The role of TAp63γ and P53 point mutations in regulating DNA repair, mutational susceptibility and invasion of bladder cancer cells
Figures
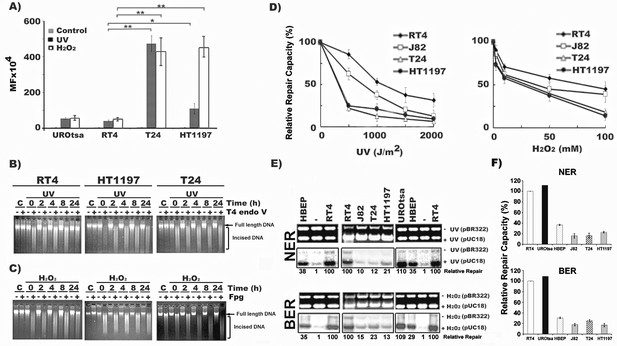
MIBC cells are hypermutable and deficient in DNA repair, whereas NMIBC cells are hyperactive in DNA repair.
Mutation susceptibility and DNA repair capacity toward UVC- and H2O2-induced DNA damage were determined in MIBC (T24, HT1197, and J82), NMIBC (RT4), immortalized normal human urothelial (UROtsa) cells and human bladder epithelial progenitor (HBEP) cells, as described (Wang et al., 2012). In (A), UVC (1500 J/m2)- and H2O2 (100 mM, 25 °C, 1 hr)-modified pSP189 plasmid DNA containing the supF were transfected into different cells and the mutations in the supF gene were detected and the mutation frequency (MF) calculated. (B & C) Detection of DNA repair in global genomic DNA of NMIBC and MIBC cells. Cells were irradiated with UVC (20 J/m2) or treated with H2O2 (100 mM, 1 hr, at 37 °C), incubated in growth medium for different times (0, 2, 4, 8, and 24 hr) and the cellular genomic DNAs were isolated. In (B), the levels of CPD in the genomic DNA were detected by T4 endonuclease V (T4 Endo V) incision method (Tang et al., 1994; Hu et al., 2002). In (C), the levels of ODD in the genomic DNA were detected by formamidopyrimidine glycosylase (Fpg) incision method (Wang et al., 2010). The enzyme-incised resultant DNAs were denatured and separated by electrophoresis, as described (Wang et al., 2010). Full length genomic DNA and enzyme-incised DNA are indicated. Symbol: C, Un-irradiated or unmodified control cells. (D, E and F) NER and BER capacities in MIBC and NMIBC cells were measured by the host cell reactivation (HCR) assay (D), and by the in vitro DNA-damage-dependent repair synthesis (DDDRS) assay (E & F) as previously described (Lee et al., 2014). For HCR assay, UVC-irradiated (0–2000 J/m2) or H2O2 (0–100 mM, 25 °C, 1 hr)-modified luciferase reporter (pGL-3-luciferase) and unmodified β-galactosidase (pSV-β-galactosidase) plasmids (internal control) were transfected into cells and luciferase and β-galactosidase activities were measured 72 hr post transfection (Wang et al., 2012; Lee et al., 2014). The HCR activity was calculated based on the ratio of luciferase activity versus β-galactosidase activity. The relative repair capacity was calculated based on the luciferase activity obtained from unmodified pGL-3-luciferase activity versus β-galactosidase activity as 100 %. For DDDRS assay, UVC-irradiated (1500 J/m2) or H2O2-modified (100 mM, 25 °C, 1 hr) pUC18 and unmodified pBR322 plasmids were used as DNA substrates for DNA repair synthesis carried out in cell extracts isolated from different BC cells (Lee et al., 2014). A typical DDDRS assay result is shown in (E), in which, the upper panels are ethidium bromide-stained gels. The lower panels are autoradiographs of the same gels. The repair activity was calculated based on the relative intensity of pUC18 band versus pBR322 band. The relative repair capacity depicted at the bottom was calculated based on assigning the repair activity of RT4 cells as 100. (F) Quantitation of DDDRS assay results from UROtsa, HBEP, NMIBC (RT4), and MIBC (T24, J82, and HT1197) cells.
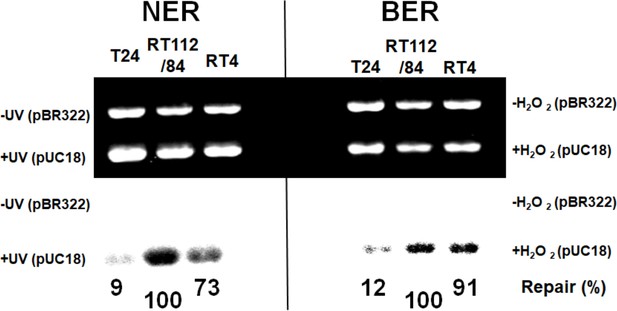
NMIBC RT112/84 cells are hyperactive in nucleotide excision repair (NER) and base excision repair (BER).
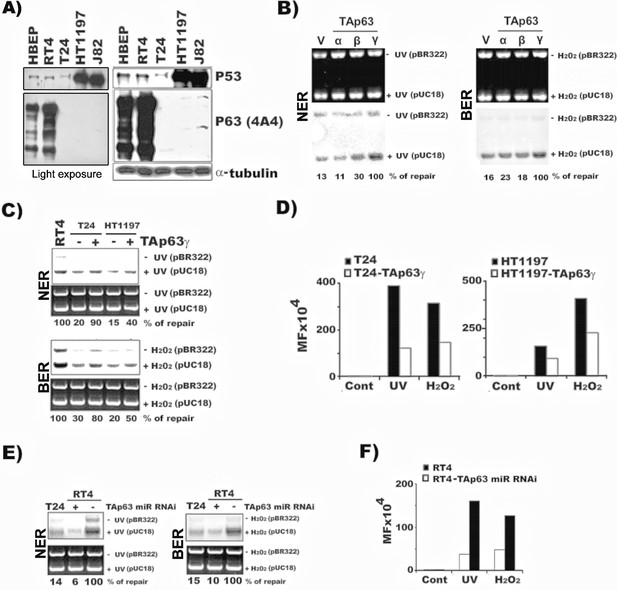
TAp63γ regulates NER and BER activity and mutational susceptibility of BC cells.
The methods for establishing the stable TAp63α, TAp63β, and TAp63γ in T24 cells (MIBC) and the TAp63γ stable transfectants in HT1197 (MIBC), knockdown TAp63γ by miR-RNAi in RT4 cells (NMIBC), and quantification of NER and BER activity and mutational susceptibility were described in text and in Figure 1. (A) Detection of p63 and p53 protein in MIBC (T24, HT1197, J82), NMIBC (RT4) and HBEP cells. Light exposure of p63 gels of RT4 and HBEP shown in the left demonstrates that p63 expression is much higher in RT4 than in HBEP. (B) Detection of NER and BER activity by DDDRS in MIBC T24 cells with and without stable TAp63α, TAp63β, and TAp63γtransfectants. Detection of mRNA of TAp63α, TAp63β, and TAp63γ in T24 cells with and without stable TAp63α, TAp63β, and TAp63γtransfectants was shown in Figure 3 and Figure 2—figure supplement 2. (C & D) Quantification of NER and BER activity by DDDRS (C) and mutational susceptibility (expressed by MF) (D) in T24 and HT1197 cells with and without stable TAp63γtransfectants. (E & F) Quantification of NER and BER activity by DDDRS (E), and mutational susceptibility (F), in NMIBC cells (RT4) with and without stable TAp63γ knockdown by miR-RNAi. Note: (1) stable TAp63α and TAp63βtransfects do not affect NER and BER activity significantly in MIBC cells (B); (2) stable TAp63γ transfectants enhance NER and BER activity (B) and reduce mutational susceptibility (D) of MIBC cells, which do not express both TAp63 and ΔNp63 isoforms (Figure 2—figure supplement 1); and (3) stable TAp63γ miR-RNAi transfectants reduce NER and BER activity (E) and enhance mutational susceptibility (F) in NMIBC cells.
-
Figure 2—source data 1
Expression of TAp63 and ΔNp63 isoforms in MIBC (T24 and J82), NMIBC (RT4) and immortalized human bladder urothelial UROtsa cells.
- https://cdn.elifesciences.org/articles/71184/elife-71184-fig2-data1-v1.zip
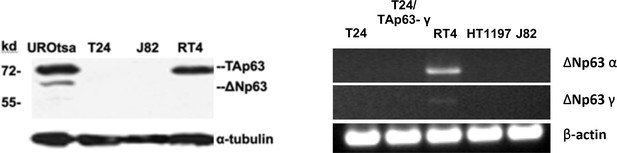
Expression of TAp63 and ΔNp63 isoforms in MIBC (T24 and J82), NMIBC (RT4) and immortalized human bladder urothelial UROtsa cells.
(A) Expression of TAp63 and ΔNp63 isoforms at protein level. No TAp63 and ΔNp63 isoforms at protein level were detected in MIBC (T24 & J82) cells. No ΔNp63 isoforms were detected in NMIBC (RT4) cells. (B) Expression of ΔNp63α and ΔNp63γ at mRNA level in MIBC (T24, HT1197, and J82), T24 with TAp63γ transfectants, and NMIBC RT4 cells. No ΔNp63α and ΔNp63γwas detected in MIBC cells. Very low level of ΔNp63γwas detected in NMIBC cells.
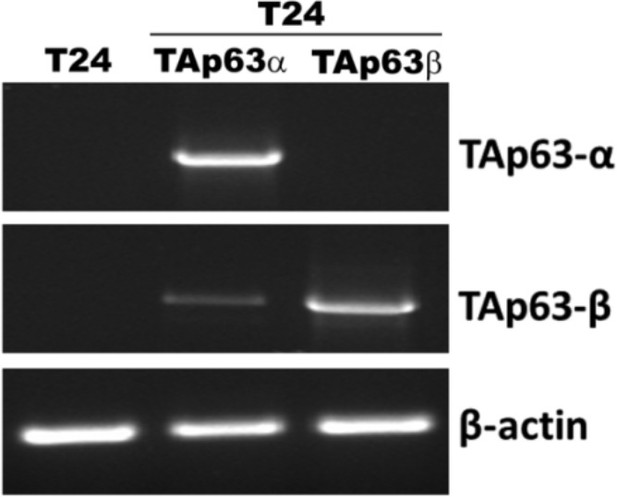
Detection of mRNA of TAp63α and TAp63β in T24 cells with and without stable TAp63α and TAp63β transfectants.
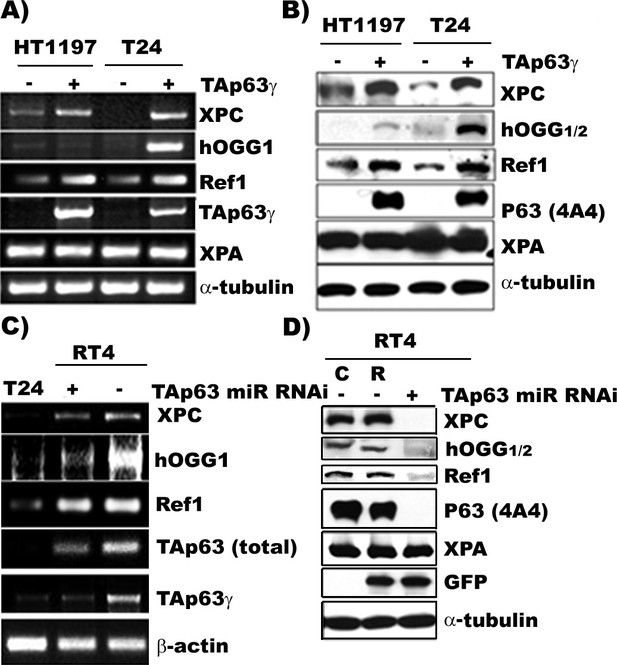
TAp63γ regulates expression of DNA repair genes, XPC, hOGG1/2, and Ref one in BC cells.
The constructs of the MIBC (T24 and HT1197) with stable TAp63γtransfectants and NMIBC (RT4) cells with stable TAp63γmiR-RNAi are the same as in Figure 2. The expression of TAp63γ, XPA, XPC, hOGG1/2, and Ref1 was determined at the mRNA (A & C) and protein (B & D) levels. The methods of detection were the same as described (Wang et al., 2012). Note: (1) XPC, hOGG1/2 and Ref1 are downregulated in MIBC cells, T24 and HT1197, and upregulated in NMIBC RT4 cells; (2) forced TAp63γ expression in MIBC cells upregulates expression of XPC, hOGG1/2, and Ref1 genes; and (3) TAp63γ knockdown by miR-RNAi downregulates these genes in NMIBC cells. Symbols: C and R in (D) represent vector control and vector with random inserts.
-
Figure 3—source data 1
Determination of DNA repair capacity and p63 protein levels in normal human urothelial mucosa.
- https://cdn.elifesciences.org/articles/71184/elife-71184-fig3-data1-v1.zip
-
Figure 3—source data 2
Determination of DNA repair capacity and p63 protein levels in normal human urothelial mucosa.
- https://cdn.elifesciences.org/articles/71184/elife-71184-fig3-data2-v1.zip
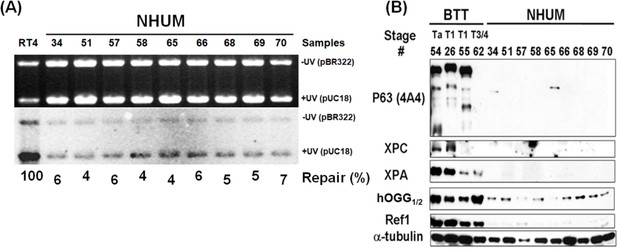
Determination of DNA repair capacity and p63 protein levels in normal human urothelial mucosa.
Human bladder tumor tissues (BTT, stage Ta, T1 and T3/4, n = 4) and normal human urothelial mucosa (NHUM, n = 9) were obtained as described in text. The DNA repair capacity (A) and the repair proteins XPC, hOGG1/2 and Ref1, and p63 (B) were detected the same as in Figure 5. Note: RT4 cells are derived from NMIBC.
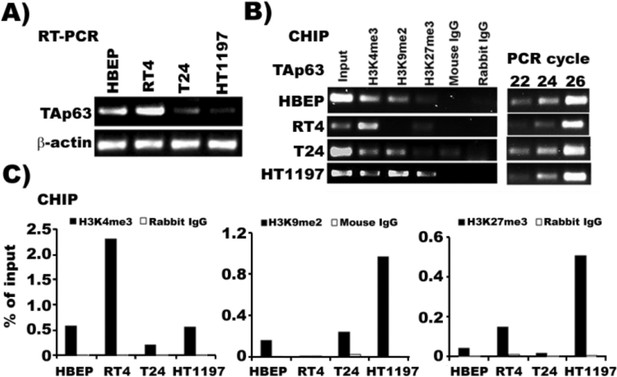
The TAp63 promoter region in MIBC cells is rich in transcription silencing markers, H3K9me2 and H3K27me3, whereas this region in NMIBC cells is rich in transcription activating marker H3K4me3.
ChIP assays were performed with chromatin extracted from human bladder epithelial progenitor (HBEP) cells, MIBC (T24 and HT1197) cells and NMIBC (RT4) cells. Chromatin-associated DNA was probed by PCR using TAp63-specific primers. ChIP assays were also performed on input chromatin (positive control) and chromatin precipitated with non-specific IgG antibodies (negative control). (A) mRNA level of TAp63 in HBEP, NMIBC RT4 cells and MIBC T24, and HT1197 cells. (B) Results of CHIP assay using either antibody against activation marker H3K4me3 or silencing marker H3H9me2 or H3H27me3 followed by PCR reaction. (C) Quantification of histone methylation.
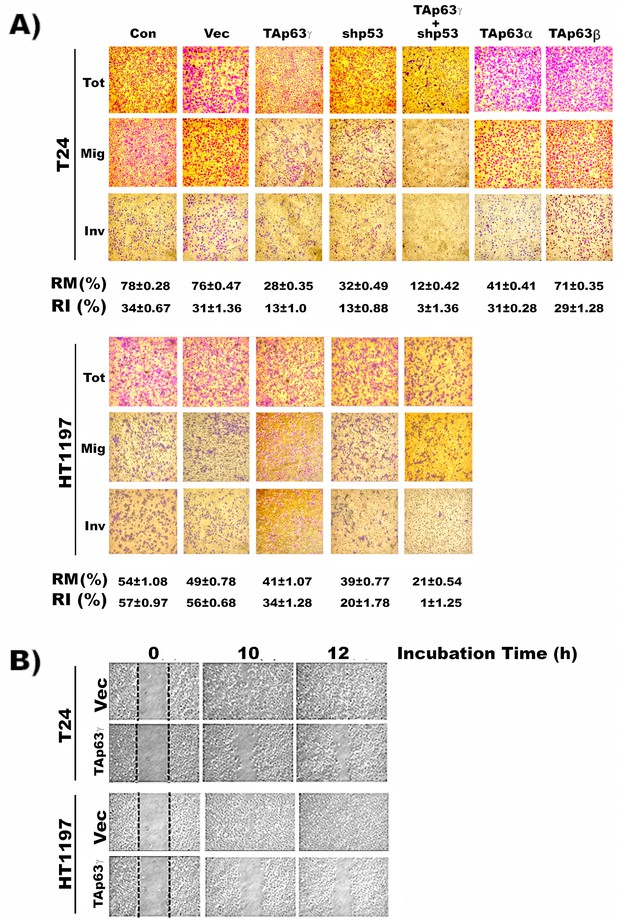
TAp63γ expression and p53m knockdown reduce invasion potential of MIBC cells.
The effect of TAp63γ and p53m on cell mobility and invasion potential were assayed by (A) migration and invasion chambers using Matrigel kits, and (B) wound healing in different cell lines which were constructed as described in the text. These cell lines are: (1) MIBC (T24 and HT1197) cells, (2) MIBC (T24 and HT1197) cells with stable control vector transfectants, (3) MIBC cells with stable TAp63γ,TAp63α, and TAp63β transfectants, (4) MIBC cells with shRNA p53 knockdown, (5) MIBC cells with stable TAp63γ transfectants and shRNA p53 knockdown. The relative migration (RM) and relative invasion (RI) potentials were quantified by the methods described in vendor’s instruction as follow:
Note: Invasion potential of HT1197 cells which over expressed p53m365 (Cooper et al., 1994) as shown in Figure 2, is higher than T24 cells which have a low expression of one wild-type allele and one allele nonsense mutation of p53.
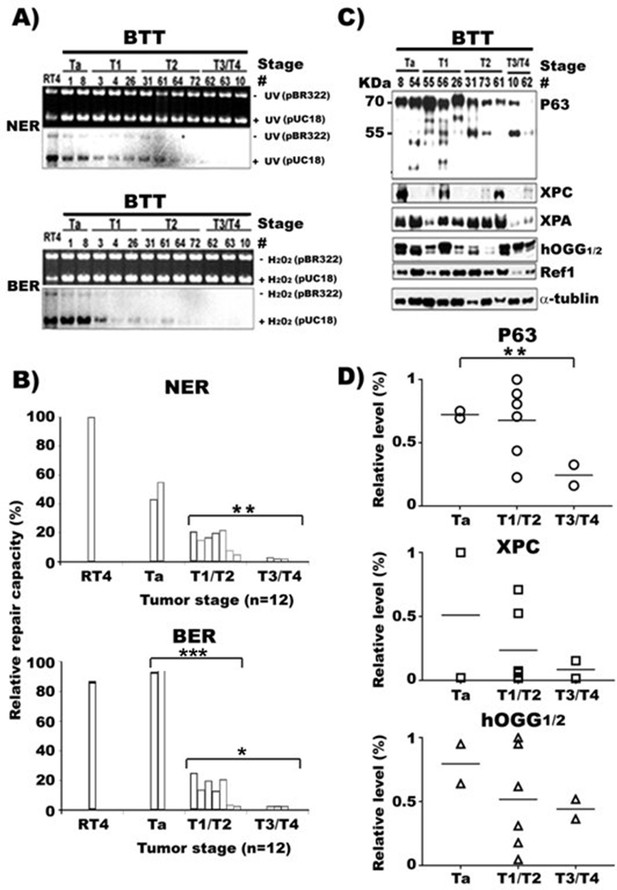
NER and BER capacity, expression of repair genes, and p63 in human bladder cancer tissues of different stages.
Cell lysates were prepared from human bladder tumor tissues (BTT) of different stages (Ta to T4, n = 16). (A) NER and BER capacity in these lysates were determined by in vitro DNA-damage-dependent-repair synthesis assay, as described in Figures 1 and 2, and relative repair capacities were calculated and depicted in (B). (C) Repair proteins XPC, hOGG1/2 and Ref1, and p63 proteins in these lysates were detected the same as described in Figure 3. (D) Quantifications of the relative levels of p63, XPC, hOGG1/2, and Ref1 proteins in different stage BC tissues. * and *** represent p value of < 0.05 and < 0.001. The p value for the NER difference of Ta vs T1/T2 in (B) is 0.073.
-
Figure 5—source data 1
Detection of P63 and diferent repair proteins in human bladder cancer tissues.
- https://cdn.elifesciences.org/articles/71184/elife-71184-fig5-data1-v1.pdf
Additional files
-
Supplementary file 1
UV- and H2O2-DNA damage induce more mutations in MIBC (T24 & HT1197) than NMIBC (RT4) cells and normal human urothelial (UROtsa) cells.
- https://cdn.elifesciences.org/articles/71184/elife-71184-supp1-v1.docx
-
Supplementary file 2
Enforced TAp63γ expression reduces UV- and H2O2-DNA damage2 induced mutations3 in MIBC (T24 & HT1197) cells.
- https://cdn.elifesciences.org/articles/71184/elife-71184-supp2-v1.docx
-
Supplementary file 3
Knockdown TAp63γ expression enhances UV- and H2O2-DNA damage induced mutations in NMIBC (RT4) cells.
- https://cdn.elifesciences.org/articles/71184/elife-71184-supp3-v1.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/71184/elife-71184-transrepform1-v1.docx





