Adiponectin receptor agonist AdipoRon improves skeletal muscle function in aged mice
Figures
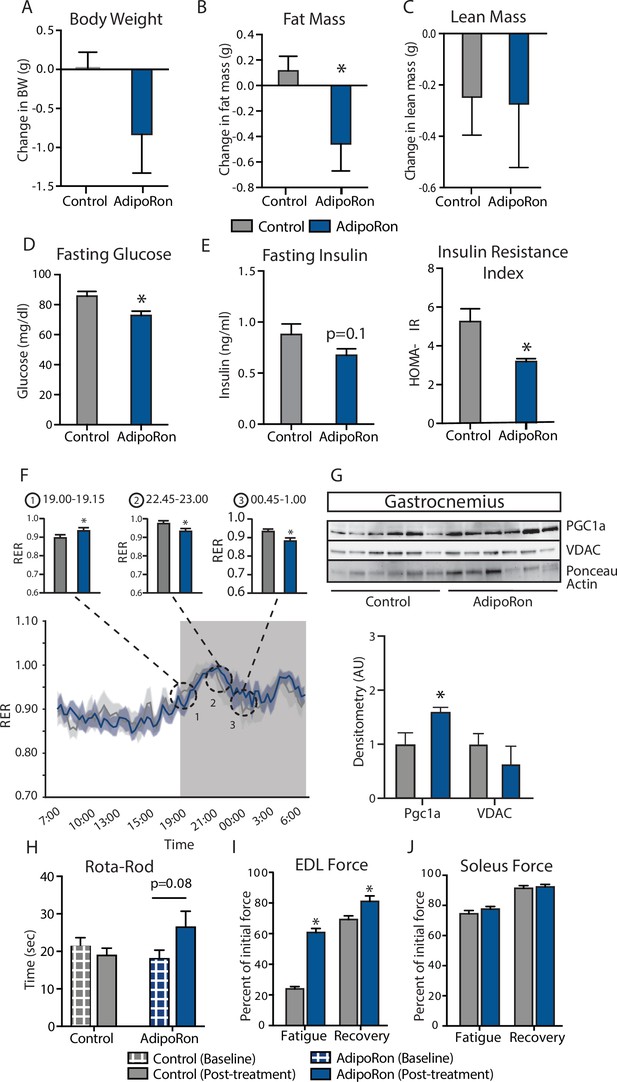
Metabolic and functional effects of chronic AdipoRon treatment in aged mice.
Chronic AdipoRon treatment (1.2 mg/kg BW, intravenous injection three times per week for 6 weeks) in aged male C57BL/6J mice (25 months old). (A) Change in body weight, (B) change in fat mass, and (C) lean mass. (D) Fasting glucose, (E) fasting insulin and HOMA-IR (Control [n=10] and AdipoRon [n=8]), (F) mean respiratory exchange ratios (RERs) showing confidence intervals during 24 hr in metabolic chambers, box signifies night (Control [n=10] and AdipoRon [n=8]), (G) immunodetection of mitochondrial marker VDAC and PGC1a protein expression in gastrocnemius (Control [n=6] and AdipoRon [n=6]), (H) in vivo latency to fall on Rota-Rod testing (Control [n=10] and AdipoRon [n=7]). Functional muscle changes assessed by measuring peak force after tetanic stimulation in ex vivo contractility experiments in isolated (I) extensor digitorum longus (EDL) and (J) soleus (Control [n=10] and AdipoRon [n=8]). Data shown as average ± SEM, significance determined by Student’s t-test (*p<0.05).
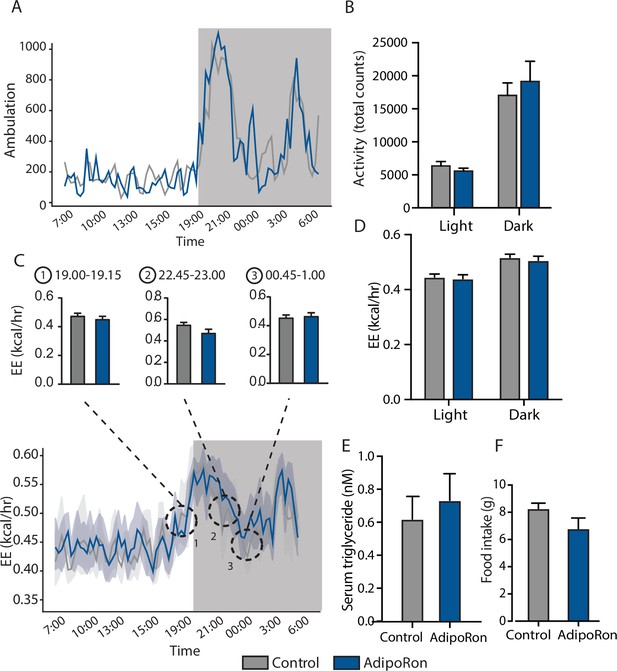
Metabolic effects of chronic AdipoRon treatment.
AdipoRon treatment (1.2 mg/kg BW, intravenous injection three times per week for 6 weeks) in aged male mice (25 months old), (A) ambulation data during 24 hr in metabolic chambers, box signifies night, (B) total activity count during light and dark phases, (C) 24 hr energy expenditure (EE), and (D) light and dark mean EE, (E) serum triglyceride levels, (F) food intake (Control [n=10] and AdipoRon [n=8]). Data shown as average ± SEM.
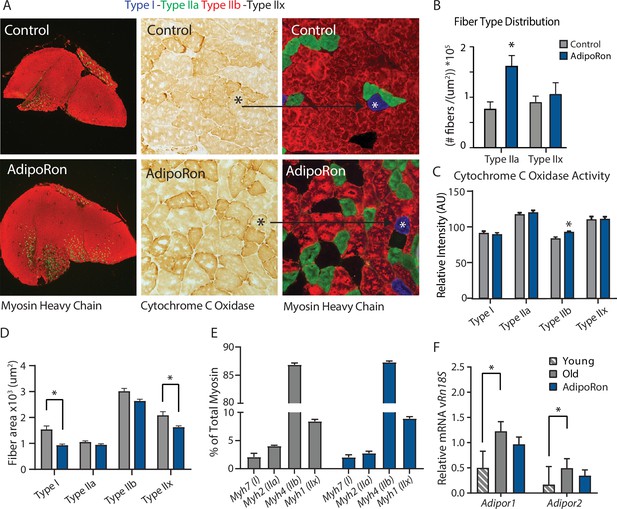
Fiber type-specific metabolic and structural changes in response to AdipoRon.
Chronic AdipoRon treatment in aged male C57BL/6J mice (25 months old). (A) Immunodetection of Type IIa (green) and Type IIb (red) shown at 2.5× magnification (Left), representative histochemical staining for Cytochrome C Oxidase activity (Middle), and immunohistochemistry for Type I (blue), Type IIa (green), Type IIb (red), and Type IIx (black) in adjacent gastrocnemius muscle sections at 20× magnification (Right). (B) Type IIa and Type IIx fiber counts normalized to (C) Cytochrome C Oxidase staining intensity analysis (D) and cross-sectional area of individual fibers. Data shown as mean ± SEM, significance determined by t-test (*p<0.05). (E) mRNA expression as percent of total myosin heavy chain isoforms (Control [n=10] and AdipoRon [n=8]). (F) Relative mRNA expression (relative to Rn18s RNA) of adiponectin receptors Adipor1 and Adipor2 from young (Control [n=6] and AdipoRon [n=6]) and aged (Control [n=10] and AdipoRon [n=8]) mice. Data shown as fold change of average difference from Rn18s ± SEM, significance determined by ANOVA (*p<0.05).
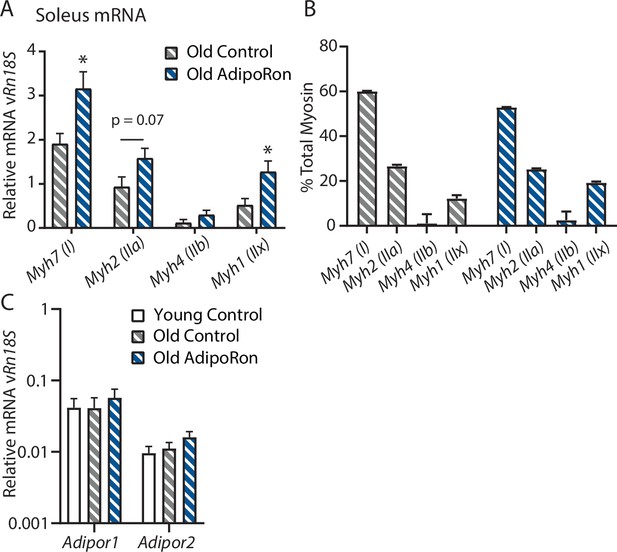
Chronic AdipoRon treatment in aged mice soleus.
(A) Relative mRNA expression myosin heavy chain isoforms versus Rn18s ribosomal RNA, (B) isoform distribution as a percent total in soleus of AdipoRon treated mice. (C) mRNA expression of adiponectin receptors Adipor1 and Adipor2 versus Rn18s in young and aged control and AdipoRon treated mice (Control [n=6] and AdipoRon [n=6]). Data shown as fold change of average difference from Rn18s RNA reference ± SEM with significance determined by Student’s t-test or ANOVA (*p<0.05).
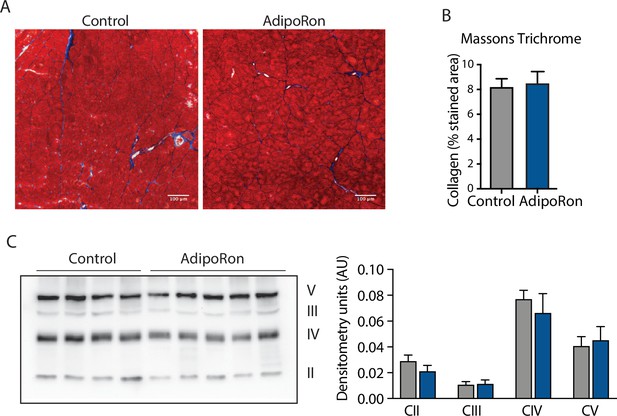
Effect of AdipoRon treatment on aged muscle fibrosis.
Chronic AdipoRon treatment effect on muscle fibrosis shown via (A) representative Masson’s trichrome stained images from control and AdipoRon gastrocnemius, (B) quantitation of intramuscular collagen content based on n=5 images per mouse, (C) immunodetection of proteins in the electron transport system (ETS) in gastrocnemius from control and AdipoRon treated animals with immunoblot quantitation relative to ponceau total protein loading control.
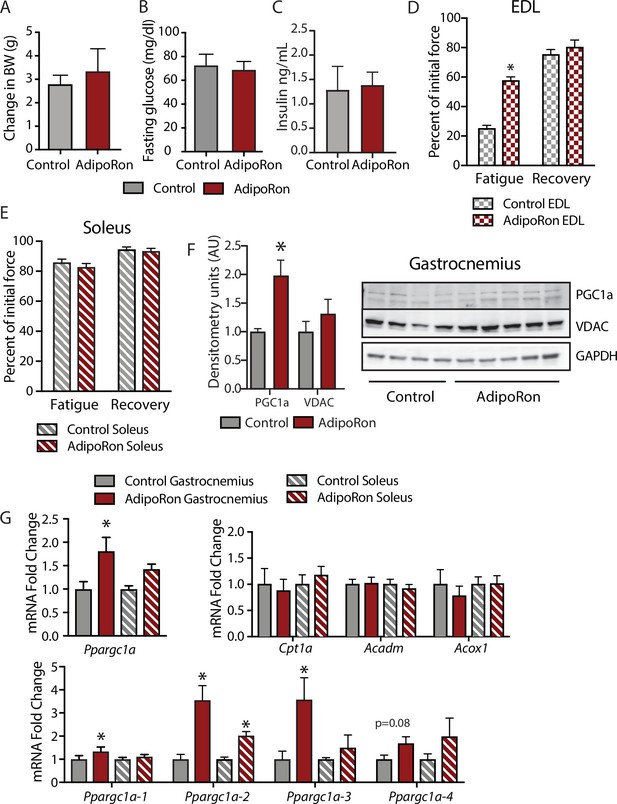
Metabolic and functional effects of chronic AdipoRon treatment in young mice.
Chronic AdipoRon treatment (1.2 mg/kg BW, intravenous injection three times per week for 6 weeks) in young male B6C3F1 mice (3.5 months). Measures of (A) body weight, (B) fasting glucose, (C) fasting insulin (Control [n=5] and AdipoRon [n=5]). Measures of peak force after tetanic stimulation in ex vivo contractility experiments in isolated EDL (D) and Soleus (E) muscles. (F) Immunodetection of protein levels of PGC1a and VDAC in gastrocnemius muscle. (G) mRNA expression of Ppargc1a, PGC-1a gene targets, and Ppargc1a isoforms in gastrocnemius and soleus muscle. Data shown as mean ± SEM, significance determined by Student’s t-test (*p<0.05). EDL, extensor digitorum longus.
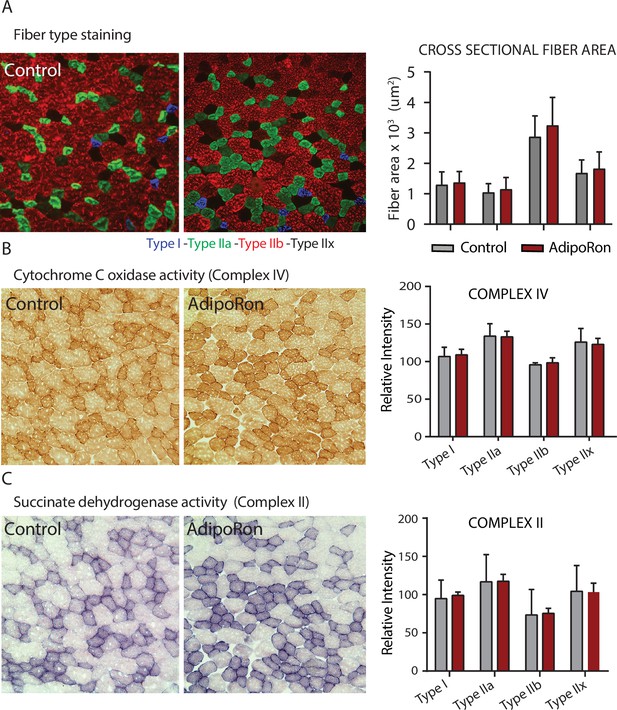
Effects of chronic AdipoRon treatment in young adult mice.
Effects of chronic AdipoRon treatment (1.2 mg/kg BW, intravenous injection three times per week for 6 weeks) in young B6C3F1 male mice (8 weeks old). (A) Representative images of fiber typing immunohistochemistry for Type I (blue), Type IIa (green), Type IIb (red), and Type IIx (black) fibers in adjacent gastrocnemius muscle sections of control and AdipoRon treated animals. (B) Histochemical staining for Cytochrome C Oxidase activity (Complex IV), (C) histochemical staining for maximal succinate dehydrogenase (Complex II, middle panel), and corresponding intensity quantitation. Control (n=4) and AdipoRon (n=4). All data shown as mean ± SEM.
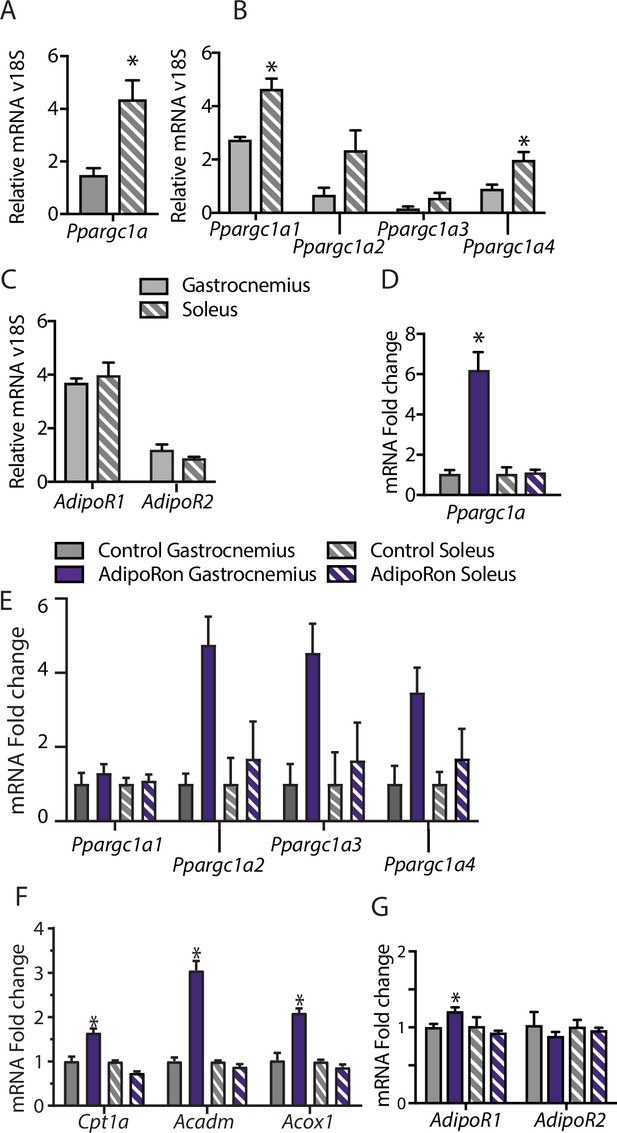
Muscle-type-specific transcriptional response to acute AdipoRon treatment.
Comparison between gastrocnemius and soleus muscles of (A) Ppargc1a, (B) Ppargc1a isoforms, and (C) adiponectin receptors Adipor1 and Adipor2 mRNA relative to Rn18s (Gastrocnemius [n=3] and Soleus [n=3]). Fold changes in gene expression in gastrocnemius and soleus muscle of 6-week-old male B6C3F1 hybrid mice 90 min post-IV injection with AdipoRon (1.2 mg/kg BW) (D) Ppargc1a, (E) Ppargc1a isoforms, (F) gene targets of PGC1a, and (G) adiponectin receptors R1 and R2 (Control [n=3] and AdipoRon [n=5]). Data shown as mean ± SEM, significance determined by Student’s t-test (*p<0.05).
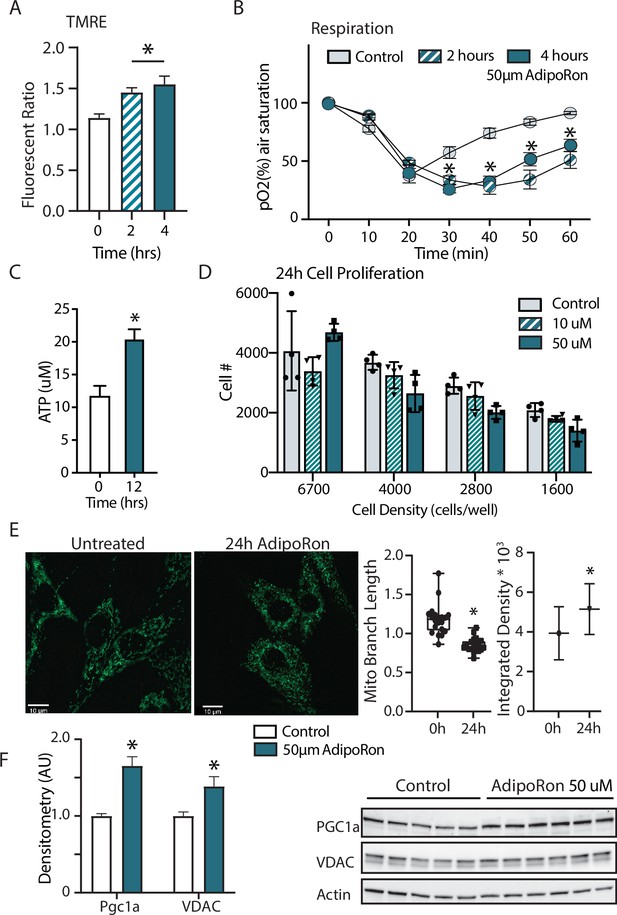
Short term impact of AdipoRon treatment on mitochondrial metabolism in murine fibroblasts.
(A) Mitochondrial membrane potential measured by TMRE assay at 2 and 4 hr of AdipoRon treatment (n=6 per group), (B) oxygen consumption measured in response to AdipoRon treatment (Control-open circle, AdipoRon 2 hr-hatched circle, AdipoRon 4 hr-filled circle) by oxoplate assay (n=5 per group), (C) ATP concentration after indicated times with 50 µm AdipoRon treatment, (D) impact of 24 hr of AdipoRon treatment on cell proliferation of differing seeding densities as assessed by CyQUANT assay (n=4 per group), (E) fluorescent detection of mitochondria in fixed NIH-3T3 following AdipoRon treatment including quantitation of integrated density (product of mean intensity and mitochondrial area), and mean mitochondrial skeleton branch length (15–20 cells per time point), (F) Western blot showing protein levels of PGC1a and VDAC after 48 hr of 50 µm AdipoRon treatment of NIH-3T3 fibroblasts (n=5–6 per group), data shown as average shown as mean ± SEM (*p<0.05 via Student’s t-test).
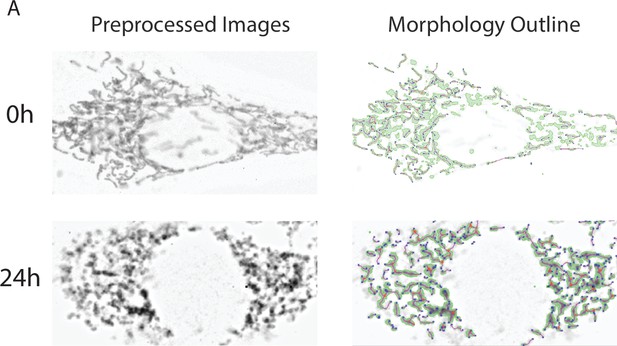
Mitochondrial morphology analysis.
(A) Representative images of cultured NIH-3T3 treated with 50 µm AdipoRon or DMSO (n = 4, 15–20 cells per treatment) shown in grayscale contrast pre-processed image, before (above) and after (below) mitochondrial footprint morphology mask and 2D skeleton identified via MINA ImageJ plugin binarization and ridge detection algorithms.
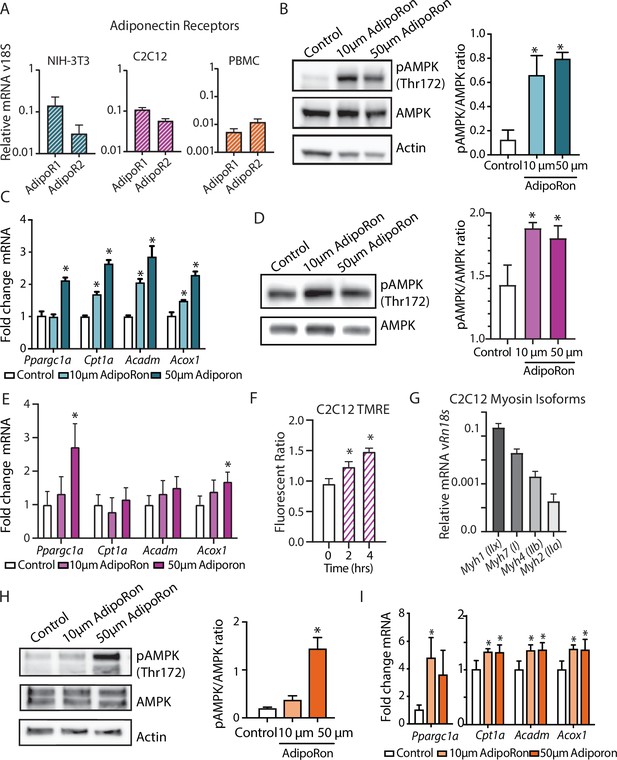
AdipoRon activates AMPK, increases the expression of Ppargc1a, and activates PGC-1a gene targets in diverse cultured cells.
(A) RT-PCR detection of adiponectin receptors in NIH-3T3 fibroblast, day 7 differentiated C2C12 myotubes, and rhesus monkey peripheral blood mononuclear cells (PBMCs) (n=7, 7, and 4, respectively), (B) phosphorylation of AMPK at Thr172 after AdipoRon treatment (10 or 50 µm) for 10 min (n=3 per group) in NIH-3T3 fibroblasts, (C) transcript levels of Ppargc1a and its gene targets after 90 min of AdipoRon treatment (10 or 50 µm, n=4–5 per group, relative to Rn18s RNA), (D) phosphorylation of AMPK at Thr172 after AdipoRon treatment for 10 min in day 7 differentiated myotubes (n=3 per group), (E) transcript levels of Ppargc1a and its gene targets after 90 min of AdipoRon treatment (10 or 50 µm, n=7 per group, relative to Rn18s RNA), (F) mitochondrial membrane potential measured by TMRE assay at 2 and 4 hr of AdipoRon treatment (n=7 per group), (G) RT-PCR detection of myosin isoforms relative to Rn18s in day 7 differentiated myotubes, (H) phosphorylation of AMPK at Thr172 after AdipoRon treatment (10 or 50 µm) for 10 min in cultured rhesus monkey PBMCs (n=3–4 per group), and (I) transcript levels of Ppargc1a and PGC1a gene targets after 60 min of AdipoRon treatment (n=3–4 per group). Data shown as average ± SEM (*p<0.05).
Additional files
-
Supplementary file 1
RT-PCR forward and reverse primer sequences and corresponding genes used for identifying transcripts in mouse tissue and diverse cell types.
- https://cdn.elifesciences.org/articles/71282/elife-71282-supp1-v2.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/71282/elife-71282-transrepform1-v2.docx





