Inhibition of itch by neurokinin 1 receptor (Tacr1) -expressing ON cells in the rostral ventromedial medulla in mice
Figures
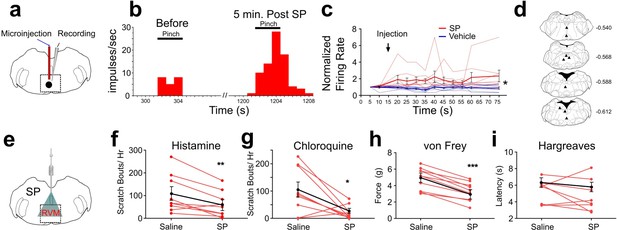
Effects of intramedullary microinjection of SP on RVM ON cells and itch and pain behavior.
(A) SP or saline was microinjected while recording from single ON cells. (B) Peristimulus-time histogram of ON cell response to pinch before (left) and 5 min after local microinjection of SP (right). (C) Normalized firing rate of ON cells following local microinjection of saline (blue, n=8) or SP (red, n=8) at time indicated by arrow. ON cells showed a significant increase in evoked firing following SP injection compared with saline (*; two-way ANOVA, F1, 14 = 8.020, p=0.0133, bolded lines: mean responses; error bars SEM). Male mice were used in these experiments. (D) Lesion sites from the RVM ON cell recordings. Numbers to right indicate Bregma coordinates. (E) An implanted intramedullary microinjection cannula allowed assessment of itch and pain behavior after injection of SP into RVM. (F, G) Graphs plot the number of scratch bouts elicited by intradermal injection of histamine (F, n=9, M = (106.2; 59.72), t=3.4113, DF = 8, 95% CI [15.06, 77.83], p=0.0092) or chloroquine (G, n=9, M = (102.9; 22.00), t=2.847, DF = 8, 95% CI [15.39, 146.5], p=0.0216) for each mouse (red dots and lines), and mean scratch bouts (black line; error bars: SEM), following intramedullary microinjection of saline or SP. Experiments with saline and SP microinjections were conducted at least 7 days apart. Microinjection of SP significantly attenuated histamine- and chloroquine-evoked scratching (F, G). (H) Mechanical withdrawal thresholds were reduced by intramedullary SP, n=10, M = (4.855; 3.253), t=8.96, DF = 8, 95% CI [1.198, 2.007], p = <0.0001 (I) Thermal withdrawal latency was not significantly affected by intramedullary SP, n=8, M = (5.271; 4.691), t=0.9784, DF = 7, 95% CI [–0.8216, 1.981], p=0.3605. (G, F) students t-test *p<0.05, **p<0.01, ***p<0.001. n=5–7 males, 3 females/ group.
-
Figure 1—source data 1
Data for electrophysiolgical recordings and behavior from SP injection into the RVM.
- https://cdn.elifesciences.org/articles/69626/elife-69626-fig1-data1-v1.xlsx
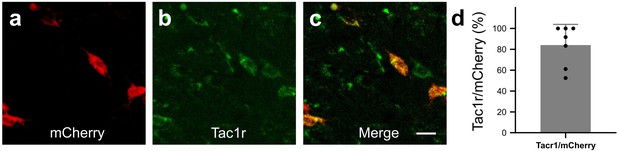
In Tacr1 cre +/-mice receiving AAV-DIO-hM3Dq-mCherry injections in the RVM, mCherry strongly colocalized with Tacr1 expression.
(A–C): Images of RVM cells expressing mCherry (A), anti-Tacr1 antibody (B), and double-labeled cells (C). (D): 84% of mCherry expressing neurons co-express Tacr1. N=7. Scale bar (25 µm).
-
Figure 2—source data 1
Data for co-expression of DREADDs to Tac1r.
- https://cdn.elifesciences.org/articles/69626/elife-69626-fig2-data1-v1.xlsx
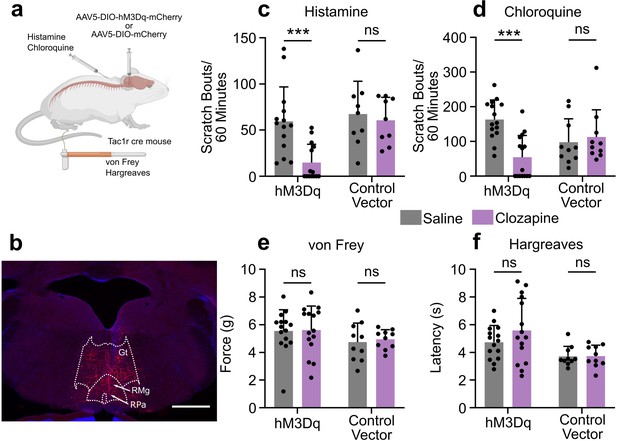
Chemogenetic activation of RVM Tacr1 expressing neurons inhibits itch related behavior.
(A) AAV5-DIO-hM3Dq-mCherry or AAV5-DIO-mCherry was injected into the RVM of Tacr1 cre mice. (B) Expression of hM3Dq-mCherry was limited to the RVM. Gt: gigantocellularis; RMg: raphe magnus; RPa: raphe pallidus. Scale bar (1 mm). (C) Administration of clozapine caused a significant reduction in histamine-evoked scratching in hM3Dq expressing mice (7 males, 7 females, M = (59.32; 14.75), t=3.715, DF = 13, 95% CI [18.65, 70.49], p=0.0026), but not in control vector mice (six males, three females, M = (67.28; 60.61), t=0.6235, DF = 8, 95% CI [–24.11, 37.44], p=0.6235). (D) Administration of Clozapine caused a significant reduction in chloroquine-evoked scratching in hM3Dq mice (seven males, eight females, M = (163.2; 54.33), t=4.72, DF = 14, 95% CI [59.40, 158.3], p=0.0003) but not in control vector mice (six males, four females, M = (97.90; 112.9), t=0.4129, DF = 9, 95% CI [–23.45, 36.78], p=0.4129). (E) Clozapine administration did not significantly change mechanical withdrawal thresholds in hM3Dq (seven males, eight females, M = (5.549; 5.610), t=0.1309, DF = 14, 95% CI [–1.060, 0.9378], p=0.8977) or control vector mice (six males, four females, M = (4.742; 4.958), t=0.6342, DF = 9, 95% CI [–0.9867, 0.5546], p=0.5418). (F) Clozapine administration did not significantly change thermal withdrawal thresholds in hM3Dq (seven males, eight females, M = (4.735; 5.587), t=1.262, DF = 14, 95% CI [–2.300, 0.5961], p=0.2276) or control vector mice (six males, four females, M = (3.718; 3.737), t=0.0597, DF = 9, 95% CI [–0.7260, 0.6886], p=0.9537). (C–F) *p<0.05, **p<0.01, ***p<0.001, Students paired t-test.
-
Figure 3—source data 1
Behavioral data of RVM Tac1r DREADDs activation with clozapine.
- https://cdn.elifesciences.org/articles/69626/elife-69626-fig3-data1-v1.xlsx
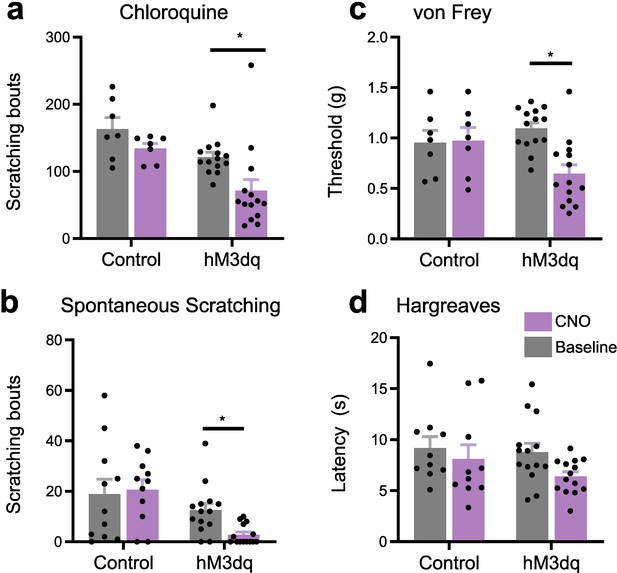
Chemogenetic activation of RVM Tacr1 expressing neurons inhibits itch-related behavior.
Tacr1creER mice were injected with AAV2-DIO-hM3Dq-mCherry. (A) CNO administration significantly reduced chloroquine evoked scratch bouts in DREADDs but not control vector mice (Control: n=7, M = (163.286; 134.143), t=1.243, DF = 19, 95% CI [–27.7, 86.06], p=0.4057. hM3dq: n=14, M = (121.214; 71.357), t=3.007, DF = 19, 95% CI [9.608, 90.11], p=0.0145). (B) CNO reduced spontaneous scratching in hM3Dq-expressing but not control vector mice (Control: n=11, M = (18.909; 20.636), t=0.4089, DF = 23, 95% CI [–11.83, 8.377], p=0.9017. hM3dq: n=14, M = (12.571; 2.786), t=2.613, DF = 23, 95% CI [0.8292, 18.74], p=0.0308). (C) CNO administration significantly reduced mechanical withdrawal thresholds in DREADDs but not control vector mice (Control: n=7, M = (0.955; 0.976), t=1.035, DF = 38, 95% CI [–0.4584, 0.1762], p=0.5199. hM3dq: n=14, M = (1.096; 0.647), t=2.411, DF = 38, 95% CI [0.01126, 0.6459], p=0.0413). (D) CNO did not affect thermal withdrawal latency in any group (Control: n=10, M = (9.193; 8.131), t=0.7995, DF = 22, 95% CI [–2.125, 4.248], p=0.678. hM3dq: n=14, M = (8.797; 6.401), t=2.135, DF = 22, 95% CI [–0.2973, 5.089], p=0.0864). (A–D) Two-way ANOVA with Sidak multiple comparisons *p<0.05, **p<0.01, ***p<0.001.
-
Figure 3—figure supplement 1—source data 1
Behavioral data of RVM Tac1r DREADDs activation with CNO.
- https://cdn.elifesciences.org/articles/69626/elife-69626-fig3-figsupp1-data1-v1.xlsx
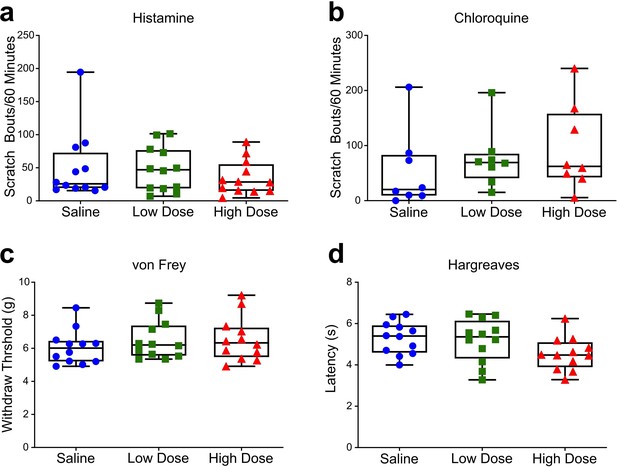
Clozapine administration does not affect acute itch or pain behavior.
Saline, low dose clozapine (0.01 mg/kg) or high dose clozapine (0.1 mg/kg) was administered systemically, followed by tests for acute itch and pain behaviors. (A, B) Clozapine did not significantly affect the number of scratch bouts elicited by intradermal injection of histamine (A, RM one-way ANOVA, F2,22 = 0.7673, p=0.4763) or chloroquine (B, RM one-way ANOVA, F2,14 = 0.7234, p=05024). (C, D) Clozapine also did not significantly affect the respective latency or threshold of hindlimb withdrawals elicited by acute thermal (C, RM one-way ANOVA, F2,22 = 0.2.900, p=0.0763) or mechanical (D, RM one-way ANOVA, F2,22 = 1.103, p=0.3496) stimuli. (A, C and D) n=6 males, 6 females; (B) n=5 males, 3 females.
-
Figure 3—figure supplement 2—source data 1
Data for Clozapine administration effects on behavior.
- https://cdn.elifesciences.org/articles/69626/elife-69626-fig3-figsupp2-data1-v1.xlsx
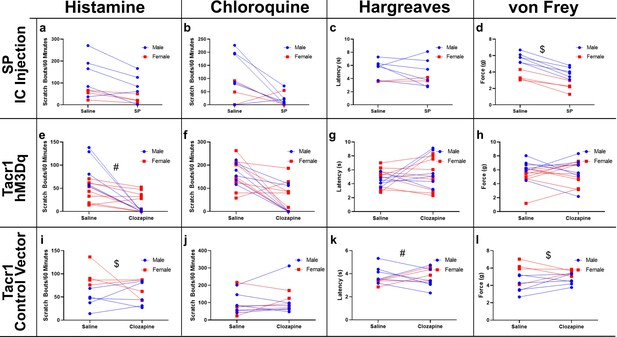
Sex differences.
Graphs are replotted with data sorted into male (blue) and females (red). (A–C) There were no significant sex differences following intramedullary microinjection of saline or SP for histamine- (A, n=9, SP: F1,7 = 11.49 p=0.0116, Sex: F1,7 = 4.325 p=0.0761, SP*Sex: F1,7 = 1.682 p=0.2357) or chloroquine-evoked scratching (B, n=9, SP: F1,7 = 5.64 p=0.0493, Sex: F1,7 = 2.094 p=0.1911, SP*Sex: F1,7 = 1.969 p=0.2033), or for thermal withdrawal latencies (C, n=8, SP: F1,6 = 0.153 p=0.7092, Sex: F1,6 = 2.208 p=0.1878, SP*Sex: F1,6 = 0.7851 p=0.4097). (D) Males exhibited a significantly higher mechanical withdrawal threshold following intramedullary saline ($, n=10, SP: F1,8 = 84.72 p = <0.001, Sex: F1,8 = 31.28 p=0.005, SP*Sex: F1,8 = 2.599 p=0.1456), while both sexes showed a significant reduction in withdrawal threshold following intramedullary SP. (E) Suppression of histamine-evoked scratching following clozapine was significantly greater in males (#; n=14, Clozapine: F1,12 = 49.73 p = <0.0001, Sex: F1,12 = 1.84 p=0.2, Clozapine*Sex: F1,12 = 21.6 p=0.0006). (F–H) There were no sex differences for chloroquine-evoked scratching (F, n=15, Clozapine: F1,13 = 22.09 p=0.0004, Sex: F1,13 = 0.1533 p=0.7018, Clozapine*Sex: F1,13 = 0.5548 p=0.4696), thermal withdrawal latency (G, n=15, Clozapine: F1,13 = 1.921 p=0.844, Sex: F1,13 = 0.0403 p=0.844, Clozapine*Sex: F1,13 = 0.6736 p=0.4266) or mechanical withdrawal threshold (H, n=15, Clozapine: F1,13 = 0.006799 p=0.9355, Sex: F1,13 = 1.51 p=0.2409, Clozapine*Sex: F1,13 = 1.116 p=0.3101). (I–L) We did observe a higher number of histamine-evoked scratch bouts in female mice when compared to male -(I, $, n=9, Clozapine: F1,7 = 0.5241 p=0.4926, Sex: F1,7 = 10.74 p=0.0135, Clozapine*Sex: F1,7 = 2.429 p=0.1631) and a higher mechanical withdrawal threshold for female versus male mice (L, $, n=10, Clozapine: F1,8 = 0.1487 p=0.7099, Sex: F1,8 = 8.878 p=0.0176, Clozapine*Sex: F1,8 = 1.671 p=0.2322). In the Hargreaves test there was no significant effect of clozapine administration or sex on thermal withdrawal latency, but we did observe a significant interaction (K, n=10, Clozapine: F1,8 = 0.3303 p=0.5813, Sex: F1,8 = 0.0842 p=0.7791, Clozapine*Sex: F1,8 = 6.277 p=0.0366). We did not observe any sex differences for chloroquine- evoked scratch bouts (J, #, n=10, Clozapine: F1,8 = 0.147 p=0.4061, Sex: F1,8 = 0.00381 p=0.9523, Clozapine*Sex: F1,8 = 0.147 p=0.7114),.
-
Figure 3—figure supplement 3—source data 1
Data for behavioral sex differences.
- https://cdn.elifesciences.org/articles/69626/elife-69626-fig3-figsupp3-data1-v1.xlsx
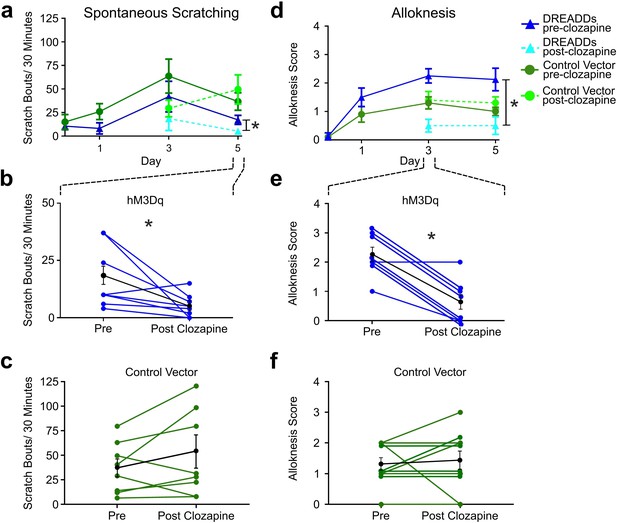
Chemogenetic activation of RVM Tacr1 neurons reduces spontaneous scratching and alloknesis in the imiquimod model of chronic psoriasisiform itch.
(A) Application of imiquimod (1%, 0.05 g, Taro) once per day produced a significant increase in spontaneous scratching at day 3 for both DREADDs (dark blue, RM ANOVA, Dunnett’s multiple comparisons Day0 vs Day 3, q=0.18511, p=0.0234) and control vector (dark green, RM ANOVA, Dunnett’s multiple comparisons Day0 vs Day 3, q=2.549, p=0.0382) mice. Following clozapine, there was a significant reduction in spontaneous scratching on day 5 for the DREADDs mice (dashed light blue, Paired t test n=8, M = (17.25; 5.250), t=2.38, DF = 7, 95% CI [-23.92,–0.07597], p=0.0489) but no significant change in control vector mice (dashed light green, Paired t test n=10, M = (36.75; 49.56), t=1.343, DF = 9, 95% CI [–35.38, 9.752], p=0.2213). (B) Graph shows individual DREADDs animals’ spontaneous scratch bouts pre- and post-clozapine. Following clozapine there was a significant reduction in scratching. Blue: individual counts; black: mean +/-SEM. Paired t test n=8, M = (17.25; 5.250), t=2.38, DF = 7, 95% CI [-23.92,–0.07597], p=0.0489 (C) Graph as in B for mice in vector control group, in which clozapine had no significant effect. Green: individual counts; black: mean +/-SEM. Paired t test n=10, M = (36.75; 49.56), t=1.343, DF = 9, 95% CI [–35.38, 9.752], p=0.2213 (D) Imiquimod induced significant increases in alloknesis scores on days 3 and 5 of treatment in DREADDs (dark blue, RM ANOVA, Dunnett’s multiple comparisons, Day0 vs Day 3, q=9.379, p=0.<0.0001, Day0 vs Day5, q=4.00, p=0.0129) and vector control (dark green, RM ANOVA, Dunnett’s multiple comparisons, Day0 vs Day 3, q=4.811, p=0.0025, Day0 vs Day5, q=5.014, p=0.0019) mice. Following clozapine administration on days 3 and 5 there were significant reductions in alloknesis scores for the DREADDs mice (dashed light blue, Paired t test n=8, M = (2.250; 0.6250) t=6.177, DF = 7, 95% CI [1.003, 2.247], p=0.0005) but not control vector groups (dashed light green, Paired t test n=10, M = (1.300; 1.400) t=0.3612, DF = 9, 95% CI [–0.7264, 0.5264], p=0.7263). (E) Clozapine resulted in a significant reduction in alloknesis scores (format as in B), Paired t test n=8, M = (2.250; 0.6250) t=6.177, DF = 7, 95% CI [1.003, 2.247], p=0.0005 (F) Clozapine had no effect on alloknesis scores in vector controls (format as in C). Paired t test n=10, M = (1.300; 1.400) t=0.3612, DF = 9, 95% CI [–0.7264, 0.5264], p=0.7263. Paired students t-test *p<0.05, **p<0.01, ***p<0.001. (A–F) n=4–5 males, 4–5 females/group.
-
Figure 4—source data 1
Data for RVM Tac1r activation in psoriasis model of chronic itch.
- https://cdn.elifesciences.org/articles/69626/elife-69626-fig4-data1-v1.xlsx
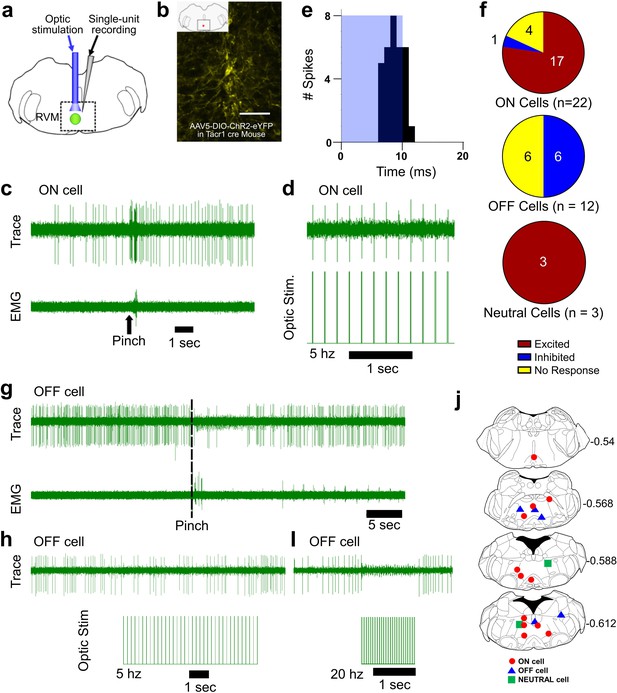
Optotagging of RVM Tacr1 neurons.
(A) RVM cells were recorded with a microelectrode coupled to an optic fiber. (B) Injection of AAV-ChR2-eYFP in the RVM of Tacr1 cre+/- mice caused strong expression of eYFP. Scale bar (100 µm). (C) ON cells were identified based on their pinch-evoked response that preceded the hindlimb withdrawal as monitored by EMG in biceps femoris. (D) Cells identified as RVM ON cells were optically stimulated (5 mW, 472 nm). This neuron faithfully responded to each pulse in a 5 hz train. (E) Light entrainment was analyzed by creating peristimulus-time histograms (PSTH) of action potentials that occurred within a 20 ms window following the onset of each light pulse. This neuron responded consistently at a latency of approximately 8.14 ms with a calculated efficiency index of 0.86. (F) Distribution of RVM ON, OFF, and Neutral cells which were excited (red), inhibited (blue), or not affected (yellow) by optic stimulation. (G) Application of a pinch stimulus elicited a hindlimb withdrawal (dotted line) and a pause in firing that is typical of OFF cells. (H) There was an intermittent decrease in OFF cell firing during 5 hz optic stimulation and a (I) total cessation of firing during 20 hz optic stimulation. (J) Lesion sites from the optotagging recordings of RVM ON (red circles), OFF (blue triangles), and NEUTRAL cells (green squares). Numbers to right indicate Bregma coordinates.
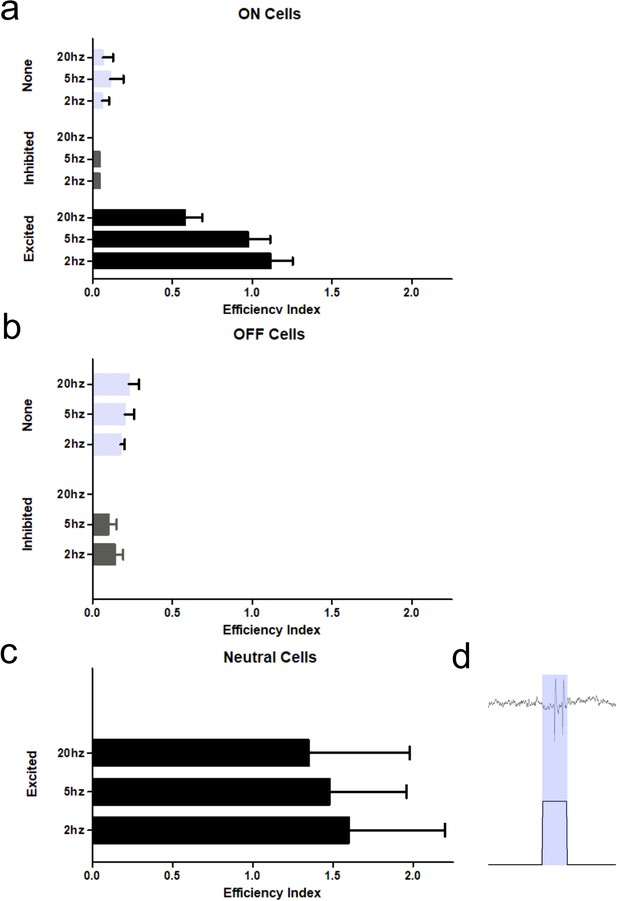
Efficiency index of classified RVM neurons.
(A–C) Identified ON (A), OFF (B), and NEUTRAL (C) cells were tested for efficiency to respond within 20 ms following the onset of an optic stimulus. Neurons which were excited by optic stimulation had a robust efficiency index (approaching or >1) compared to neurons inhibited or unaffected (none) by optic stimulation. (D) Neutral cell doublet.
-
Figure 5—figure supplement 1—source data 1
Data for efficiency index analsyis of optotagging experiments.
- https://cdn.elifesciences.org/articles/69626/elife-69626-fig5-figsupp1-data1-v1.xlsx
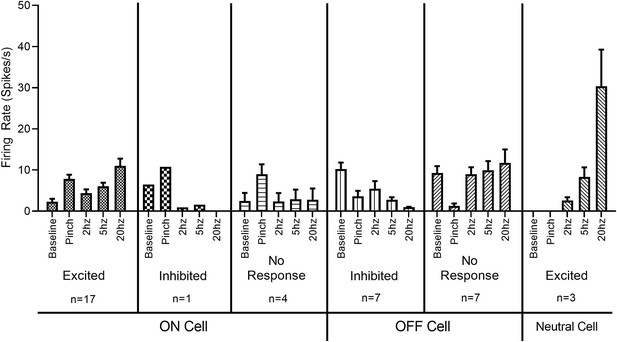
Firing rates of classified RVM neurons in response to optic stimulation.
-
Figure 5—figure supplement 2—source data 1
Data for firing rate analsyis of optotagging experiments.
- https://cdn.elifesciences.org/articles/69626/elife-69626-fig5-figsupp2-data1-v1.xlsx
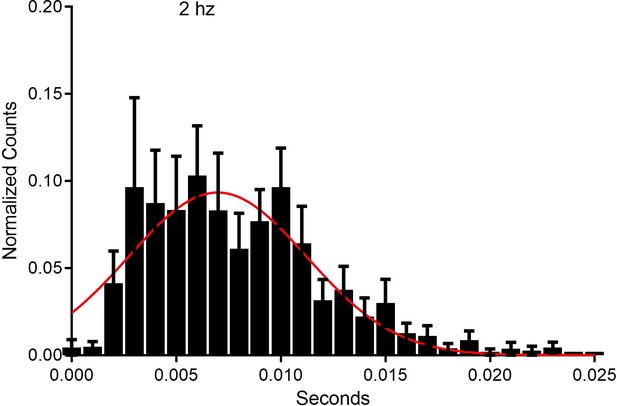
Response latency of ON cells to optic stimulation.
RVM neurons (n=17) which responded to optic stimulation at 2 hz, had averaged response latencies of approximately 7.6+/-1.12 ms.
-
Figure 5—figure supplement 3—source data 1
Data for response latency of optotagging experiments.
- https://cdn.elifesciences.org/articles/69626/elife-69626-fig5-figsupp3-data1-v1.xlsx
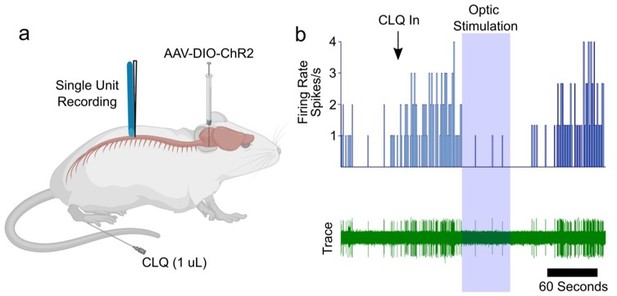
Response of spinal neuron to intradermal injection of chloroquine (CLQ) is suppressed by optogenetic stimulation in RVM.
(a) diagram of experimental design. AAV5-DIO-ChR2-eYFP was injected into the RVM of Tacr1 cre mice. (B) A CLQ responsive neuron was identified and inhibition observed following blue light stimulation to the spinal cord to activate ChR2-expressing descending fibers.
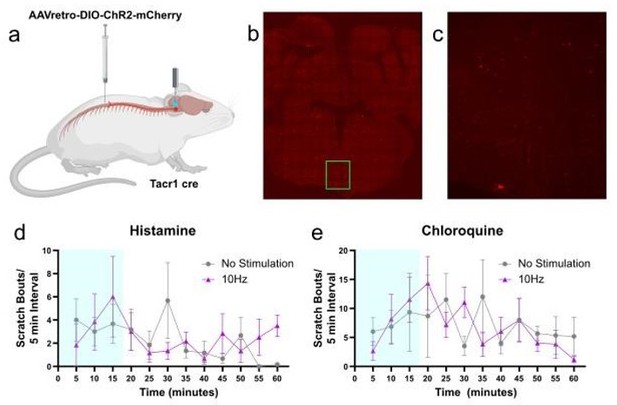
Inability of optogenetic stimulation of spinally projecting RVM Tacr1 expressing neurons to suppress scratching behavior.
(a) retrograde AAV was injected into the dorsal spinal cord of Tacr1 cre mice. An optic fiber was implanted into the RVM following viral injection. (b-c) Expression of mCherry was present, but weak, in the RVM. (d,e) Blue light stimulation did not significantly reduce histamine (d) or chloroquine (e) evoked scratching behavior. n = 6 group.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (M. musculus) | Tacr1 cre | Dong Lab | ||
| Strain, strain background (M. musculus) | Tacr1 creER | Dong Lab (Huang et al., 2016) | ||
| Strain, strain background (AAV) | aav5-hSyn-DIO-hM3Dq-mCherry | Addgene | 44361-AAV5 | |
| Strain, strain background (AAV) | aav5-hSyn-DIO-mCherry | Addgene | 50459-AAV5 | |
| Strain, strain background (AAV) | aav5-Ef1a-hChR2-mCherry | Addgene | 20297-AAV5 | |
| Antibody | Mouse monoclonal: anti-Tacr1 conjugated 488 | SCBT | sc-365091 AF488 | (1:50) |
| Chemical compound, drug | Substance P (SP) | Tocris | 1156/5 | |
| Chemical compound, drug | Histamine Dihydrochloride (HA) | Sigma-Aldrich | H7250 | |
| Chemical compound, drug | Chloroquine Diphosphate (CLQ) | Sigma-Aldrich | C6628 | |
| Chemical compound, drug | Pentobarbital Sodium | Sigma-Aldrich | P3761 | |
| Chemical compound, drug | Buprenorphine Hydrochloride | Amerisoucebergen | NDC42023-179-05 | |
| Software, algorithm | 5 Prism | Graphpad |






