Golgi membrane protein Erd1 Is essential for recycling a subset of Golgi glycosyltransferases
Figures
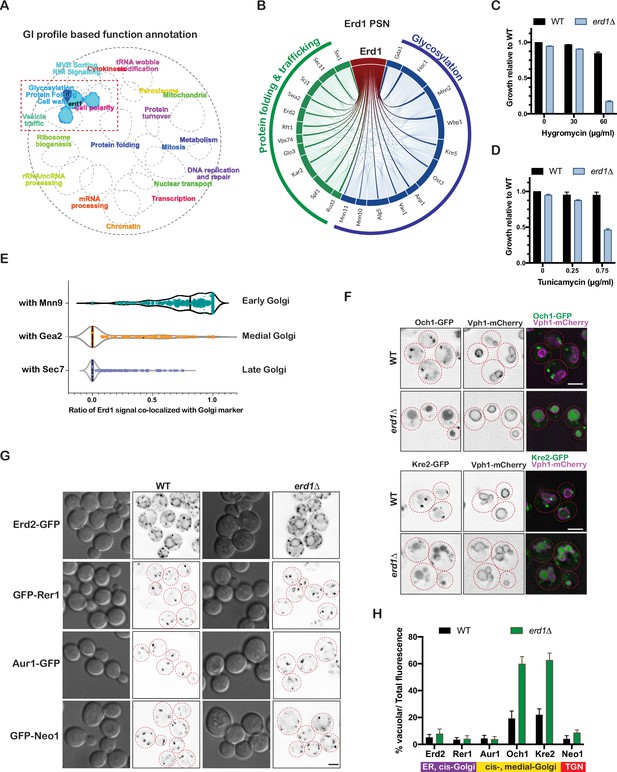
Erd1 is required for Golgi protein glycosylation.
(A) Spatial analysis of functional enrichment (SAFE) analysis based on the genetic interaction profile of erd1 mutant (stringent cut-off (p < 6e-11)). (B) Profile similarity network (PSN) of Erd1 showing genes with similar genetic interactions (similarity cut-off 0.25). (C) Growth of wild type and erd1 mutant in the presence of indicated concentrations of HygromycinB in YPD liquid cultures at 30° C after 24 hr. (D) Sensitivity of wild type and erd1 mutant to indicated concentrations of Tunicamycin in YPD liquid cultures at 30° C. (E) Violin plots for the ratio of co-localized Erd1-mNeonGreen fluorescence with early (Mnn9-mCherry), medial (Gea2-3xmMars), and late (Sec7-6xDsRed) Golgi markers (n = 250 puncta for each condition). The median is indicated with dashed lines. (F) Live-cell fluorescence imaging of Och1-GFP, Kre2-GFP, and vacuole membrane marker (Vph1-mCherry) in wild type and erd1 mutant. Red dashed lines indicate the cell boundaries based on DIC images. (G) Live-cell fluorescence imaging of Erd2-GFP, GFP-Rer1, Aur1-GFP and GFP-Neo1 in wild type and erd1 mutant. (H) Quantification of the percent vacuolar to total GFP fluorescence for reporters in (F) and (G). Scale bars: 2.5µ m.
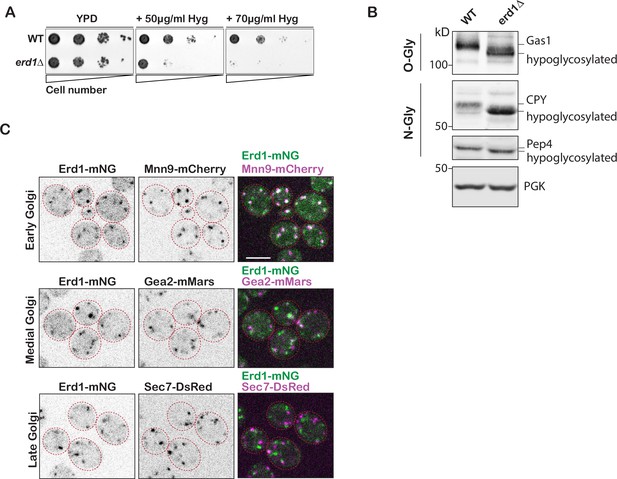
Erd1 localizes to the cis-Golgi and is required for protein glycosylation.
(A) Growth of wild type and erd1 mutant on YPD media with or without the indicated concentrations of HygromycinB at 26° C after 2 days. (B) Immunoblot analysis on yeast cell lysates from wild type and erd1 mutant for O-linked glycosylation reporter (Gas1), N-linked glycosylation reporters (CPY, Pep4) or loading control (PGK). (C) Live-cell confocal fluorescence imaging of Erd1-mNeonGreen along with early (Mnn9-mCherry), medial (Gea2-3xmMars), and late (Sec7-6xDsRed) Golgi markers. Scale bars: 2.5µm.
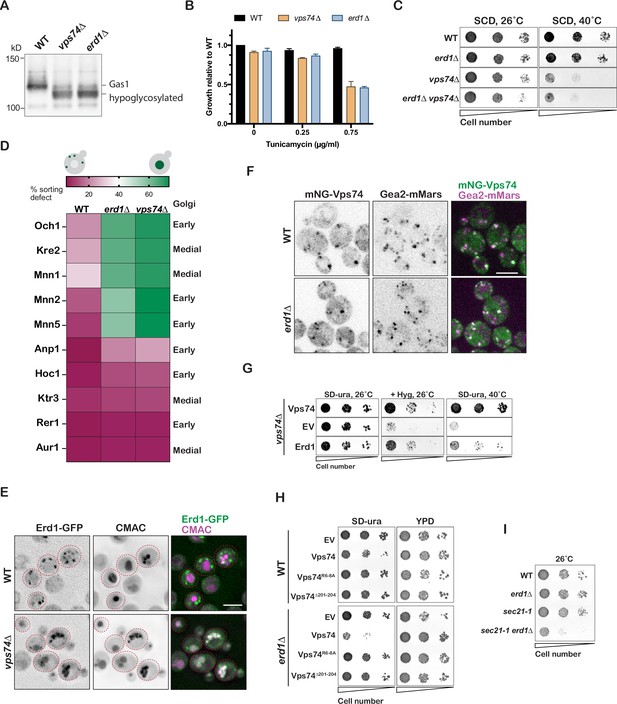
Erd1 is required for Vps74-COPI dependent recycling of specific Golgi glycosyltransferases.
(A) Immunoblot analysis on yeast cell lysates from wild type, erd1, and vps74 mutant for glycosylation reporter, Gas1. (B) Growth of wild type, erd1, and vps74 mutant in the presence of indicated concentrations of Tunicamycin in YPD liquid cultures at 30° C after 24 hr. (C) Growth of serial dilutions of wild type, erd1, vps74, and erd1vps74 mutants on synthetic media at 26 °C and 40° C after 2 days. (D) Quantification of percent vacuolar fluorescence to total fluorescence of the indicated GFP tagged early and medial Golgi proteins in wild type, erd1, and vps74 mutant. (E) Live-cell fluorescence imaging of Erd1-GFP and vacuolar dye, CMAC in wild type and the vps74 mutant. (F) Live-cell fluorescence imaging of mNeonGreen-Vps74 and medial Golgi marker, Gea2-3xMars in wild type and the erd1 mutant. (G) Growth of serial dilutions of vps74 mutant transformed with empty vector (EV) or plasmids overexpressing Vps74 and Erd1 on YPD with 50 µg/ml hygromycinB at 26 °C or synthetic media lacking uracil at 26 °C or 40° C after 2–3 days. (H) Growth of serial dilutions of wild type and erd1 mutant transformed with the indicated Vps74 mutants at 26° C after 3 days. (I) Growth of serial dilutions of wild type, erd1, sec21-1, and sec21-1 erd1 mutants at 26 °C after 3 days. Scale bars: 2.5µ m.
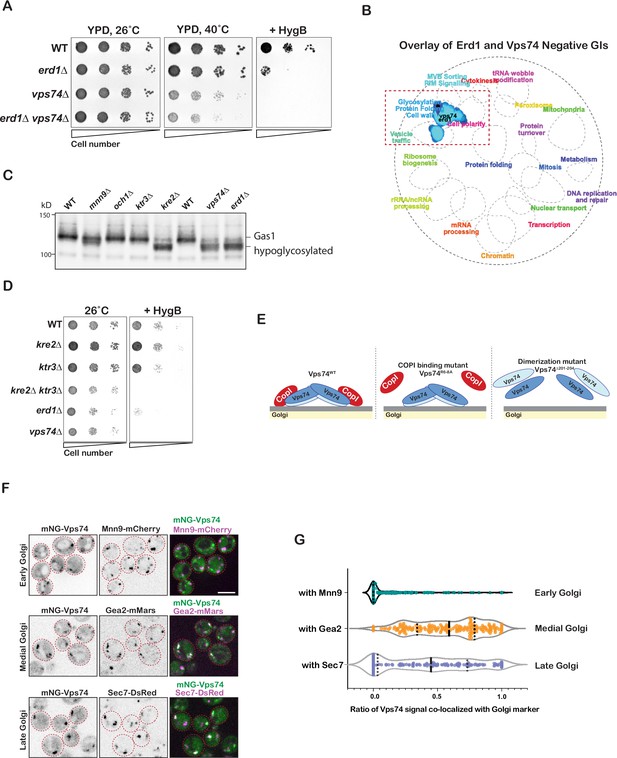
Erd1 and Vps74 mutants exhibit similar glycosylation defects.
(A) Growth of serial dilutions of wild type, erd1, and vps74 mutants on YPD with or without 60 µg/ml hygromycinB at the indicated temperature after 2 days. (B) SAFE analysis of genetic interaction profiles of erd1 and vps74 mutant (cut-off (p < 3e-8)). (C) Immunoblot analysis on yeast cell lysates from wild type, mnn9, och1, ktr3, kre2, vps74 and erd1 mutants for glycosylation reporter, Gas1. (D) Growth of serial dilutions of wild type, kre2, ktr3, kre2ktr3, erd1 and vps74 mutants on YPD with or without 60 µg/ml hygromycinB at 26° C after 2 days. (E) Schematic depicting the known defects in the Vps74 mutants tested in Figure 2H. (F) Live-cell confocal fluorescence imaging of mNeonGreen-Vps74 along with early (Mnn9-mCherry), medial (Gea2-3xmMars), and late (Sec7-6xDsRed) Golgi markers. (G) Violin plots for the ratio of co-localized mNeonGreen-Vps74 with the Golgi markers in (F). Dashed lines indicate the median values, and dotted lines indicate the 95 CIs. Scale bars: 2.5µ m.
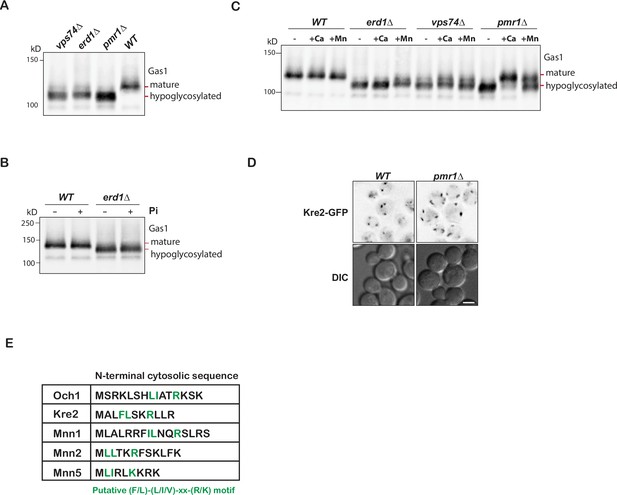
Pmr1 is required for glycosylation but not glycosyltransferase recycling.
(A) Immunoblot analysis on yeast cell lysates from wild type, pmr1, vps74 and erd1 mutants for glycosylation reporter, Gas1. (B) Immunoblot analysis on indicated yeast cell lysates from untreated or cells grown in media supplemented with 50 mM phosphate buffer pH 6.2 for 4 hr at 30° C. (C) Immunoblot analysis on indicated yeast cell lysates from untreated or cells grown in media supplemented with 50 mM CaCl2, and 600 µM MnCl2 for 4 hr at 30 °C. (D) Live cell imaging of wild type or pmr1 mutant expressing Kre2-GFP. Scale bars: 2.5µ m. (E) List of Golgi glycosyltransferases dependent on Erd1 and Vps74 for their recycling. The (F/L)-(L/I/V)-x-x-(R/K) motif known to bind Vps74 is highlighted in green.
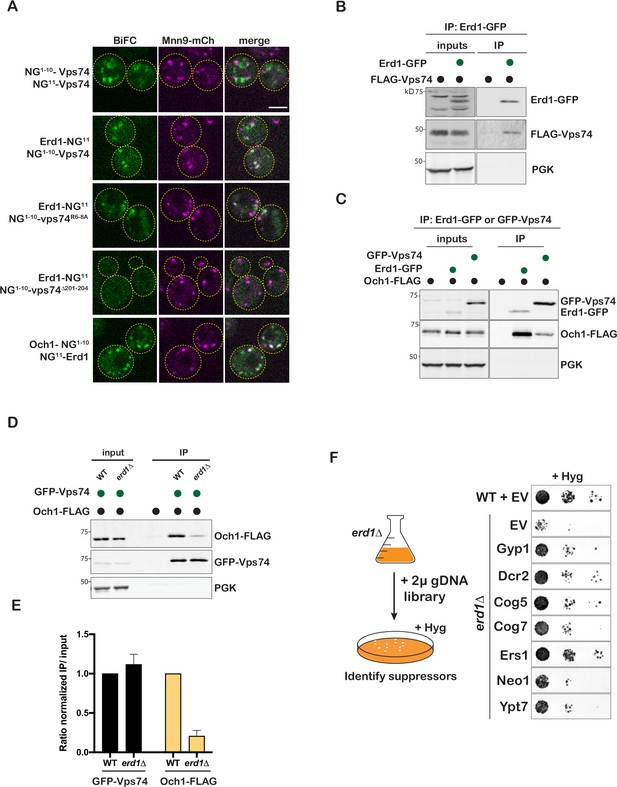
Erd1 interacts with glycosyltransferases and Vps74.
(A) Bimolecular fluorescence complementation of mNeongreen tested in cells with plasmids expressing split-mNG fragments fusions to the indicated proteins, in a strain also expressing the early Golgi marker, Mnn9-mCherry. BiFC signal is shown in green, and Mnn9-mCherry is shown in magenta. (B) Coimmunporecipitation analysis to test the interaction between Erd1-GFP and FLAG-Vps74 (FLAG tag listed in all cases is 6 x His-TEV cleavage site-3xFLAG) from yeast cell lysates. (C) Coimmunporecipitation analysis to test the interaction between Erd1-GFP and Och1-FLAG, and GFP-Vps74 and Och1-FLAG from yeast cell lysates. (D) Coimmunporecipitation analysis to test the interaction between GFP-Vps74 and Och1-FLAG from wild type and erd1 yeast cell lysates. (E) Quantification of ratio of the signal in the IP to input normalized to the loading control for GFP-Vps74 and Och1-FLAG. (F) Growth of serial dilutions of erd1 mutant transformed with plasmids expressing Gyp1, Dcr2, Cog5, Cog7, Ers1, Neo1, Ypt7 identified in the dosage suppressor screen on YPD with 60 µg/ml hygromycin at 26 °C for 2 days.
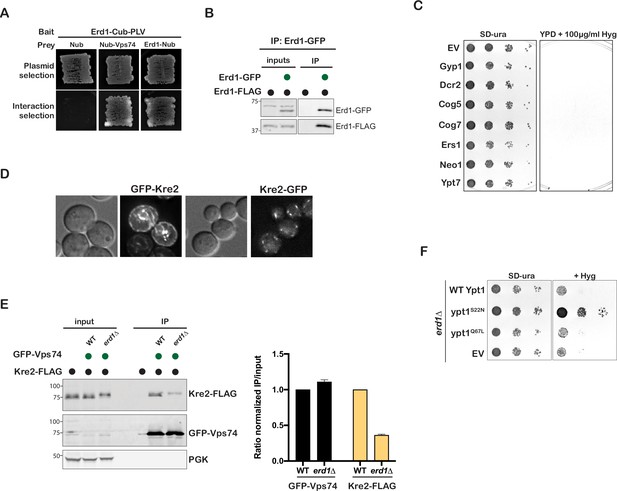
Erd1 is required for Golgi protein trafficking.
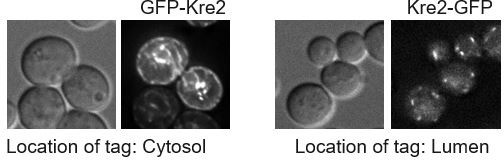
(A) Split-ubiquitin Y2H assay to test interaction between Erd1 with Vps74 and with itself. (B) Coimmunporecipitation analysis to test the interaction between Erd1-GFP and Erd1-FLAG. (C) Growth of serial dilutions of wild type cells transformed with plasmids expressing Gyp1, Dcr2, Cog5, Cog7, Ers1, Neo1, Ypt7 identified in the dosage suppressor screen on YPD with 100 µg/ml hygromycin at 26 °C for 2 days. (D) Live cell microscopy of wild type cells expressing Kre tagged with GFP at the N terminal end (GFP-Kre2) or at the C-terminus (Kre-GFP). (E) Coimmunporecipitation analysis to test the interaction between GFP-Vps74 and Kre2-FLAG from wild type and erd1 yeast cell lysates. Quantification of ratio of the signal in the IP to input normalized to the loading control for GFP-Vps74 and Kre2-FLAG. (F) Growth of serial dilutions of erd1 mutant transformed with EV, wild type Ypt1, GDP-locked ypt1 S22N, and GTP-locked ypt1 Q67L mutants on SD-ura and YPD with 60 µg/ml HygromycinB at 26 °C for 2 days.
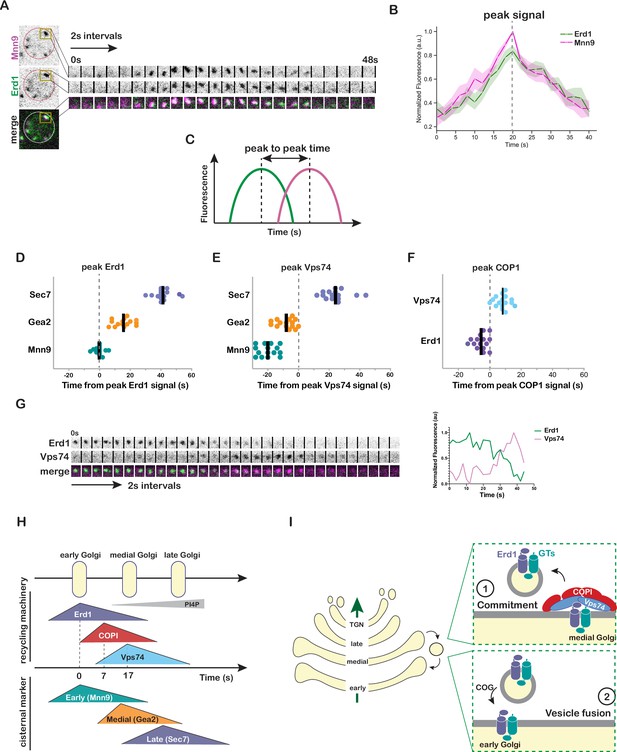
Kinetic analysis of Erd1 and Vps74 in the context of Golgi cisternal maturation.
(A) Time-lapse imaging series (2 s intervals) of the indicated single Golgi compartment in cells expressing Erd1-mNeonGreen and Mnn9-mCherry. (B) Averaged normalized fluorescence traces with 95% CI of time-lapse imaging analysis of 10 puncta described in (A). Dashed line represents the time corresponding to the peak signal of Erd1 and Mnn9. (C) Schematic describing the estimation of peak-to-peak fluorescence times of two markers from time-lapse imaging analysis. (D) Quantification of peak-to-peak times for Erd1-mNeonGreen with respect to early (Mnn9-mCherry), medial (Gea2-3xMars), and late (Sec7-6xDsRed) Golgi markers (n > 15 puncta for each condition). Peak Erd1 is set at t0 and indicated with the dashed line. The bold line represents the median value for each condition. (E) Quantification of peak-to-peak times for mNeonGreen-Vps74 with respect to early (Mnn9-mCherry), medial (Gea2-3xMars), and late (Sec7-6xDsRed) Golgi markers. Peak Vps74 is set at t0 and indicated with the dashed line. The bold line represents the median value for each condition. (F) Quantification of peak-to-peak times for Erd1 and Vps74 with respect to COP1-mCherry. Peak COP1 is set at t0 and indicated with the dashed line. (G) Time-lapse imaging series (2 s intervals) of the indicated single Golgi compartment in cells expressing Erd1-mNeonGreen and mScarlet-Vps74 (left) and normalized fluorescence of time-lapse imaging (right). (H) Schematic depicting the dynamics of tagged Erd1, Vps74 and COP1 at maturing Golgi compartments marked by Mnn9 (early), Gea2 (medial), and Sec7 (late) based on A-F. (I) A simplified model for the proposed role of Erd1 in the recycling of Golgi glycosyltransferase at two steps - (1) at the step of commitment of the cargos for recycling by allowing stable complex formation with the cytosolic recycling machinery, and (2) at the final step of vesicle tethering and fusion at the early Golgi by facilitating interaction with the tethering machinery.
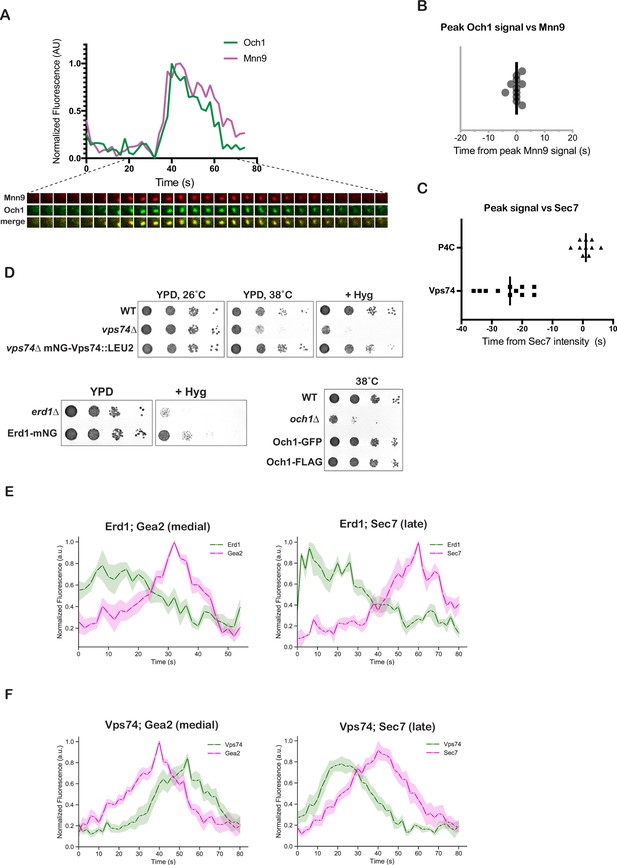
Kinetic analysis of Erd1 and Vps74 with Golgi markers.
(A) Representative traces and images from time-lapse imaging series of Och1-mNeonGreen and Mnn9-mCherry at a maturing Golgi compartment. (B) Quantification of peak-to-peak times for Och1 and Mnn9. (C) Quantification of peak-to-peak times for PI4P marker (GFP-P4C), and mNeonGreen-Vps74 versus Sec7-6xDsRed. The bold line represents the median value for each condition. (D) Tagged Vps74, Erd1, and Och1 are functional. Growth of serial dilutions of the mutants or tagged Vps74, Erd1, and Och1 constructs as indicated. (E) Averaged normalized fluorescence traces from time-lapse imaging analysis of 10 puncta for Erd1-Gea2 and Erd1-Sec7 described in Figure 4D. (F) Averaged normalized fluorescence traces from time-lapse imaging analysis of 10 puncta for Vps74-Gea2 and Vps74-Sec7 described in Figure 4E.
Additional files
-
Supplementary file 1
List of strains and plasmids used in this study.
- https://cdn.elifesciences.org/articles/70774/elife-70774-supp1-v1.xlsx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/70774/elife-70774-transrepform1-v1.docx