activation.
Male wild-type (WT), Chil1-/-, Cd44-/- mice were treated with APAP (n = 3 mice/group). (A) glutathione (GSH) levels in the liver were measured at indicated time points by HPLC. (B) Hepatic protein levels of cytochrome P450 2E1 (CYP2E1) were measured by Western blotting after mice were fasted overnight without APAP treatment. (C) N-acetyl-p-benzoquinone imine (NAPQI)-protein adducts in liver were measured by Western blotting 2 hr after APAP treatment.
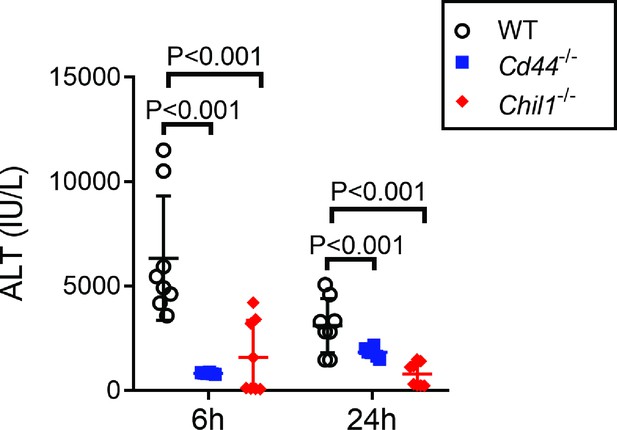
Female Chil1-/- and Cd44-/- mice develop reduced liver injury compared to female wild-type (WT) mice.
Female WT, Chil1-/- and Cd44-/- mice were treated with acetaminophen (APAP). Serum ALT levels were measured at 6 and 24 hr after APAP treatment (n = 6–8 mice/group). One-way ANOVA was performed.
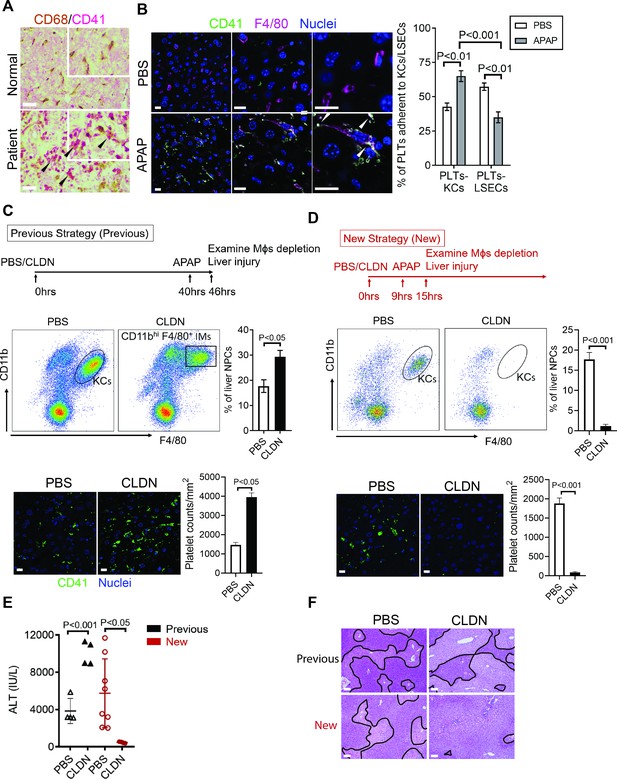
Hepatic Mɸs promote platelet recruitment.
(A) Immunohistochemical (IHC) staining for macrophages (CD68+) and platelets (CD41+) in normal liver biopsies (Normal) and those from patients with AILI (Patient) (n = 10/group). Scale bar, 25 μm. (B) Immunofluorescence (IF) staining for intrahepatic platelets (CD41+) and Kupffer cells (KCs) (F4/80+) in male C57B/6 mice treated with PBS or acetaminophen (APAP) for 3 hr. Scale bar, 25 μm. Arrowheads indicate platelets adherent to KCs. Quantification of platelets adherent to KCs or liver sinusoidal endothelial cells (LSECs). (C–F) Male C57B/6 mice were injected with either empty liposomes containing PBS (PBS) or liposomes containing clodronate (CLDN), followed by APAP treatment. (C, D) Non-parenchymal cells (NPCs) were isolated and underwent flow cytometry analysis. Indicated cells were gated on single live CD45+CD146- cells. IF staining was performed to detect intrahepatic platelets (CD41+). Scale bar, 25 μm. (E) Serum levels of ALT and (F) liver histology with necrotic areas outlined. Scale bar, 250 μm (n = 6 mice/group in B-F). Two-tailed, unpaired Student’s t-test was performed in B-D, F.
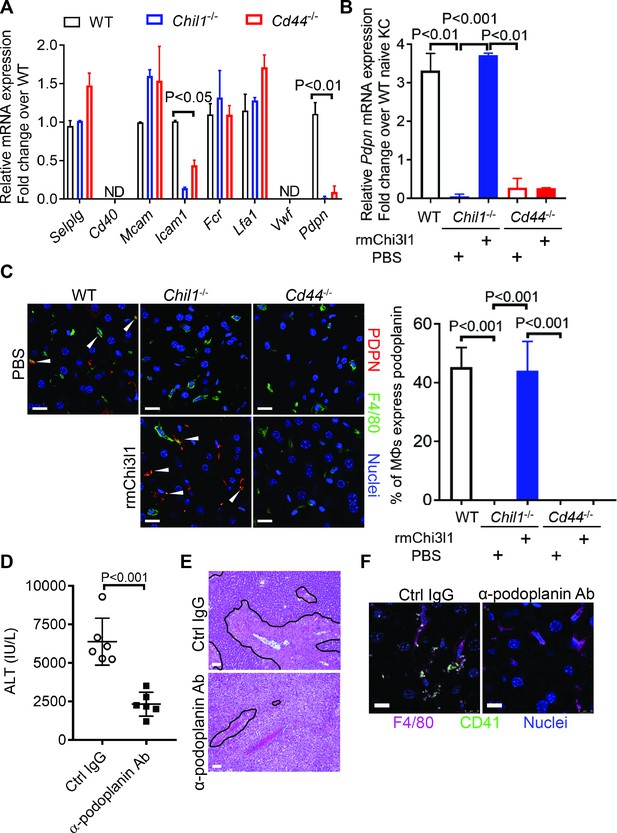
Chitinase 3-like-1 (Chi3l1)/CD44 signaling in Mɸs upregulates podoplanin expression and platelet adhesion.
(A) Male WT, Chil1-/-, Cd44-/- mice were treated with acetaminophen (APAP) (n = 4 mice/group). After 3 hr, mice were sacrificed and Mɸs were isolated to measure mRNA levels of various adhesion molecules, including selectin P ligand (Selplg), Cd40, melanoma cell adhesion molecule (Mcam), Fc receptor (Fcr), intercellular adhesion molecule 1 (Icam1), lymphocyte function-associated antigen 1 (Lfa1), von Willebrand factor (Vwf), and podoplanin (Pdpn). (B, C) Wild-type (WT) mice were treated with APAP. Chil1-/- and Cd44-/- mice were treated with PBS or rmChi3l1 followed by APAP challenge simultaneously and mice were sacrificed 3 hr after APAP (n = 3 mice/group). (B) Mɸs were isolated and mRNA levels of Pdpn in Mɸs were analyzed by quantitative reverse transcription polymerase chain reaction (qRT-PCR). (C) Immunofluorescence (IF) staining of liver sections for podoplanin and F4/80 is shown and the proportions of Mɸs that express Pdpn were quantified, Scale bar, 25 μm. (D–F) Chil1-/- mice reconstituted with rmChi3l1 were treated with either Ctrl IgG or α-podoplanin Ab for 16 hr and subsequently challenged with APAP. (D) Serum levels of ALT and (E) liver histology were evaluated 24 hr after APAP treatment (n = 6 mice/group). Scale bar, 250 μm. (F) IF staining for intrahepatic platelets (CD41+) and Mɸs (F4/80+) was performed 3 hr after APAP (n = 3 mice/group). Scale bar, 25 μm. One-way ANOVA were performed in A–C. Two-tailed, unpaired Student’s t-test was performed in D.
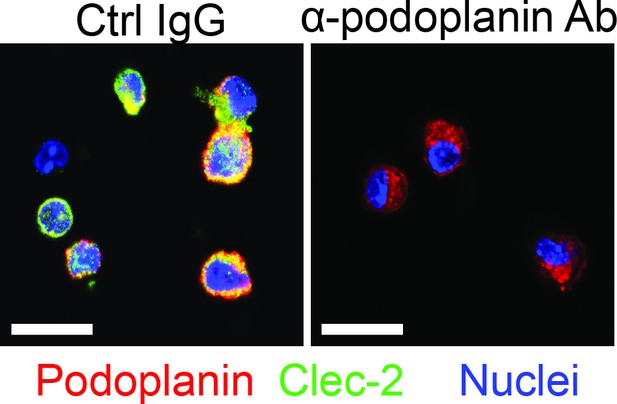
Podolanin expressing on Mɸs mediates interactions with platelets.
Mɸs were isolated from wild-type (WT) mice treated with acetaminophen (APAP) for 3 hr. The cells were treated in vitro with either control IgG (Ctrl IgG) or an anti-podoplanin antibody (α-podoplanin Ab) before incubation with platelets. Immunofluorescence (IF) staining was performed to detect podoplanin on Mɸs and C-type lectin-like receptor 2 (Clec-2) on platelets. Scale bar, 25 μm.
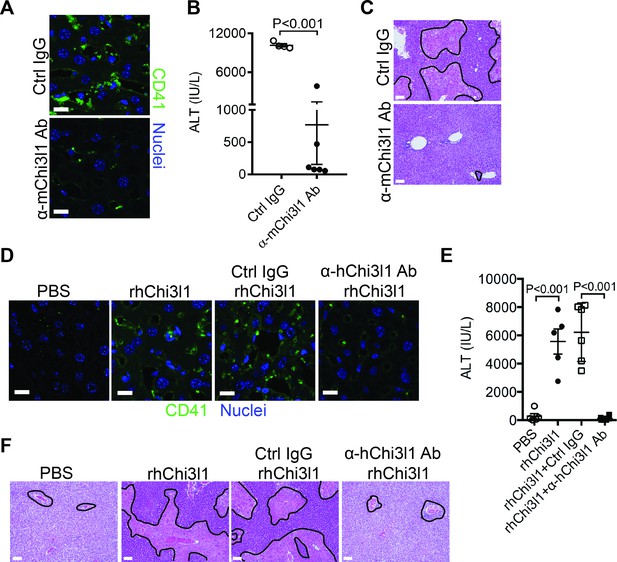
Evaluation of the therapeutic potential of targeting chitinase 3-like-1 (Chi3l1) in the treatment of acetaminophen-induced liver injury (AILI).
(A–C) Male C57B/6 mice were treated with acetaminophen (APAP) for 3 hr, followed by intraperitoneally (i.p.) injection of either a control IgG (Ctrl IgG) or an anti-mouse Chi3l1 Ab (α-mChi3l1 Ab, C59). (A) Immunofluorescence (IF) staining for intrahepatic platelets (CD41+) was performed 6 hr after APAP treatment (n = 3 mice/group). Scale bar, 25 μm. (B) Serum levels of ALT and (C) liver histology were evaluated 24 hr after APAP treatment (n = 4–6 mice/group). Scale bar, 250 μm. (D–F) Chil1-/- mice were treated with APAP plus PBS or recombinant human Chi3l1 (rhChi3l1) for 3 hr as indicated and APAP plus rhChi3l1 treatment group were either without treatment or treated with a control IgG (Ctrl IgG) or an anti-human Chi3l1 Ab (α-hChi3l1 Ab, C7). (D) IF staining was performed to identify intrahepatic platelets (CD41+) 6 hr after APAP treatment. Scale bar, 25 μm. (E) Serum levels of ALT and (F) liver histology were evaluated 24 hr after APAP treatment. Scale bar, 250 μm (n = 5–10 mice/group in D–F). Two-tailed, unpaired Student’s t-test was performed in B. One-way ANOVA were performed in E.
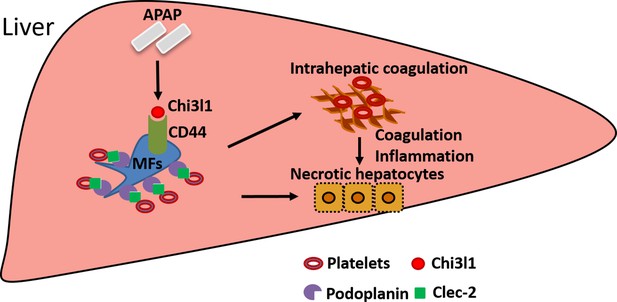
Schematic summary of the main findings.
Acetaminophen (APAP) overdose induces chitinase 3-like-1 (Chi3l1) expression, which binds CD44 on Mɸs and promotes Mɸs-mediated platelets recruitment through podoplanin/Clec-2 (C-type lectin-like receptor 2) interaction. Recruited platelets further contribute to APAP-induced liver injury (AILI).
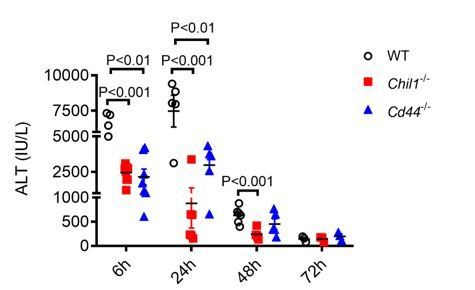
Deletion of Chi3l1- or CD44 does not affect liver recovery after APAP-induced injury.
Male WT, Chil1-/- and Cd44-/- mice were treated with APAP. Serum ALT levels were measured at 6hrs, 24hrs, 48hrs and 72hrs after APAP treatment (n=5-8 mice/group). One-way ANOVA was performed.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (Mus musculus) | C57BL/6J | Jackson Laboratory, PMID:14759567 | Stock #:000664 MGI Cat# 3849035, RRID:MGI:3849035 | |
| Genetic reagent (Mus musculus) | Cd44-/- mice (also called Cd44tm1Hbg/Cd44tm1Hbg) | Jackson Laboratory | Stock #:005878 MGI Cat# 4941902, RRID:MGI:4941902 | |
| Genetic reagent (Mus musculus) | Chil1-/-mice | PMID:19414556 | MGI #:3846223 RRID:MGI:3846223 | Dr Jack A. Elias (Brown University) |
| Chemical compound, drug | Acetaminophen | Sigma-Aldrich | A7085 | 210 mg/kg for male mice, 325 mg/kg for female mice |
| Peptide, recombinant protein | Recombinant mouse Chi3l1 | Sino Biological | 50929-M08H | 500 ng/mouse in 100 μl PBS |
| Commercial assay or kit | ALT diagnostic assay kit | Teco Diagnostics, | A526-120 | |
| Antibody | Syrian hamster polyclonal IgG Ctrl IgG for anti-podoplanin antibody | Bioxcell InvivoMab | BE0087, RRID:AB_1107782 | 100 μg/mouse |
| Antibody | Syrian hamster monoclonal anti-mouse podoplanin antibody | Bioxcell InvivoMab | BE0236, RRID:AB_2687718 | 100 μg/mouse |
| Antibody | Rat monoclonal Ctrl IgG for anti-mouse CD41 antibody | BD Biosciences | 553922, Clone R334, RRID:AB_479672 | 2 mg/kg |
| Antibody | Rat monoclonal anti-mouse CD41 antibody | BD Biosciences | 553847, Clone MWReg 30, RRID:AB_395084 | 2 mg/kg, 1:200 for IF |
| Peptide, recombinant protein | Recombinant human Chi3l1 | Sino Biological | 11227-H08H | 1 μg/mouse in 100 μl |
| Antibody | Rabbit polyclonal anti-human CD41 | Proteintech | 24552–1-AP, RRID:AB_2879604 | 1:200 for IHC |
| Antibody | Mouse monoclonal anti-human CD68 | Thermo Fisher | MA5-13324, RRID:AB_10987212 | 1:100 for IHC |
| Antibody | Rabbit polyclonal anti-human Chi3l1 | Proteintech | 12036–1-AP, RRID:AB_2877819 | 1:100 for IHC |
| Antibody | Rat monoclonal anti-mouse F4/80, Alexa 647 conjugated | Biolegend | 123122, RRID:AB_893480 | 1:100 for IF |
| Antibody | Rat monoclonal anti-mouse CD44 | abcam | ab112178, clone KM81, RRID:AB_10864553 | 1:200 for IF |
| Antibody | Golden Syrian Hamster monoclonal anti-mouse podoplanin | Novus, biological | NB600-1015, RRID:AB_2161937 | 1:100 for IF |
| Antibody | Rabbit polyclonal anti-mouse Clec-2 | Biorbyt | orb312182, RRID:AB_2891123 | 1:100 for IF |
| Antibody | Donkey anti-rat polyclonal immunoglobulin, Alexa 488-conjugated | Invitrogen | A-21208, RRID:AB_141709 | 1:1000 for IF |
| Antibody | Goat anti-rabbit polyclonal immunoglobulin, Alexa 488-conjugated | Invitrogen | A-11034, RRID:AB_141709 | 1:1000 for IF |
| Antibody | Goat anti-rabbit polyclonal immunoglobulin, Alexa 594-conjugated | Invitrogen | A-11012, RRID:AB_141359 | 1:1000 for IF |
| Antibody | Goat anti-hamster polyclonal immunoglobulin, Alexa 594-conjugated | Invitrogen | A-21113, RRID:AB_2535762 | 1:1000 for IF |
| Antibody | Hoechst | Invitrogen | H3570, RRID:AB_10626776 | 1:10000 for IF |
| Peptide, recombinant protein | TRITC-labeled Albumin | Sigma-Aldrich | A2289-10MG RRID:AB_2891111 | 10 μl/mouse for intravital microscopy |
| Antibody | Rat monoclonal anti-mouse anti-F4/80 antibody, BV421-labeled | Biolegend | 123132, RRID:AB_11203717 | 15 μl/mouse for intravital microscopy |
| Antibody | Rat monoclonal anti-mouse CD41 antibody, DyLight 649-labeled | emfret ANALYTICS | X649, RRID:AB_2861336 | 30 μl/mouse for intravital microscopy |
Real-time PCR primers used.
| Gene | Forward (F)/reverse (R) primer | Primer sequences |
|---|---|---|
| Pdpn | F | ACCGTGCCAGTGTTGTTCTG |
| R | AGCACCTGTGGTTGTTATTTTGT | |
| Psgl-1 | F | GAAAGGGCTGATTGTGACCCC |
| R | AGTAGTTCCGCACTGGGTACA | |
| Cd40 | F | TGTCATCTGTGAAAAGGTGGTC |
| R | ACTGGAGCAGCGGTGTTATG | |
| Mcam | F | GTGGCGTTGACATCGTTGG |
| R | CTATGTACTTCGTATGCAGGTCG | |
| Icam-1 | F | GTGATGCTCAGGTATCCATCCA |
| R | CACAGTTCTCAAAGCACAGCG | |
| Fcr | F | AGGGCCTCCATCTGGACTG |
| R | GTGGTTCTGGTAATCATGCTCTG | |
| Lfa1 | F | CCAGACTTTTGCTACTGGGAC |
| R | GCTTGTTCGGCAGTGATAGAG | |
| Vwf | F | CTCTTTGGGGACGACTTCATC |
| R | TCCCGAGAATGGAGAAGGAAC |
Additional files
-
Source data 1
Source data for all numerical bar graph shown in the manuscript including figure supplements.
- https://cdn.elifesciences.org/articles/68571/elife-68571-data1-v2.xlsx
-
Supplementary file 1
Representative list of potential chitinase 3-like-1 (Chi3l1)-interacting proteins detected by mass spectrometry.
Non-parenchymal cells were isolated from C57B/6 mice treated with acetaminophen (APAP) for 3 hr and the cell lysate was incubated with rmChi3l1 overnight. Proteins potentially bound to rmChi3l1 were immune-precipitated with an anti-His antibody and subjected to mass spectrometry analyses.
- https://cdn.elifesciences.org/articles/68571/elife-68571-supp1-v2.xlsx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/68571/elife-68571-transrepform-v2.docx





