A highly conserved host lipase deacylates oxidized phospholipids and ameliorates acute lung injury in mice
Figures
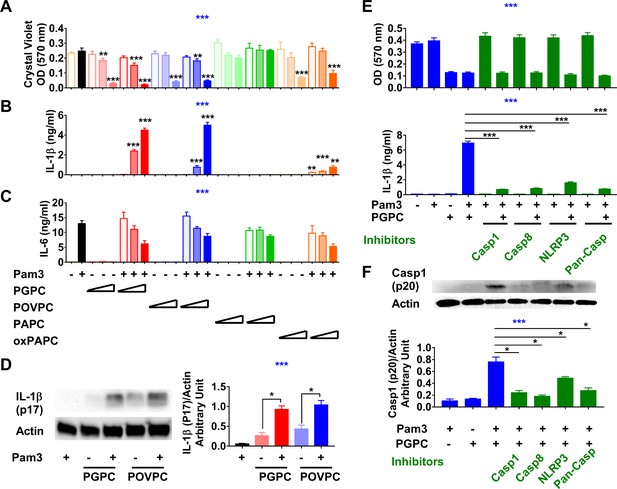
Low concentrations of oxidized phospholipids (oxPLs) induce macrophage cell death but IL-1β release requires priming.
(A–C) Resident peritoneal macrophages were incubated for 7 hr with or without priming with 10 ng/ml Pam3. 2, 5, or 12.5 μg/ml PGPC, POVPC, PAPC, or oxPAPC were added to the media. After incubation for 18 hr, cells were washed and then stained with crystal violet (OD 570 nm). IL-1β and IL-6 were measured in the culture media using ELISA (B, C). Data were combined from at least two experiments, n ≥ 3. (D) Macrophages were treated with 10 ng/ml Pam3 for 7 hr and then 12.5 μg/ml PGPC or POVPC was added. After 18 hr incubation, the culture medium and cell lysate were combined, concentrated, and subjected to western blot analysis for mature IL-1β and actin. One representative experiment of four is shown (left). The western blot results were quantitated using ImageJ and expressed as IL-1β (p17)/actin (right). n = 4. (E, F) 10 μM VX-765 (caspase 1 inhibitor), 20 μM Z-IETD-FMK (caspase 8 inhibitor), 20 μM Z-VAD-FMK (Pan-Caspase inhibitor), or 100 μM MCC950 (NLRP3 inflammasome inhibitor) was added 1 hr before priming. After 7 hr priming with 10 ng/ml Pam3, macrophages were treated with 12.5 μg/ml PGPC for 18 hr. Cells were then stained with crystal violet and IL-1β in culture medium was measured (E). Data were combined from three experiments, each with n = 4. A mixture of culture medium and cell lysate was subjected to western blot analysis for caspase 1 (p20) and actin (F). One representative experiment of four is shown. The western blot results were quantitated using ImageJ and expressed as caspase 1 (p20)/actin, n = 4. One-way ANOVA was used to test the difference among groups (blue stars in A–C, E, F), and the Mann–Whitney test was used to test the difference between groups (black stars). In (A–C), the statistical tests were made between oxPL-treated cells and untreated cells with or without priming respectively. *p<0.05; **p<0.01; ***p<0.001.
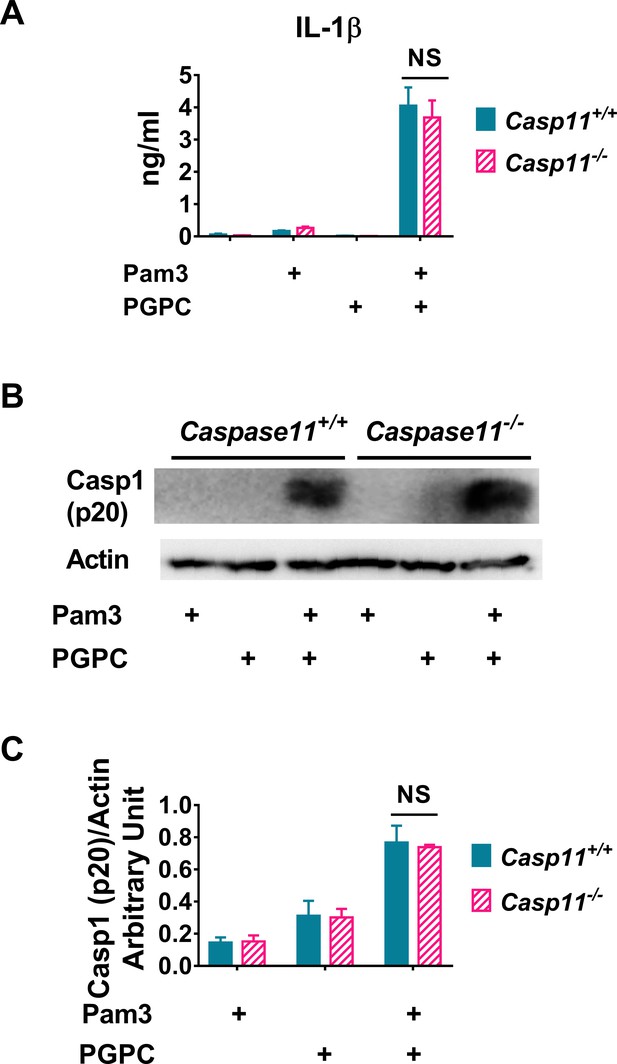
Caspase 11 is not required for PGPC-induced IL-1β release in resident peritoneal macrophages.
Resident peritoneal macrophages from Caspase 11+/+ or Caspase 11-/- mice were treated with 10 ng/ml Pam3 for 7 hr, and then 12.5 μg/ml PGPC was added and incubated for 18 hr. (A) IL-1β in culture medium was determined. Data were combined from three experiments, n = 3 in each experiment. (B) Cleaved caspase 1 (p20) and actin in the mixture of culture medium and cell lysate were measured using western blot. (C) Western blot results from four experiments were quantitated using ImageJ.
-
Figure 1—figure supplement 1—source data 1
- https://cdn.elifesciences.org/articles/70938/elife-70938-fig1-figsupp1-data1-v1.xlsx
-
Figure 1—figure supplement 1—source data 2
- https://cdn.elifesciences.org/articles/70938/elife-70938-fig1-figsupp1-data2-v1.pdf
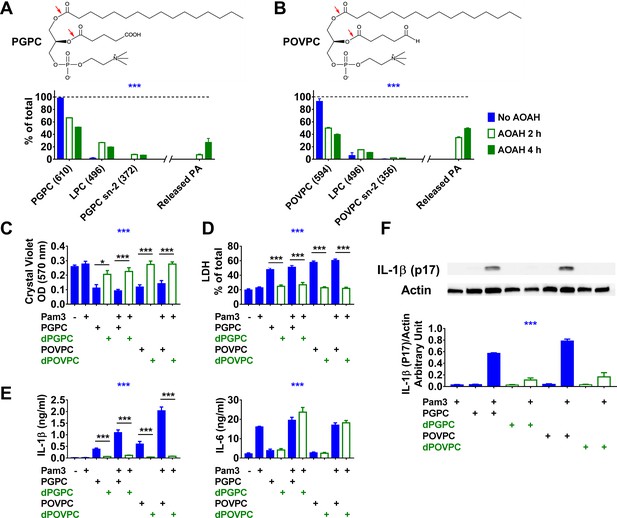
Acyloxyacyl hydrolase (AOAH) treatment decreases the bioactivities of PGPC and POVPC.
(A, B) Chemical diagrams of PGPC and POVPC are shown. Red arrows indicate AOAH cleavage sites. PGPC and POVPC were incubated with rhAOAH at 37℃ for 2 or 4 hr in 100 mM NaCl with 10 mM sodium acetate, pH 6.2, 0.1% Triton X-100, and 0.2 mg/ml fatty acid-free human BSA. The reaction products missing sn-2 oxidized FA (MW 496, LPC) or missing sn-1 palmitic acid (PA; MW 372 for PGPC or MW 356 for POVPC) were analyzed (see Materials and methods); the released PA was also measured. n = 4 (PGPC) or 3 (POVPC). (C–F) Resident peritoneal macrophages were treated or untreated with 10 ng/ml Pam3 for 7 hr. PGPC, POVPC, or AOAH-deacylated PGPC or POVPC (dPGPC or dPOVPC) (12.5 μg/ml) was then added. After incubation for 18 hr, cells were stained with crystal violet (OD 570 nm) and the OD was measured (C). Released LDH was measured (D). Concentrations of IL-1β and IL-6 (E) were measured in the culture media using ELISA. Data were combined from three experiments, each with n = 3 or 4 (C–E). Combined culture medium and cell lysate were subjected to western blot analysis and quantitated (F), n = 3. Two-way ANOVA was used to test the difference among groups (no AOAH, AOAH 2 and 4 hr, blue stars in A, B). One-way ANOVA was used to test the difference among groups (blue stars in C–F), and the Mann–Whitney test was used to test the difference between groups (black stars). *p<0.05; **p<0.01; ***p<0.001.
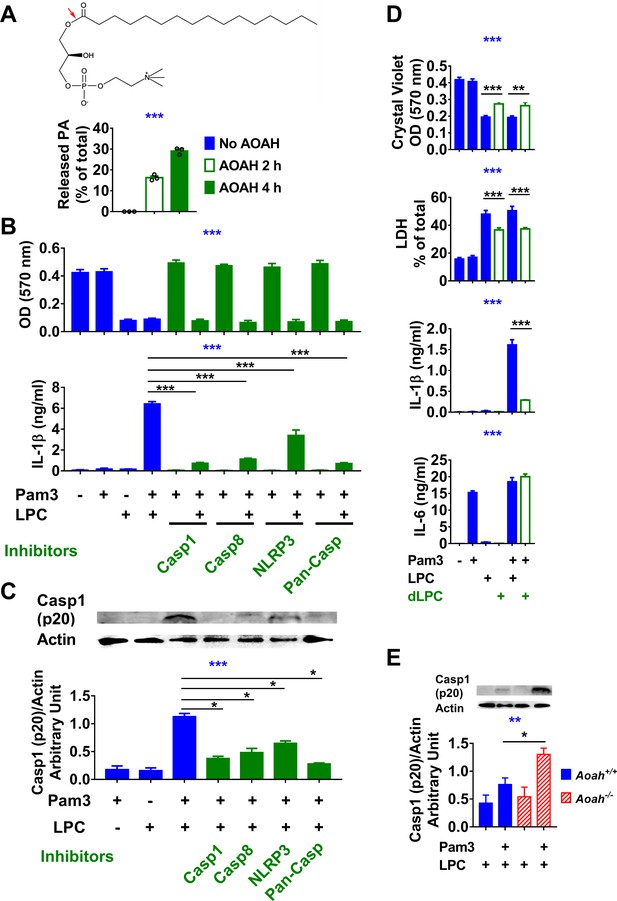
Acyloxyacyl hydrolase (AOAH) treatment decreases the bioactivities of lysophosphatidycholine (LPC).
(A) The chemical diagram of LPC is shown. The red arrow indicates the AOAH cleavage site. After LPC was incubated with purified rhAOAH for 2 or 4 hr, the released palmitate was measured. No palmitate was detected when rhAOAH was absent. n = 3. (B, C) Resident peritoneal macrophages were treated with 10 μM VX-765, 20 μM Z-IETD-FMK, 20 μM Z-VAD-FMK, or 100 μM MCC950 for 1 hr, and then primed with 10 ng/ml Pam3 for 7 hr before 12.5 μg/ml (25 μM) LPC was added to the media. After 18 hr incubation, cells were stained with crystal violet (OD 570 nm) and medium IL-1β was measured (B). Data were combined from three experiments, each with n = 3. Cleaved caspase 1 (p20) in the combined medium and cell lysate was analyzed by western blot, and the blots were quantitated using ImageJ, n = 4 (C). (D) Macrophages were treated or untreated with 10 ng/ml Pam3 for 7 hr before 12.5 μg/ml (25 μM) LPC or AOAH-treated LPC (deacylated LPC, dLPC) was added. After incubation for 18 hr, cells were stained with crystal violet and the medium was used to measure LDH activity, IL-1β and IL-6 concentrations. Data were combined from two (LDH) or four (crystal violet, IL-1β and IL-6) experiments, each with n = 4. (E) Aoah+/+ and Aoah-/- macrophages were treated with 10 ng/ml Pam3 for 7 hr and then 5 μg/ml LPC was added. After 18 hr incubation, the media were subjected to western blot analysis for cleaved caspase 1 (p20), and the cell lysates were used for actin. Western blot results from four experiments were quantitated using ImageJ. One-way ANOVA was used to test the difference among groups (blue stars in A–E), and the Mann–Whitney test was used to test the difference between groups (black stars). *p<0.05; **p<0.01; ***p<0.001.
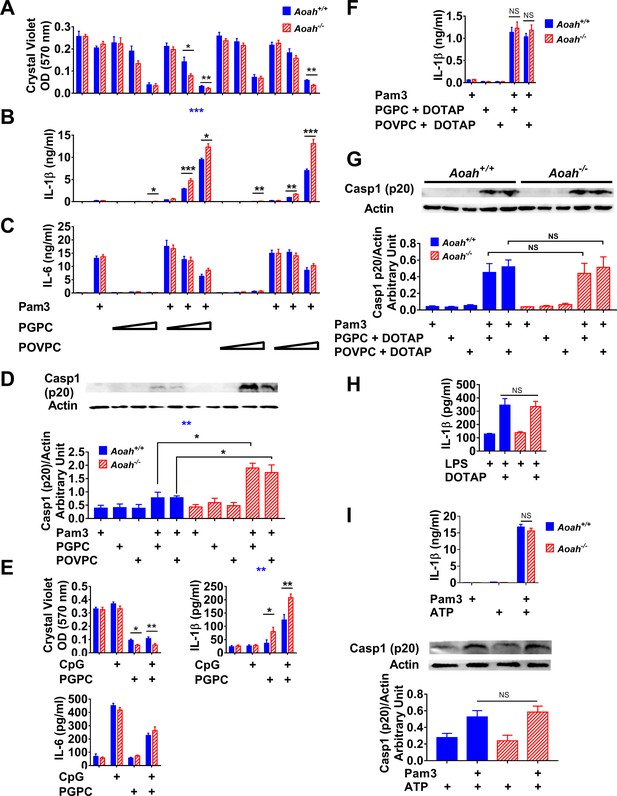
Acyloxyacyl hydrolase (AOAH) reduces oxidized phospholipid (oxPL)-induced cell death and inflammasome activation in macrophages.
(A–C) Aoah+/+ and Aoah-/- resident peritoneal macrophages were incubated for 7 hr with or without priming with 10 ng/ml Pam3. 2, 5, or 12.5 μg/ml PGPC or POVPC were then added to the media. After incubation for 18 hr, cells were washed and then stained with crystal violet (A). IL-1β (B) and IL-6 (C) were measured in the culture media using ELISA. Data were combined from three experiments, each with n = 4 wells/group. (D) Macrophages were primed with 10 ng/ml Pam3 and then 5 μg/ml PGPC or POVPC was added. After 18 hr incubation, the culture media were concentrated and subjected to western blot analysis for cleaved caspase 1 (p20). Cell lysate was used for actin detection. Western blot results from four experiments were quantitated using ImageJ and plotted below the western images, n = 4. (E) Macrophages were primed with 1 μM CpG DNA for 7 hr and then 12.5 μg/ml PGPC was added. After 18 hr incubation, the cells were washed and stained with crystal violet and the media were collected for IL-1β and IL-6 measurement using ELISA. Data were combined from three experiments, each with n = 3–4. (F, G) Macrophages were primed with 10 ng/ml Pam3 and then 5 μg/ml PGPC or POVPC encapsulated in DOTAP was added. After 18 hr incubation, the media were collected for IL-1β detection using ELISA (F) and subjected to western blot analysis for cleaved caspase 1 (p20). Most cells remained alive after the treatment. Cell lysates were collected for actin detection (G). Data were combined from 2 (F, n = 3 in each experiment) or four experiments (G). (H) Macrophages were treated with 1 μg/ml lipopolysaccharide (LPS) for 21 hr or primed with 1 μg/ml LPS for 3 hr and then 1 μg/ml LPS mixed with DOTAP was added to deliver LPS into the cytosol to activate caspase 11. After 18 hr incubation, IL-1β was measured in the culture medium. Data were combined from two experiments, each with n = 5. (I) Macrophages were primed with 10 ng/ml Pam3 for 7 hr and then 2 mM ATP was added to induce inflammasome activation. After 18 hr incubation, the culture medium was analyzed using ELISA. Data were combined from two experiments, each with n = 3/group. The mixture of medium and cell lysate was used for western blot analysis, and the results from three experiments were quantitated using ImageJ. n = 4/group. Two-way ANOVA was used to test the difference between Aoah+/+ and Aoah-/- groups with multiple treatments (blue stars), and the Mann–Whitney test was used to test the difference between groups that received the same treatment (black stars). *p<0.05; **p<0.01; ***p<0.001.
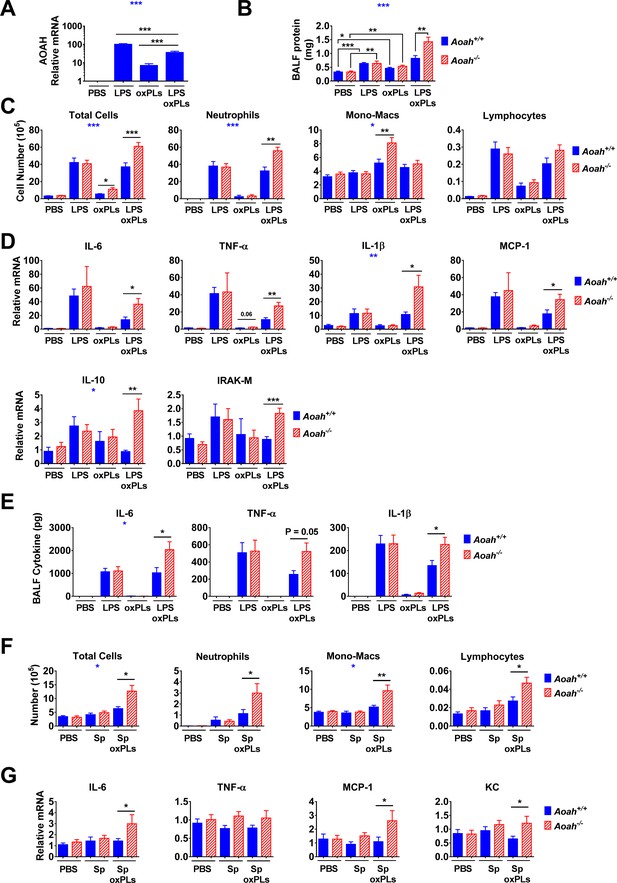
Acyloxyacyl hydrolase (AOAH) reduces inflammatory responses and lung injury induced by oxidized phospholipids (oxPLs).
(A–E) Aoah+/+ mice were instilled i.n. with 10 μg lipopolysaccharide (LPS), oxPLs (25 μg PGPC + 25 μg POVPC), or LPS + oxPLs. 18 hr later, AOAH expression in alveolar macrophages (AMs) was measured using qPCR. Data were combined from three experiments, n = 9 mice/group (A). Bronchoalveolar lavage fluid (BALF) protein amount was measured (B). BAL immune cells were analyzed using cytospin followed by Wright–Giemsa staining (C). Lung cytokine/chemokine and IRAK-M mRNA were measured using qPCR (D). BALF cytokines were measured using ELISA (E). Data were combined from two (E) or three (B–D) experiments, each with n = 3 mice/group. (F, G) Mice were instilled with 40 μl PBS containing 6 × 10 exp6 heat-inactivated Streptococcus pneumoniae, with or without oxPLs (25 μg PGPC and 25 μg POVPC). 18 hr later, their BALF cells were analyzed (F). Lung cytokine/chemokine expression was measured using qPCR (G). Data were combined from at least two experiments, each with n = 3 mice/group. One-way ANOVA was used to test the difference among groups (A, blue stars), two-way ANOVA was used to test the difference between Aoah+/+ and Aoah-/- groups with multiple treatments (B–G, blue stars), and the Mann–Whitney test was used to test the difference between groups that received the same treatment (black stars). *p<0.05; **p<0.01; ***p<0.001.
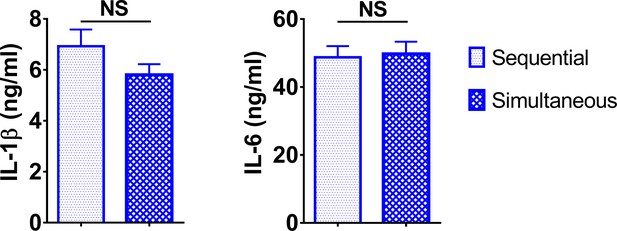
Sequential and simultaneous treatment of macrophages with lipopolysaccharide (LPS) and PGPC induced similar IL-1β production.
Peritoneal macrophages were treated with 10 ng/ml LPS for 7 hr followed by treatment with 5 μg/ml PGPC for 18 hr (sequential) or 10 ng/ml LPS mixed with 5 μg/ml PGPC for 18 hr (simultaneous). Medium IL-1β and IL-6 were measured using ELISA. Data were combined from two experiments, n = 8 (sequential) or 13 (simultaneous).
-
Figure 5—figure supplement 1—source data 1
- https://cdn.elifesciences.org/articles/70938/elife-70938-fig5-figsupp1-data1-v1.xlsx

Acyloxyacyl hydrolase (AOAH) reduces bioactive oxidized phospholipids (oxPLs) in the airspaces after HCl and oxPL instillation.
(A) Aoah+/+ and Aoah-/- mice were instilled with 40 μl 0.2 M HCl and oxPLs (25 μg PGPC + 25 μg POVPC). 18 hr later, bronchoalveolar lavage fluid (BALF) was obtained. Protein in BALF was quantitated. 17 hr after i.n. HCl and oxPL instillation, Evans blue was injected i.v. 1 hr after injection, the lung extravascular dye was extracted and measured. Data were combined from two or three experiments, each with n = 3 mice/group. (B–F) In the same experiments as in (A), cells in BALF were analyzed after cytospin and Wright–Giemsa staining (B). Lung cytokine/chemokine expression was measured by qPCR (C). Data were combined from at least two experiments, each with n = 3 mice/group (B, C). Cytokines in BALF were analyzed using ELISA (D). E06-detectable oxPLs in BALF were measured (E). BALF was used to stimulate Pam3-primed macrophages for 18 hr in the presence of 5 μg/ml E06 or a control mouse IgM. IL-1β in the media was measured using ELISA (F). (D–F) Data were combined from two experiments, n = 4–12 mice/group. Two-way ANOVA was used to test the difference between Aoah+/+ and Aoah-/- groups with multiple treatments (blue stars), and the Mann–Whitney test was used to test the difference between two groups that received the same treatment (black stars). *p<0.05; **p<0.01; ***p<0.001.
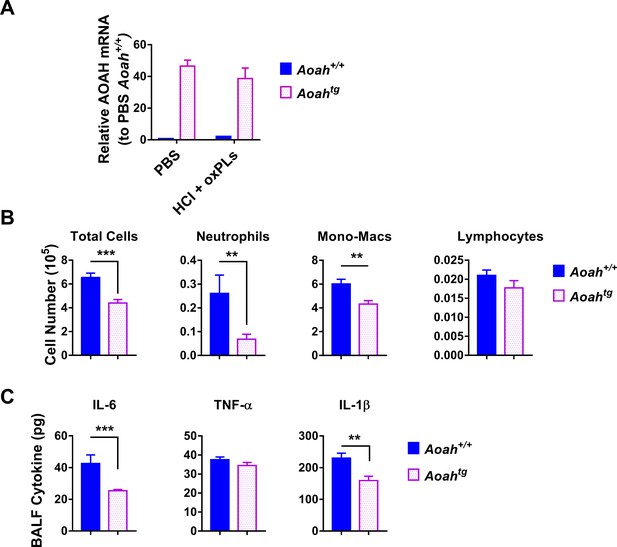
Mice that overexpress acyloxyacyl hydrolase (AOAH) in macrophages have reduced airway inflammation after HCl and oxidized phospholipid (oxPL) challenge.
Aoah+/+ and Aoah transgenic mice were instilled with 40 μl 0.2 M HCl and oxPLs (25 μg PGPC + 25 μg POVPC). 18 hr later, bronchoalveolar lavage fluid (BALF) was obtained. (A) AOAH mRNA in the lungs of Aoah+/+ and AoahTg mice was measured. n = 5/group. (B) Immune cells in BALF were analyzed after cytospin and staining. (C) BALF cytokines were measured using ELISA. Data were combined from three experiments, n = 9–15/group (B, C).
-
Figure 6—figure supplement 1—source data 1
- https://cdn.elifesciences.org/articles/70938/elife-70938-fig6-figsupp1-data1-v1.xlsx
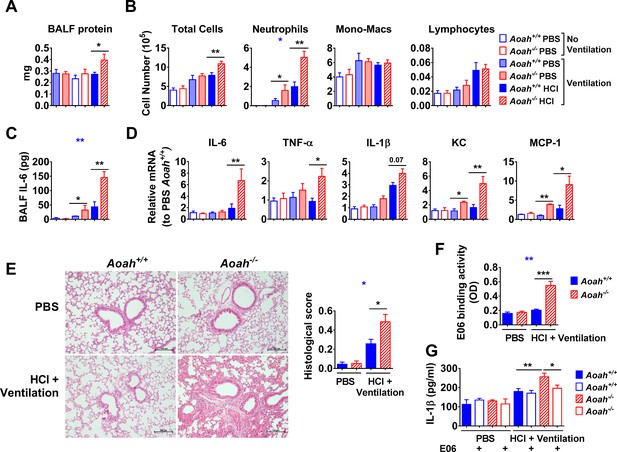
Acyloxyacyl hydrolase (AOAH) regulates inflammatory responses induced by endogenous oxidized phospholipids (oxPLs).
Aoah+/+ and Aoah-/- mice were instilled with 40 μl PBS or 0.2 M HCl, and then they were ventilated for 1 hr. 24 hr later, the mice were analyzed. (A) Bronchoalveolar lavage fluid (BALF) protein amount was measured. n = 4 or 5. (B) After cytospin and Wright–Giemsa staining, immune cells in BALF were counted. (C) IL-6 in BALF was measured using ELISA. TNF-α and IL-1β were undetectable. (D) Cytokine/chemokine expression was measured in the lungs. Data (B–D) were combined from at least two experiments, each with n = 3 mice/group. (E) Mouse lungs were excised and fixed in paraformaldehyde. The fixed lungs were sectioned and stained with hematoxylin-eosin. One representative picture of eight mice in each group is shown. The lung samples were scored according to the lung injury scoring system recommended by the American Thoracic Society, n = 8 mice/group. (F) BALF was collected and E06 binding activity was measured. (G) BALF was used to stimulate Pam3-primed macrophages, and the IL-1β in culture medium was measured. Data were combined from at least two experiments, each with n = 4 mice/group (F, G). Two-way ANOVA was used to test the difference between Aoah+/+ and Aoah-/- groups with multiple treatments (blue stars), and the Mann–Whitney test was used to test the difference between groups that received the same treatment (black stars). *p<0.05; **p<0.01; ***p<0.001.
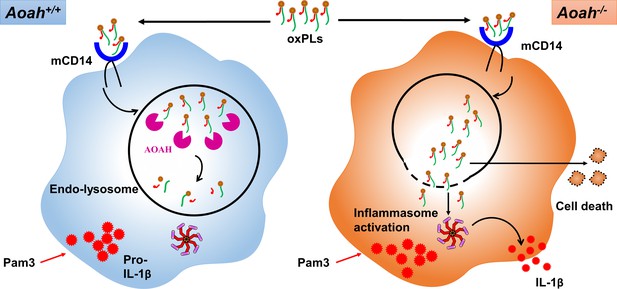
Acyloxyacyl hydrolase (AOAH) deacylates and inactivates oxidized phospholipids (oxPLs).
After oxPLs enter endolysosomes in a CD14-dependent pathway, AOAH in endolysosomes deacylates and inactivates oxPLs. When AOAH is lacking, oxPLs leak into cytosol or endolysosome membrane ruptures, leading to exaggerated inflammasome activation and IL-1β release.





