Membrane estrogen receptor alpha (ERα) participates in flow-mediated dilation in a ligand-independent manner
Figures
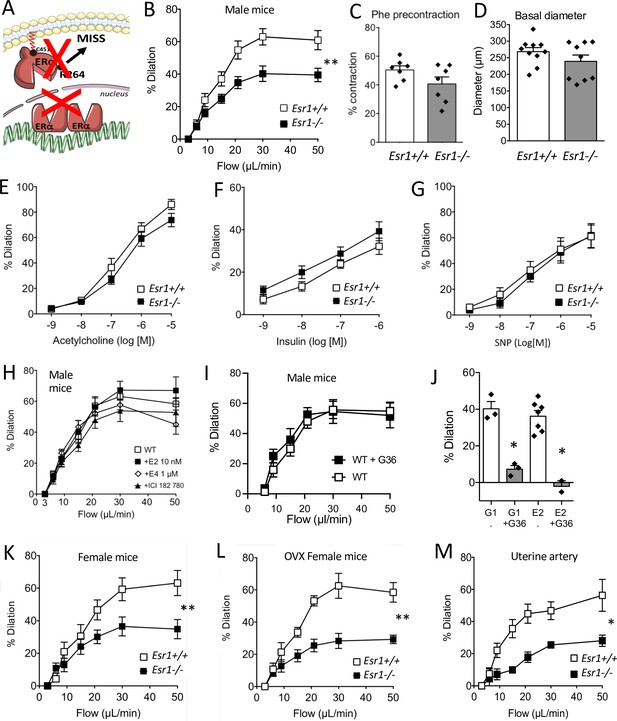
Involvement of ERα in flow-mediated dilation (FMD).
FMD was measured in mesenteric resistance arteries isolated from male mice lacking ERα (Esr1-/-) and male wild-type littermates (Esr1+/+) (A). (B) FMD was determined in response to stepwise increases in luminal flow in male Esr1-/- and Esr1+/+ mice. (C) Precontraction with phenylephrine (Phe) before measurement of FMD. (D) Basal diameter of the arteries used for FMD measurment. Besides FMD, acetylcholine- (E), insulin- (F), and sodium nitroprusside- (SNP, G) mediated dilation was measured in mesenteric resistance arteries isolated from male Esr1-/- and Esr1+/+ mice. FMD was also measured in wild-type (WT) mice in the presence (20 min incubation) or absence of 17-β-estradiol (E2, 0.01 µmol/L, H), estetrol (E4, 1 µmol/L, H), ICI 182 780 (1 µmol/L, H) and the GPER antagonist G-36 (10 µM, I). (I) G-1 (10 µM)- and E2 (0.01 µM)-mediated dilation in the presence or absence of G-36 (1 µM). FMD was then measured in mesenteric arteries isolated from intact (K) and ovariectomized (OVX, L) female Esr1-/- and Esr1+/+ mice as well as in and uterine arteries from Esr1-/- and Esr1+/+ mice (M). Flow rate rate was 3, 6, 9, 12, 15, 30, and 50 µl/min corresponding to 0.8, 1.2, 2, 2.8, 4, 8, and 12 dyn/cm2. Means ± the SEM are shown (n = 7–18 mice per group). Two-way ANOVA for repeated measurements: p = 0.0072 (interaction: p < 0.0001, B), p = 0.0087 (interaction: p < 0.0001, K), p = 0.0030 (interaction: p < 0.0001, L), p = 0.0119 (interaction: 0.0107, M). NS: two-way ANOVA for repeated measurements, panel E to I. NS: Two-tailed Mann-Whitney test, panels C and D. See source data in Figure 1—source data 1.
-
Figure 1—source data 1
Data and statistical analysis from experiments plotted in Figure 1B—M.
- https://cdn.elifesciences.org/articles/68695/elife-68695-fig1-data1-v2.xlsx
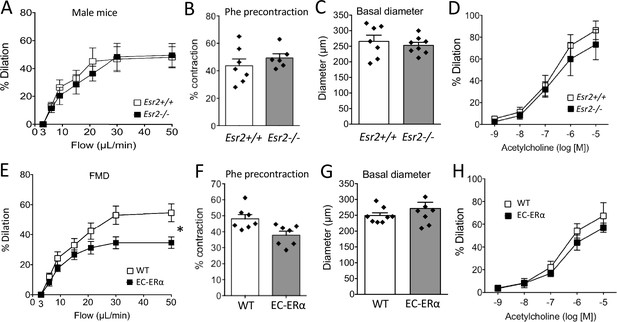
Involvement of ERβ and endothelial ERα in flow-mediated dilation (FMD).
(A to D) FMD, precontraction, basal diameter and acetylcholine-mediated dilation measured in male mice lacking ERβ (Esr2-/-) and their littermate control (Esr2+/+). (E to H) FMD, precontraction, basal diameter and acetylcholine-mediated dilation measured in TekCre/+:Esr1-/- male mice lacking endothelial ERα (EC-ERα) and TekCre/-:Esr1lox/lox their littermate controls (WT). Flow rate rate was 3, 6, 9, 12, 15, 30, and 50 µl/min corresponding to 0.8, 1.2, 2, 2.8, 4, 8, and 12 dyn/cm2.+ source data 2. Means ± the SEM are shown (n = 6 or 7 mice per group). Two-way ANOVA for repeated measurements: p = 0.0273 (interaction: p = 0.0069**, E). NS: two-way ANOVA for repeated measurements, panel A, D, and H. NS: two-tailed Mann-Whitney test, B, C, F, and G. Data and analysis in Figure 2—source data 1.
-
Figure 2—source data 1
Data and statistical analysis from experiments plotted in Figure 2A—H.
- https://cdn.elifesciences.org/articles/68695/elife-68695-fig2-data1-v2.xlsx
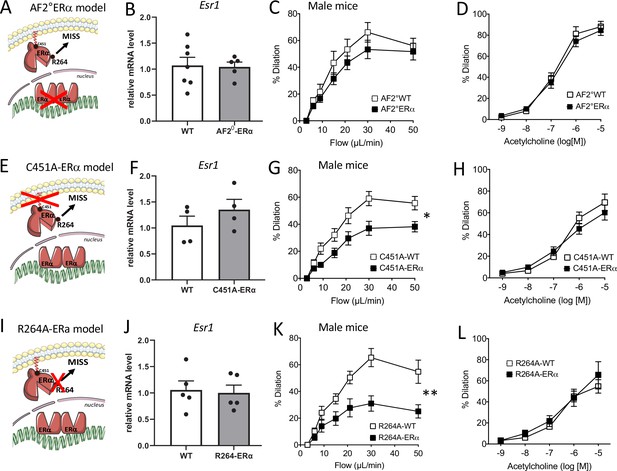
Flow-mediated dilation in mice lacking nuclear or membrane-associated ERα.
Esr1 expression level in aortic endothelial cells (expression relative to the housekeeping genes Gapdh, Hprt and Gusb), flow-mediated dilation (FMD) acetylcholine-mediated dilation were measured in mesenteric resistance arteries isolated from AF2-WT and AF20ERα male mice (A to D), C451A-WT and C451A-ERα male mice (E to H) and R264A-WT and R264A-ERα male mice (I to L). Means ± the SEM is shown (n = 13 AF20ERα, n = 5 AF2-WT mice, n = 8 C451A-ERα, n = 6 C451A-WT mice, n = 9 R264A-WT and n = 10 R264A-ERα mice). Flow rate rate was 3, 6, 9, 12, 15, 30, and 50 µl/min corresponding to 0.8, 1.2, 2, 2.8, 4, 8, and 12 dyn/cm2. Two-way ANOVA for repeated measurements: panel C, p = 0.2681 (interaction: p = 07302), panel G, p = 0.0114 (interaction: p = 0.002), panel K, p = 0.0015 (interaction: p = 0.0002). Panels D, H, and L: NS. NS, two-tailed Mann-Whitney test (panels B, F and J).
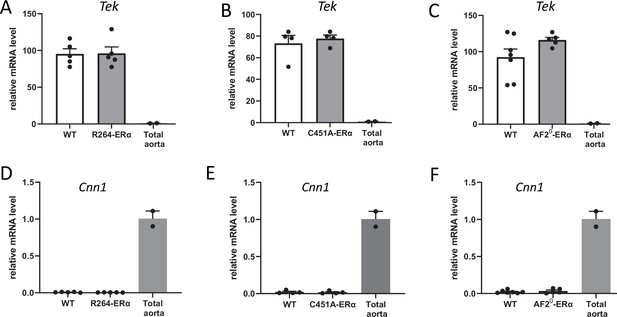
Markers of endothelial and smooth muscle cells in mouse aortic endothelial cells.
Data and analysis in Figure 3—figure supplement 1—source data 1.
-
Figure 3—figure supplement 1—source data 1
Data and statistical analysis from experiments plotted in Figure 3B–D,F–H, and J–L and for Figure 3—figure supplement 1A–F.
- https://cdn.elifesciences.org/articles/68695/elife-68695-fig3-figsupp1-data1-v2.xlsx
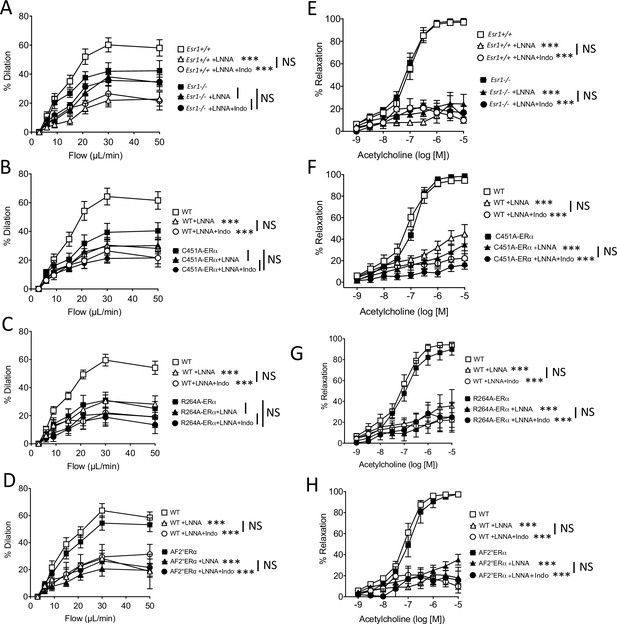
with one supplement: Effect of the blockade of NO synthesis and cyloxygenase on flow-mediated dilation.
Flow-mediated dilation (FMD) was determined in pressurized mesenteric resistance arteries isolated from male Esr1+/+ and Esr1-/- (A), C451A-WT and C451A-ERα (B), R264A-WT and R264A-ERα (C), AF20WT and AF20ERα mice (D), before and after addition of the NO synthesis blocker L-NNA (100 µM, 30 min) and then of the combination of L-NNA plus indomethacin (indo, 10 µM, 30 min). Acetylcholine-mediated relaxation was measured in the same groups in the presence and in the absence of L-NNA and of L-NNA plus indomethacin (E to H). Flow rate rate was 3, 6, 9, 12, 15, 30, and 50 µl/min corresponding to 0.8, 1.2, 2, 2.8, 4, 8, and 12 dyn/cm2. Means ± the SEM are shown (n = 6–8 per group). ***p < 0.001, two-way ANOVA for repeated measurements, L-NNA or L-NNA+ indo versus untreated arteries within each group. Data and analysis in Figure 4—source data 1.
-
Figure 4—source data 1
Data and statistical analysis from experiments plotted in Figure 4A–H.
- https://cdn.elifesciences.org/articles/68695/elife-68695-fig4-data1-v2.xlsx
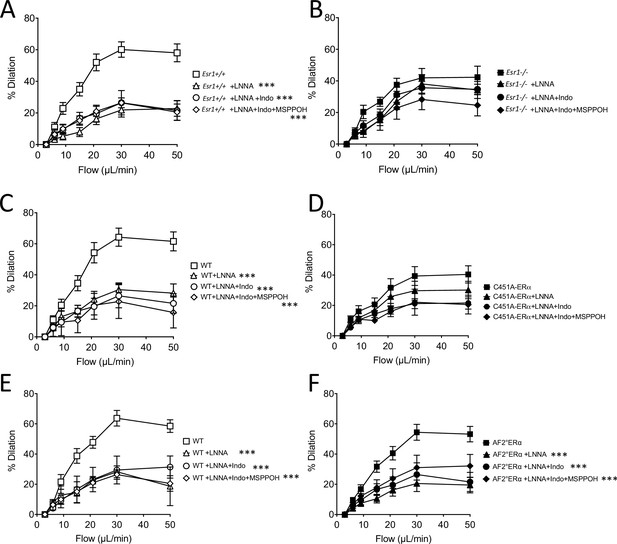
Effect of the blockade of NO synthesis (L-NNA), cyclooxygenase (indomethacin) and EETs production (MSPPOH) on flow-mediated dilation.
Data and analysis in Figure 4—figure supplement 1—source data 1.
-
Figure 4—figure supplement 1—source data 1
Data and statistical analysis from experiments plotted in Figure 4—figure supplement 1A–G.
- https://cdn.elifesciences.org/articles/68695/elife-68695-fig4-figsupp1-data1-v2.xlsx
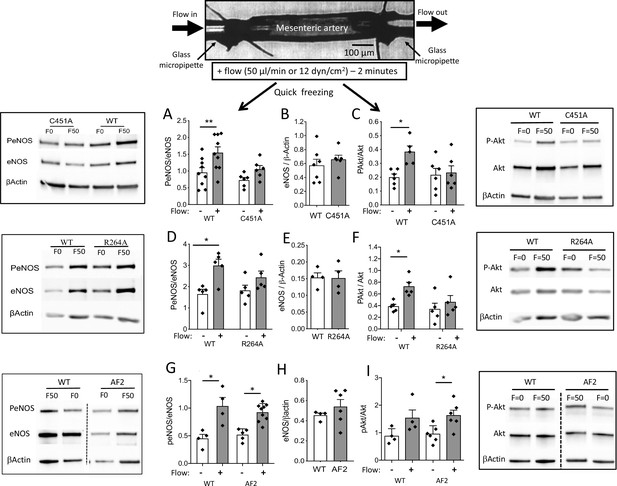
eNOS and Akt phosphorylation in response to flow in perfused isolated mesenteric resistance arteries.
As illustrated on the scheme shown on the top of the figure, mesenteric resistance arteries were cannulated in vitro on glass micropipettes and perfused with physiological salt solution. Flow (50 µl/min or 12 dyn/cm2) was applied for 2 min before quick freezing of the artery. In control experiments no flow was applied. Western-blot analysis of eNOS, phospho (Ser1177)-eNOS (P-eNOS), Akt, phospho-Akt and β-actin in mesenteric arteries isolated from male C451A-ERα mice (C451A, A to C), R264A-ERα (R264A, D to F), AF20ERα (AF2, G to I) and their littermate control (WT) was then performed. The ratio of P-eNOS / eNOS is shown in A, D and G. The expression level of eNOS/β-actin in unstimulated arteries is shown in B, E and H. The ratio of P-Akt / Akt is shown in C, F and I. Means ± the SEM are shown (n = 6 C451A-WT, n = 9 C451A-ERα, n = 5 R264A-ERα, n = 5 R264A-WT, n = 6 AF20ERα and n = 4 AF2-WT mice). *p < 0.05 (panel C: p = 0.0374, panel D: p = 0.015, panel F: p = 0.0177, panel G: WT, p = 0.0234, AF2, p = 0.0465, panel I: p = 0.0152) and **p < 0.01 (panel A: p = 0.0045), two-tailed Mann-Whitney test. Data and analysis in Figure 5—source data 1.
-
Figure 5—source data 1
Data and statistical analysis from experiments plotted in Figure 5A–I.
- https://cdn.elifesciences.org/articles/68695/elife-68695-fig5-data1-v2.xlsx
-
Figure 5—source data 2
All the blots for Figure 5.
- https://cdn.elifesciences.org/articles/68695/elife-68695-fig5-data2-v2.pdf
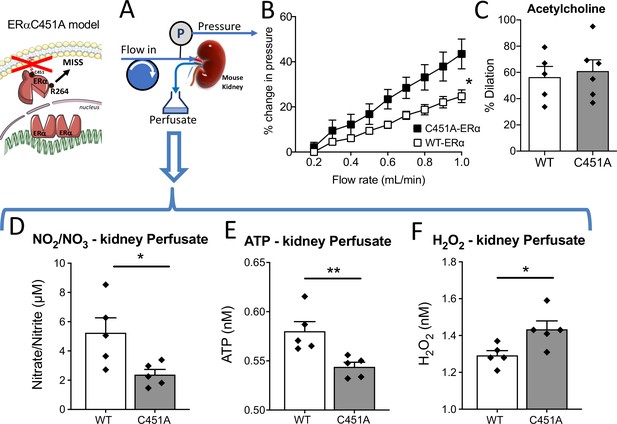
Isolated and perfused kidney from C451A-ERα mice.
In the isolated and perfused kidney (A), the flow-pressure relationship was determined in C451A-ERα and WT mice (B). (C) Acetylcholine (1 µM)-mediated relaxation. The levels of nitrate-nitrite (D), ATP (E) and H2O2 (F) level were quantified in the perfusate collected from the kidney. Means ± the SEM are shown (n = 5 C451A-WT and 7 C451A -ERα mice). *p < 0.05, two-way ANOVA for repeated measurements (panel B, C451 vs WT: p = 0.0308 Interaction: p = 0.0008). Two-tailed Mann-Whitney tests (panels C to F: p > 0.999, p = 0.0317, p = 0.0079 and p = 0.0317, respectively). Data and analysis in Figure 6—source data 1.
-
Figure 6—source data 1
Data and statistical analysis from experiments plotted in Figure 6B–F.
- https://cdn.elifesciences.org/articles/68695/elife-68695-fig6-data1-v2.xlsx
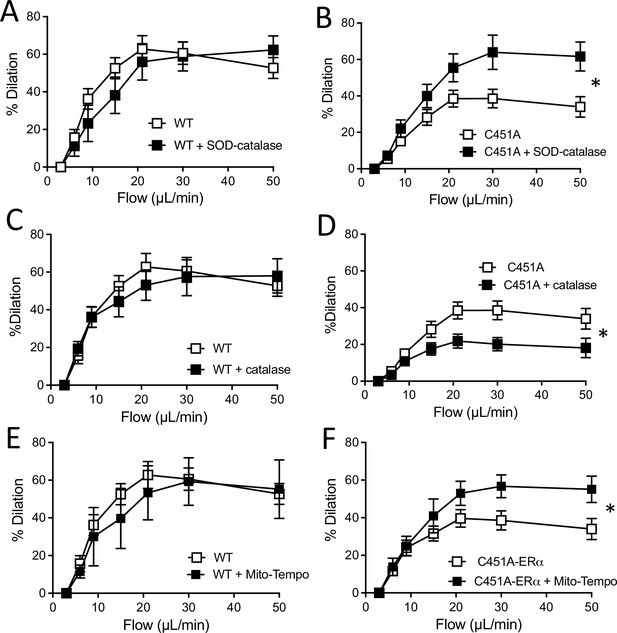
Flow-mediated dilation and oxidative stress.
Flow-mediated dilation was determined in mesenteric resistance arteries isolated from male WT and C451A-ERα mice before and after addition of PEG-SOD and catalase (SOD-catalase, A and B), catalase (C and D) or Mito-Tempo (E and F). Flow rate was 3, 6, 9, 12, 15, 30, and 50 µl/min corresponding to 0.8, 1.2, 2, 2.8, 4, 8, and 12 dyn/cm2. Means ± the SEM are shown (n = 3–9 mice per group, see details in Figure 7—source data 1). *p < 0.05, two-way ANOVA for repeated measurements (panel A to F: p = 0.5887, p = 0.0321, p = 0.7170, p = 0.0311, p = 0.7641 and p0.0354, respectively).
-
Figure 7—source data 1
Data and statistical analysis from experiments plotted in Figure 7A–L.
- https://cdn.elifesciences.org/articles/68695/elife-68695-fig7-data1-v2.xlsx
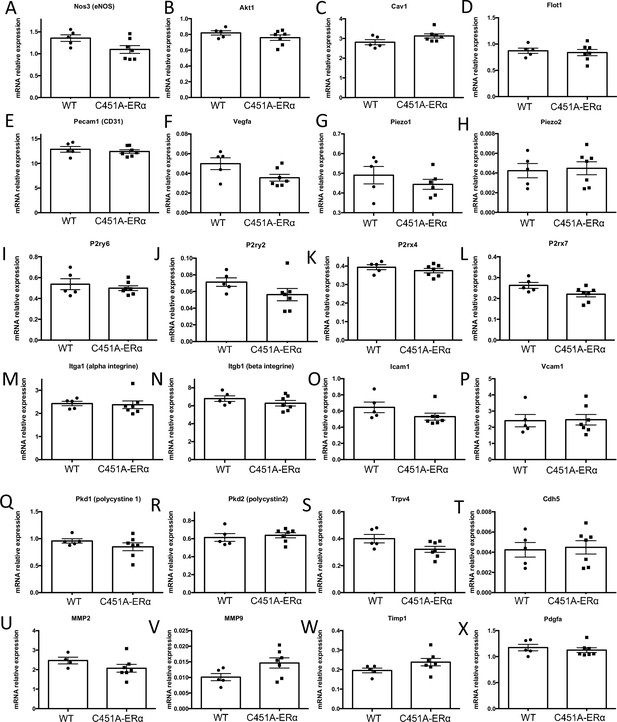
Gene expression profile in the mesenteric isolated from mice lacking membrane-ERα.
Data and analysis in Figure 7—figure supplement 1—source data 1.
-
Figure 7—figure supplement 1—source data 1
Data from experiments plotted in Figure 7—figure supplement 1A–X and in Figure 7—figure supplement 2A–T.
- https://cdn.elifesciences.org/articles/68695/elife-68695-fig7-figsupp1-data1-v2.xlsx
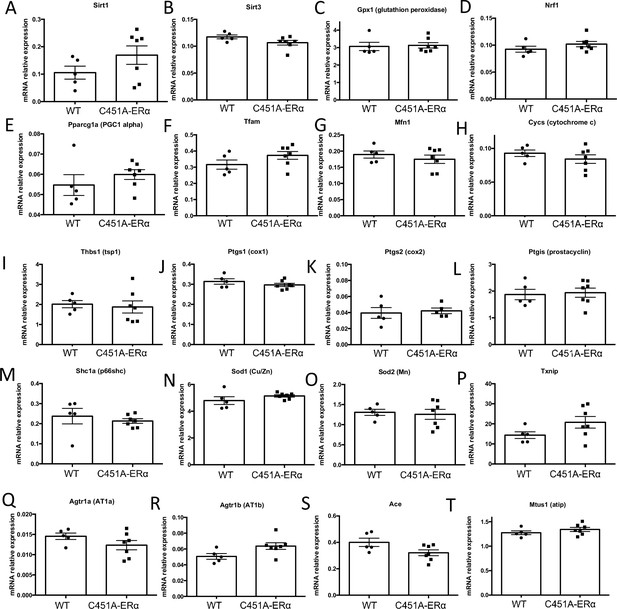
Gene expression profile in the mesenteric isolated from mice lacking membrane-ERα.
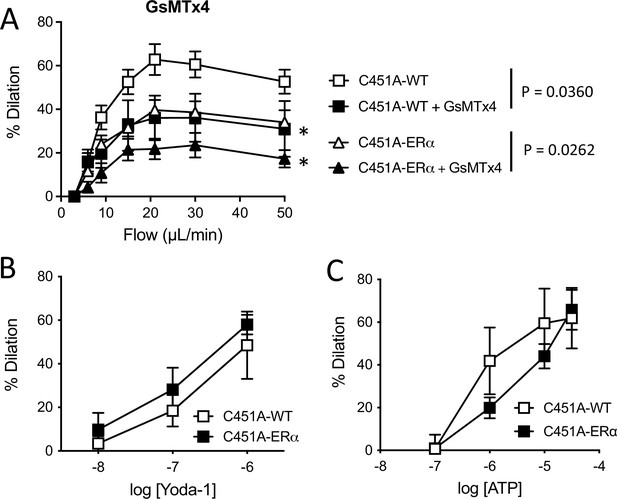
Mechanosensitive channels and ATP in FMD.
Data and analysis in Figure 7—figure supplement 3—source data 1.
-
Figure 7—figure supplement 3—source data 1
Data and statistical analysis from experiments plotted in Figure 7—figure supplement 3A–C.
- https://cdn.elifesciences.org/articles/68695/elife-68695-fig7-figsupp3-data1-v2.xlsx
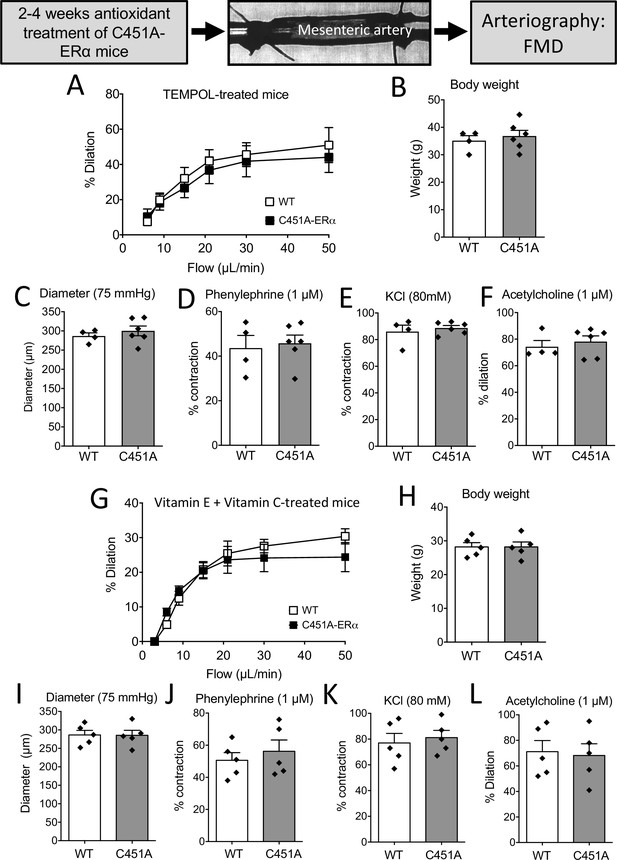
FMD after antioxidant treatments in mice lacking membrane-ERα.
FMD was determined in mesenteric resistance arteries isolated from male WT and C451A-ERα mice treated for 2 weeks with the anti-oxidant TEMPOL (A to F) or with a combination of vitamin E and vitamin C for 4 weeks (G to L). At the end of the treatments arteries were collected and mounted in an arteriograph for the measurement of FMD (A and G), body weight (B and H), arterial diameter (C and I), phenylephrine (1 µM, D and J)- and KCl (80 mM, E and K)-mediated contraction and acetylcholine (1 µM)-mediated dilation (F and L). Flow rate was 3, 6, 9, 12, 15, 30, and 50 µl/min corresponding to 0.8, 1.2, 2, 2.8, 4, 8, and 12 dyn/cm2. Means ± the SEM are shown (n = 4 C451A-WT and 6 C451A-ERα mice treated with TEMPOL and n = 5 mice per group treated with vitamin E and vitamin C). NS, two-way ANOVA for repeated measurements (panel A: p = 0.6345 and G: p = 0.6482). NS, Two-tailed Mann-Whitney tests (panels B to F and H to L). Data and analysis in Figure 8—source data 1.
-
Figure 8—source data 1
Data and statistical analysis from experiments plotted in Figure 8A–L.
- https://cdn.elifesciences.org/articles/68695/elife-68695-fig8-data1-v2.xlsx
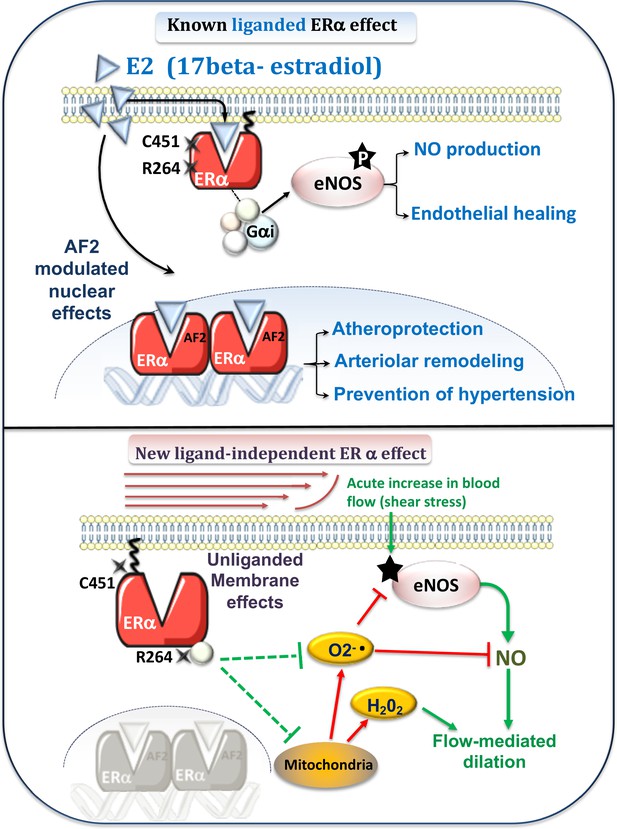
Schematic representation of the known E2-mediated ERα-dependent protective effects (upper panel) and of the new pathways described in the present study (lower panel).
Previous works (upper panel) have demonstrated the role of E2 and the nuclear activating function AF2 of ERα against atherosclerosis and hypertension (Guivarc’h et al., 2018) as well as in flow-mediated outward remodeling (Tarhouni et al., 2013; Guivarc’h et al., 2018). E2-stimulated membrane-located ERα is involved in E2-dependent NO production and in endothelial healing (Adlanmerini et al., 2014). New pathway described in the present work (lower panel): Flow, by stimulation of the surface of the endothelial cell by shear stress, activates the NO pathway (e.g. phosphorylation of eNOS: P-eNOS). This results in the production of NO, which in turn induces relaxation of the smooth muscle and thus dilation. In parallel, flow activates membrane-associated ERα, which reduces oxidative stress (O2-. and H2O2) due to NADPH-oxidase activity or of mitochondrial origin. This results in enhanced NO bioavailability. The absence of membrane-associated ERα could lead to the production of O2-., which attenuates NO-dependent dilation despite a remaining dilation due to a rise in H2O2 production.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background(Mus musculus, males and females) | Esr1-/-C57BL/6 J(Symbol: Esr1tm1.1Mma, Synonyme: ERalpha Knockout) | Mouse Clinical Inst., Strasbourg, France, Dupont et al., 2000 | MGI:2386760 | |
| Strain, strain background(Mus musculus, males) | AF2°ERα,C57BL/6 J(Symbol: Esr1tm1.1Ohl Synonym: ERalpha-AF2°) | Mouse Clinical Inst., Strasbourg, France, Billon-Galés et al., 2009 | MGI:4950046 | |
| Strain, strain background(Mus musculus, males) | C451A-ERα, C57BL/6 N(Symbol: Esr1tm1.1Ics Synonyme: C451A-ERalpha knock-in) | Mouse Clinical Inst., Strasbourg, France, Adlanmerini et al., 2014 | MGI:5574591 | |
| Strain, strain background(Mus musculus, males) | TekCre/+:Esr1f/f, C57BL/6(B6.Cg-Tg(Tek-cre)12Flv/J backcrossed with Esr1tm1.2MmaSynonym:Tie2Cre ERαlox/lox) | Esr1lox/lox: Mouse Clinical Institut, Strasbourg, France.TekCre: Jackson Lab (Bar Harbor, Me), Billon-Galés et al., 2009TekCre:Koni et al., 2001Esr1lox/lox:Dupont et al., 2000 | TekCre:Esr1lox/lox: | MGI:3775510 |
| Strain, strain background(Mus musculus, males) | Esr2-/-,C57BL/6 J (Symbol: Esr2tm1MmaSynonym: ERbeta) | Mouse Clinical Inst., Strasbourg, France, Dupont et al., 2000 | MGI:2386761 | |
| Strain, strain background(Mus musculus, males) | R264A-ERα, C57BL/6 N | Mouse Clinical Inst., Strasbourg, France, Adlanmerini et al., 2020 | No MGI ID yet | |
| Antibody | Anti-eNOS, (mouse monoclonal, clone3) | BD Biosciences | Cat# 610297, RRID:AB_397691 | WB (1:1000) |
| Antibody | Anti-phospho-eNOS, pS1177 (Mouse monoclonal,Clone 19/eNOS/S1177) | BD Biosciences | Cat# 612392, RRID:AB_399750 | WB (1:1000) |
| Antibody | Anti-beta-actin, (Mouse monoclonal, clone AC-74) | Sigma-Aldrich | Cat#: 5316; RRID:AB_476743 | WB (1:5000) |
| Antibody | Anti-Akt Pan, (rabbit monoclonal, clone C67E7) | Cell signalling technologyOzyme | Cat#: 4691; RRID:AB_915783 | WB (1:1000) |
| Antibody | Anti-phospho-Akt, S473, (rabbit monoclonal, clone D9E) | Cell signalling technology Ozyme | Cat#: 4060; RRID:AB_2315049 | WB (1:2000) |
| Antibody | Anti-mouse IgG (H + L) Secondary antibody HRP (Goat polyclonal) | Thermo scientific | Cat#: 31430; RRID:AB_228307 | WB (1:5000) |
| Antibody | Anti-rabbit IgG(H + L) Secondary antibody HRP (Goat polyclonal) | Thermo scientific | Cat#: 31460; RRID:AB_228341 | WB (1:10000) |
| Chemical compound, drug | vitamin C | Sigma Aldrich Merck, Favre et al., 2011 | A5960 | |
| Chemical compound, drug | vitamin E | Sigma Aldrich Merck, Favre et al., 2011 | T3251 | |
| Chemical compound, drug | Mito-tempo | Sigma Aldrich Merck, Freed et al., 2014 | SML0737 | |
| Chemical compound, drug | catalase | Sigma Aldrich Merck, Bouvet et al., 2007 | C3155 | |
| Chemical compound, drug | PEG-superoxide dismutase (SOD) | Sigma Aldrich Merck, Bouvet et al., 2007 | S9549 | |
| Chemical compound, drug | Estetrol (E4) | Sigma Aldrich Merck, Abot et al., 2014 | SML1523 | |
| Chemical compound, drug | ICI 182 780 | Tocris Biotechne, Meyer et al., 2010 | 1047 | |
| Chemical compound, drug | G-1 ((±)–1-[(3aR*,4S*,9bS*)–4-(6-Bromo-1,3-benzodioxol-5-yl)–3 a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinolin-8-yl)]- ethanone | Cayman chemical Bertin Bioreagent, Meyer et al., 2010 | 10008933 | |
| chemical compound, drug | G-36 ((±)-(3aR*,4S*,9bS*)–4-(6-Bromo-1,3-benzodioxol-5-yl)–3 a,4,5,9b-tetrahydro-8-(1-methylethyl))–3H-cyclopenta[c]quinoline | Cayman chemical Bertin Bioreagent, Meyer et al., 2016 | 14,397 | |
| Sequence-based reagent | N-(methylsulfonyl)–2-(2-propynyloxy)-benzenehexanamide (MSPPOH) | Cayman chemical Bertin Bioreagent, Dietrich et al., 2009 | 75,770 | |
| Chemical compound, drug | Grammostola spatulata mechanotoxin 4 (GsMTx4) | Alomone Labs, John et al., 2018 | STG-100 | |
| Chemical compound, drug | YODA1 | Bertin Bioreagent, Lhomme et al., 2019 | SML1558 | |
| Chemical compound, drug | ATPγS | Tocris Biotechne, Kukulski et al., 2009 | 4080 | |
| Chemical compound, drug | 4-hydroxy-2,2,6,6-tetramethylpiperidine (TEMPOL) | Sigma Aldrich Merck, Freidja et al., 2014 | 176,141 | |
| commercial assay or kit | Nitric oxide metabolite detection kit | Cayman Chemical | 780,051 | |
| commercial assay or kit | Hydrogen peroxide assay kit | Abcam | Ab102500 | |
| commercial assay or kit | ATP determination kit | Invitrogen Molecular Probes | A22066 |
Additional files
-
Supplementary file 1
Primer sequences used for the RT-qPCR.
- https://cdn.elifesciences.org/articles/68695/elife-68695-supp1-v2.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/68695/elife-68695-transrepform1-v2.docx





