‘Fearful-place’ coding in the amygdala-hippocampal network
Figures
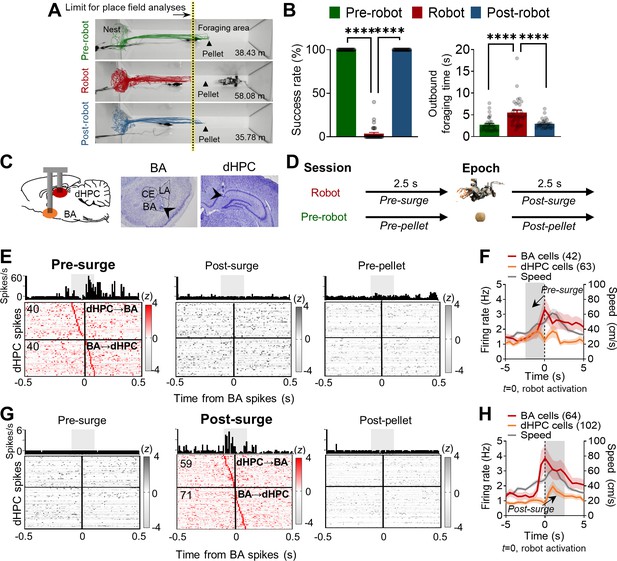
Simultaneous basal amygdala (B) and dorsal hippocampus (dHPC) recordings in foraging rats facing a predatory threat.
(A) Overlaid images of the foraging apparatus and representative trajectories of a rat during the pre-robot, robot, and post-robot sessions. The number below each apparatus indicates the total distance traveled from the representative trajectory data. Contrary to 100% successful trials during the pre-robot and post-robot sessions, this rat made 14 attempts to procure the pellet during the robot session but failed each time. The limits of place field analyses for all sessions were matched to the yellow-tinted dotted line denoting the extent of the place cells recorded during the robot session. (B) Left panel: the mean success rate of pellet acquirement (± SEM) during pre-robot, robot, and post-robot session (****p<0.0001, n = 32 recording days from four rats). Right panel: the mean outbound foraging time (± SEM) from the gate opening for animals to reach the pellet (pre-robot and post-robot sessions) or the robot trigger location (robot session) (****p<0.0001, n = 32 recording days from four rats). Each circle represents each recording day’s data. (C) A schema of simultaneous recordings (left) and photomicrographs of tetrode tips in BA (middle) and dHPC (right). (D) Simultaneously recorded 1999 BA-dHPC unit pairs were evaluated at the four different time epochs: 2.5 s before each robot activation (pre-surge; robot session), 2.5 s after each robot activation (post-surge; robot session), 2.5 s before each pellet procurement (pre-pellet; pre-robot session), and 2.5 s after each pellet acquirement (post-pellet; pre-robot session). (E) Cross-correlograms (CCs) of all BA-dHPC cell pairs (n = 80) that showed significant spike synchrony during the pre-surge epoch (left) but not during the post-surge (middle) and pre-pellet (right) epochs. CCs of a representative pair are shown above each epoch data, 10 ms bin (the gray shaded area indicates the time window for statistical significance, –100 ms to +100 ms from the BA spikes). The vertical lines (0) indicate the time when the reference BA spikes occurred. The horizontal lines indicate the borders between the presumable dHPC→BA pairs (above the line) and BA→dHPC pairs (below the line). (F) The mean firing rates of BA and dHPC cells showed significant spike synchrony during the pre-surge epoch (the gray shaded area, BA = 42 cells, dHPC = 63 cells; overlapping cells were counted once) and the mean speed of the animals (n = 20 recording days from three rats). The dark lines and shaded bands represent the mean and SEM. (G) CCs of all BA-dHPC cell pairs (n = 130) that showed significant spike synchrony during the post-surge epoch (middle) but not during the pre-surge (left) and post-pellet (right) epochs. CCs of a representative pair are shown above each epoch data. (H) The mean firing rates of BA and dHPC cells showed significant spike synchrony during the post-surge epoch (the gray shaded area, BA = 64 cells, dHPC = 102 cells; overlapping cells were counted once) and the mean speed of the animals (n = 20 recording days from three rats). LA: lateral amygdala; CE: central amygdala.
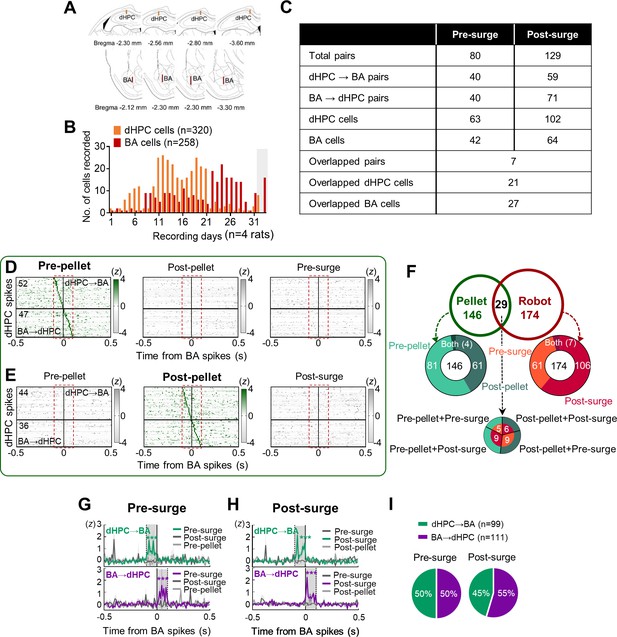
Simultaneous recordings from the basal amygdala (BA) and dorsal hippocampus (dHPC).
(A) Histological reconstructions of the trajectory of the recording sites (bars) in the dHPC and the BA. (B) The number of simultaneously recorded cells during each recording day. The gray shaded area represents 2 days where cells were recorded from only one region. (C) A detailed composition of the significant pairs: the actual number of dHPC and BA cells and the number of overlapped pairs/cells between epochs. (D) Cross-correlograms (CCs) of all BA-dHPC cell pairs (n = 99) that showed significant spike synchrony during the pre-pellet epoch (left) but not during the post-pellet (middle) and pre-surge (right) epochs (10 ms bin). The red dashed rectangles indicate the time window for statistical significance, –100 ms to +100 ms from the BA spikes. The vertical lines (0) indicate the time when the reference BA spikes occurred. The horizontal lines indicate the borders between the presumed dHPC→BA pairs (above the line) and BA→dHPC pairs (below the line). (E) CCs of all BA-dHPC cell pairs (n = 80) that showed significant spike synchrony during the post-pellet epoch (middle) but not during the pre-pellet (left) and post-surge (right) epochs. (F) The proportions of BA-dHPC pairs that showed significant spike synchrony during the pellet procurements (pre-pellet, post-pellet; n = 146), robot encounters (pre-surge, post-surge; n = 174), or both pellet-robot (n = 29). (G) The Z-scored dHPC spikes at the time of BA spikes are shown in upper (dHPC→BA pairs, ****p<0.0001) and bottom (BA→dHPC pairs, ****p<0.0001) during the pre-surge. The dark lines represent the mean, and the shaded bands represent SEM. (H) The Z-scored dHPC spikes at the time of BA spikes are shown in upper (dHPC→BA pairs, ****p<0.0001) and bottom (BA→dHPC pairs, ****p<0.0001) during the post-surge. (I) The percentage of the dHPC→BA and BA→dHPC cell pairs in the pre-surge and post-surge peaked CCs (p=0.787).
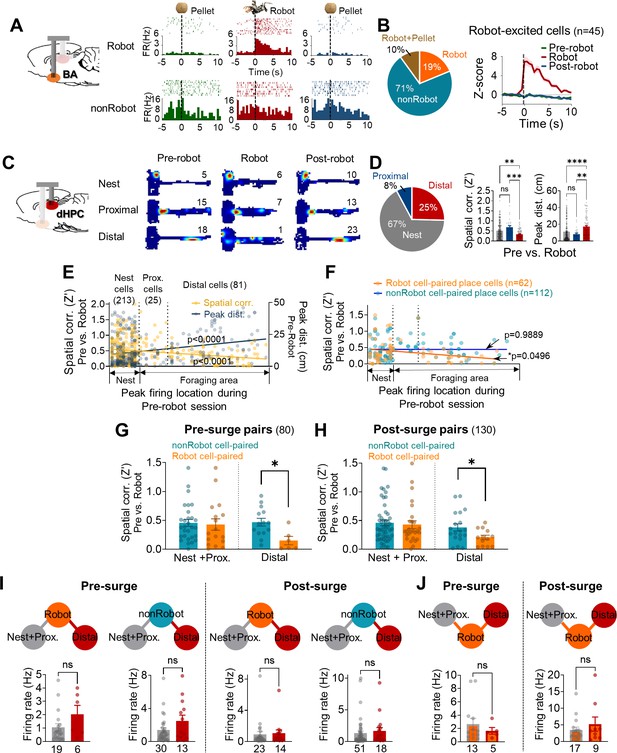
Spike synchrony between dorsal hippocampus (dHPC) units and basal amygdala (BA) units during the robot-predator encounter.
(A) The BA raster plots and peri-event histograms (PETHs) of representative Robot cell (top) and nonRobot cell (bottom) during pre-robot, robot, and post-robot sessions. (B) The percentage of different categories of BA cells (left) and the normalized population activity of all robot-excited cells during all three sessions (n = 45). (C) dHPC place fields from the nest, proximal, and distal cells during pre-robot, robot, and post-robot sessions (the numerical value represents the peak firing rate). (D) Left: the percentage of three place cell types. Middle: the pixel-by-pixel spatial correlation (Z’) values between the pre-robot and robot sessions of three place cell types (**p=0.0062, ***p=0.0003, nest = 213 cells, proximal = 25 cells, distal = 81 cells). Right: the peak distances between the pre-robot and robot sessions of three place cell types (**p=0.0047, ****p<0.0001). (E) Spatial correlations (yellow, r = −0.2203, p<0.0001) and peak distances (navy, r = 0.2594, p<0.0001) of all place cells between pre-robot and robot sessions are plotted as a function of the peak firing location during the pre-robot session (left, nest; right, end of the foraging distance; circles individual data with regression lines). (F) Spatial correlations (pre-robot vs. robot sessions) of place cells that co-fired with Robot (orange circles) or with nonRobot (blue circles) cells are plotted as a function of the peak firing location during the pre-robot session (62 Robot cell-paired place cells, linear regression, r = −0.2446, *p=0.0496; 112 nonRobot cell-paired place cells, linear regression, r = −0.001316, p=0.9889). (G) The spatial correlations between the pre-robot and robot sessions of the nest + proximal cells paired with Robot vs. nonRobot cells during the pre-surge (p=0.7961) and the distal cells paired with Robot vs. nonRobot cells during the pre-surge (*p=0.0119). (H) The spatial correlations between the pre-robot and robot sessions of the nes + proximal cells paired with Robot vs. nonRobot cells during the post-surge (p=0.5939) and the distal cells paired with Robot vs. nonRobot cells during the post-surge (*p=0.0430). (I) The mean firing rates between the Robot cell-paired nest + proximal cells and the Robot cell-paired distal cells during the pre-surge epoch (the first and second graphs) and during the post-surge epoch (the third and fourth graphs). The numeric values represent the number of cells in each cell type. (J) The mean firing rates of nest + proximal or distal cell-paired Robot cells during the pre-surge epoch (left) and during the post-surge epoch (right). The numbers below each graph represent the number of cells, and each circle represents individual cell data. Data are presented as mean ± SEM.
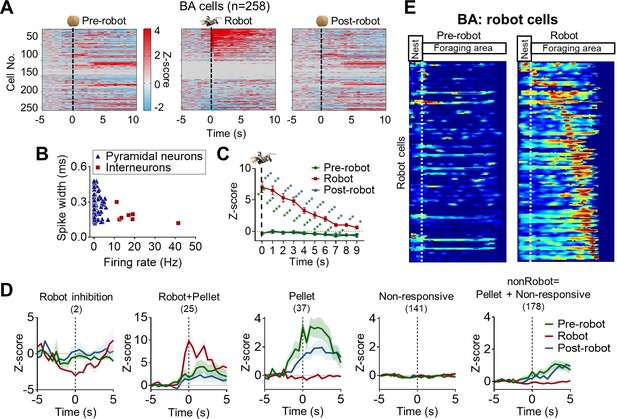
The basal amygdala and the risky foraging behaviors.
(A) Z-scored activities of all basal amygdala (BA) cells (258 cells, pyramidal cells + interneurons) during –5 s to 10 s after the pellet procurement for the pre-robot (left) and post-robot (right) sessions and during –5 s to 10 s after the robot activation for the robot session (middle). Individual cell activity is presented in the same row of each session. (B) BA units were classified as putative pyramidal neurons (blue triangles) and putative interneurons (red square). Only putative pyramidal cells (n = 250) were included in further analyses. (C) Comparisons of Z-scored activities of Robot cells after the pellet acquirement and robot activation. The robot-induced increased activities lasted over a period of 10 s, which was beyond the robot activation (green asterisks: pre-robot vs. robot; blue asterisks: robot vs. post-robot). (D) Z-scored activities of Robot-inhibited cells, Robot + Pellet cells, Pellet cells, Non-responsive cells, and nonRobot cells ( = Pellet cells + Non-responsive cells) during –5 s to 5 s after the pellet acquirement for the pre- and post-robot sessions and the robot activation for the robot session. (E) The firing maps of all Robot cells in the BA during the pre-robot (left) and robot (right) trials. All cells were aligned to the peak firing location during the robot session. The x-axis denotes the distance from the nest. The white dotted line in each figure separates the nest and the foraging area. For each map, red represents the maximal firing rate.
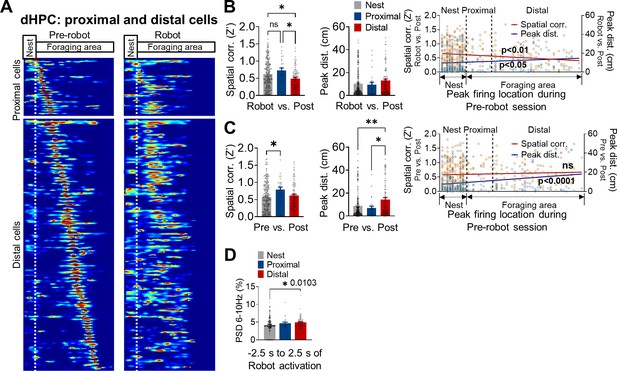
The dorsal hippocampus and the risky foraging behaviors.
(A) The place maps of the proximal and distal cells during the pre-robot (left) and robot (right) sessions. All cells are aligned to the peak firing location of the pre-robot session. The x-axis denotes the distance from the nest. The white dotted line in each figure separates the nest and the foraging area. Each map shows its maximal firing rate in red. (B) Differences in the Z’ value (left) and peak distance (middle) between the robot and post-robot sessions are plotted in the order of X position of rats (spatial correlations: individual data, orange squares; regression line, dark red; r = −0.1755, **p=0.0016; peak distances: individual data, blue circles; regression line, dark blue; r = 0.11, *p=0.0493). (C) Differences in the Z’ value (left) and peak distance (middle) between the pre- and post-robot sessions are plotted in the order of X position of rats (spatial correlations: individual data, orange squares; regression line, dark red, r = 0.05898, p=0.2929; peak distances: individual data, blue circles; regression line, dark blue; r = 0.2239, ****p<0.0001). (D) The percentage of theta power from 6 to 10 Hz band during –2.5 s to 2.5 s of the robot activation.
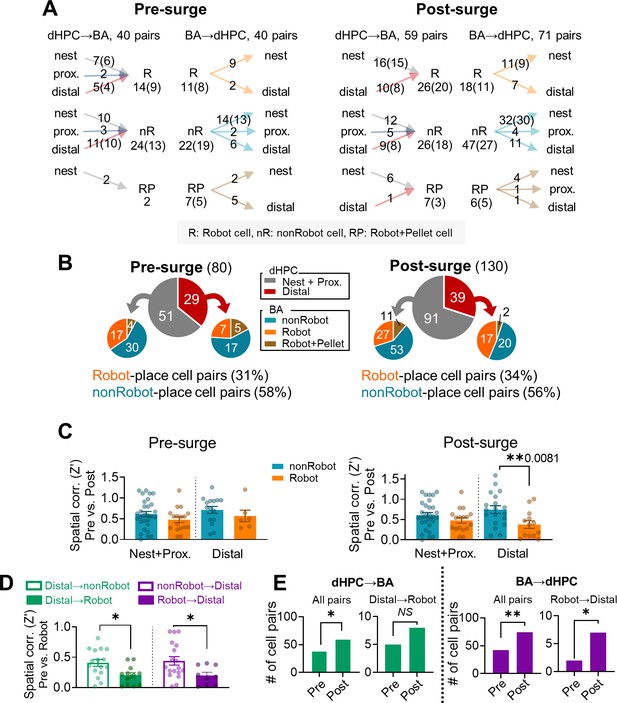
Simultaneous recordings from the basal amygdala (BA) and dorsal hippocampus (dHPC) during the risky foraging behaviors.
(A) All pairs that fired synchronously during the pre-surge (left) and the post-surge (right) epochs during the robot session. If a cell was in more than one pair, the cell was counted only once and indicated in parentheses. The arrows indicate the direction of information flow. (B) The subdivision of cell pairs that showed significant synchrony during the pre-surge epoch (left) and post-surge epoch (right). (C) Spatial correlations between the pre-robot and post-robot sessions in the nest + proximal and distal cells that had significant spike synchrony with Robot or nonRobot during the pre-surge epoch (left) or post-surge epoch (right, **p=0.0081). (D) Distal cells leading Robot cells (distal→Robot) remapped more during the robot session compared with those paired with nonRobot cells (distal→nonRobot, *p=0.0123). Distal cells led by Robot cells (Robot→distal) remapped more during the robot session compared with those paired with nonRobot cells (nonRobot→distal, *p=0.0321). (E) The numbers of both dHPC→BA and BA→dHPC cell pairs increased during the post-surge epoch compared to the pre-surge epoch (dHPC→BA pairs, χ² = 5.042, p=0.025; BA→dHPC pairs, χ² = 8.828, p=0.003), but the number of Robot cell-distal cell pairs significantly increased during the post-surge epoch only in the BA leading direction (distal→Robot pairs, χ² = 0.692, p=0.405; Robot→distal pairs, χ² = 4.500, p=0.034).
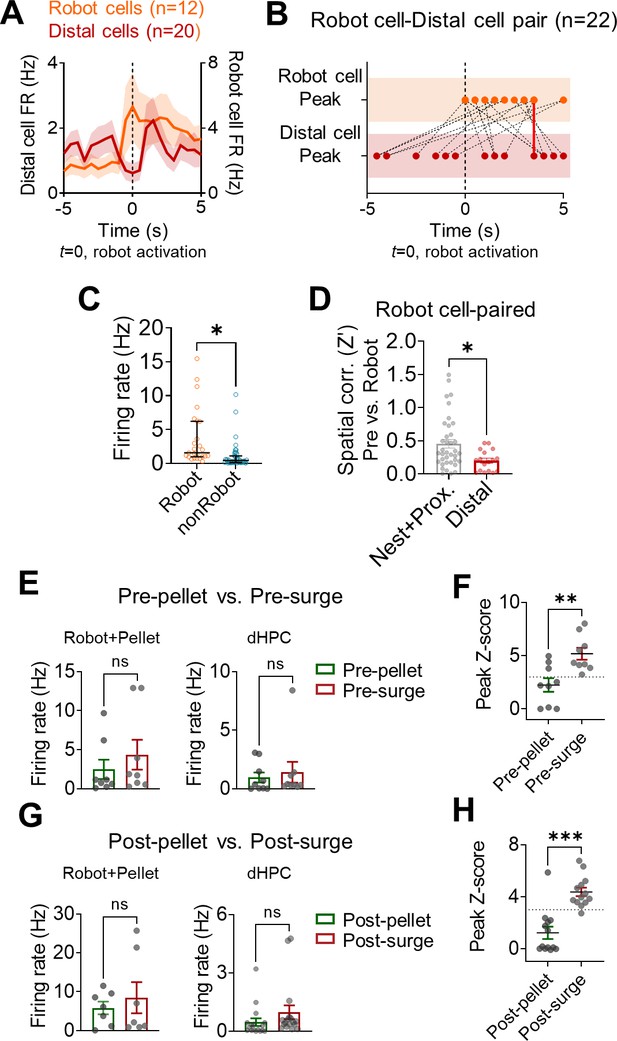
The relationships between firing rates and cross-correlations.
(A) The mean firing rates of Robot and distal cells that showed significant spike synchrony during the pre- and post-surge epochs (overlapping cells were counted once). (B) The peak firing times of Robot cells (top row) and distal cells (bottom row) from the significantly synchronized pairs. Red lines indicate the pairs with peak firings in the same bin (two lines overlapped, 500 ms bin, 3.5 s after the robot activations). (C) The firing rate differences between dorsal hippocampus (dHPC)-Robot paired cells and dHPC-nonRobot paired cells (****p<0.0001). The median and interquartile ranges are indicated in black lines. (D) Spatial correlations between the pre-robot and post-robot sessions in the nest + proximal and distal cells that had significant spike synchrony with Robot cells (*p=0.0126). (E) The mean firing rates of Robot + Pellet (left) and dHPC (right) cells during the pre-pellet vs. pre-surge epochs. (F) The peak Z-scored dHPC spikes at the time of basal amygdala (BA) spikes during the pre-pellet and pre-surge epochs (**p=0.0044). (G, H) Same analyses as (E, F) but from the pairs that showed significant spike synchrony during the post-pellet epoch (***p=0.0006).
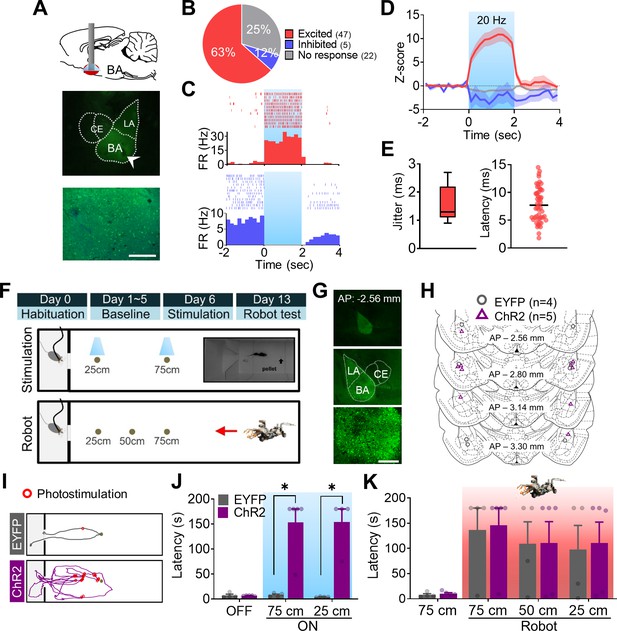
Optogenetic manipulations of the amygdala alter the foraging behaviors of naïve rats in the absence of external threats.
(A) A schema of optic fiber implant (top) and a photomicrograph of optic fiber tip in the basal amygdala (BA; middle) and overlaid image of EYFP and DAPI (bottom). (B) The percentage of cells that responded differentially to the photostimulation. (C) Peri-event histogram (PETH) and raster plot of a representative excited cell (upper) and inhibited cell (bottom) in response to the photostimulation. The blue shaded area indicates the photostimulation period (2 s, 20 Hz). (D) Z-scored firing rates of each cell type (red, excited; blue, inhibited; gray, no response). The dark lines and shaded bands represent the mean and SEM, respectively. (E) Jitter and latency of light-evoked responses. (F) Illustrations of the experimental design. A pellet was set at 25 cm, 50 cm, or 75 cm distance per trial (inset: the actual foraging apparatus). (G) A representative viral expression in the BA at different magnifications. (H) Placements of optic fiber tips bordering above or within the BA. Gray and purple circles indicate EYFP-expressing (n = 4) and channelrhodopsin (ChR2)-expressing (n = 5) rats, respectively. (I) Representative trajectory plots of EYFP- and ChR2-expressing rats. Red circles indicate the stimulation delivery locations during a 75 cm distance trial. (J) The latency of procuring pellets without photostimulations (OFF, p=0.7843) and with photostimulations during the 75 cm (ON-75 cm, *p=0.0159) and 25 cm (ON-25 cm, *p=0.0286) distance trials. (K) Latency of procuring pellets during the Robot test. The red shaded area indicates the trials with the robot-predator. Data are presented as mean ± SEM, and individual data are represented as distinct symbols. LA: lateral amygdala; CE: central amygdala. Scale bars, 200 µm.
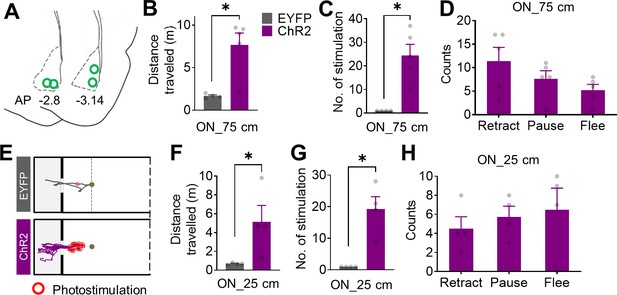
Locomotor data during the optogenetic stimulation.
(A) Histological reconstruction of the optrode tip placements (green circles). (B) During the stimulation session, the ChR2-expressing rats traveled more than the EYFP-expressing rats due to multiple failed attempts at the pellet (ChR2 = 5, EYFP = 4; Mann–Whitney test, U = 0, *p=0.016). (C) The ChR2-expressing rats received a greater number of photostimulations than the EYFP-expressing rats because each photostimulation reliably produced fleeing behavior that prevented securing 75 cm placed pellet (Mann–Whitney U test, U = 0, *p=0.016). (D) Specific types of defensive behaviors elicited by photostimulation. (E) Representative tracking data of the EYFP- and ChR2-expressing rats during photostimulation to closely (25 cm) placed pellet. Red circles indicate the locations of photostimulation delivery. (F) The ChR2-expressing rats traveled more than the EYFP-expressing rats during the stimulation session with a pellet at 25 cm (ChR2 = 4, EYFP = 4; Mann–Whitney test, U = 0, *p=0.0286). (G) The ChR2-expressing rats received more photostimulations than the EYFP-expressing rats with a pellet at 25 cm (Mann–Whitney U test, U = 0, *p=0.0286). (H) Types of defensive behaviors elicited by photostimulation with a pellet at 25 cm. Data are presented as mean ± SEM, and individual data are represented as symbols.
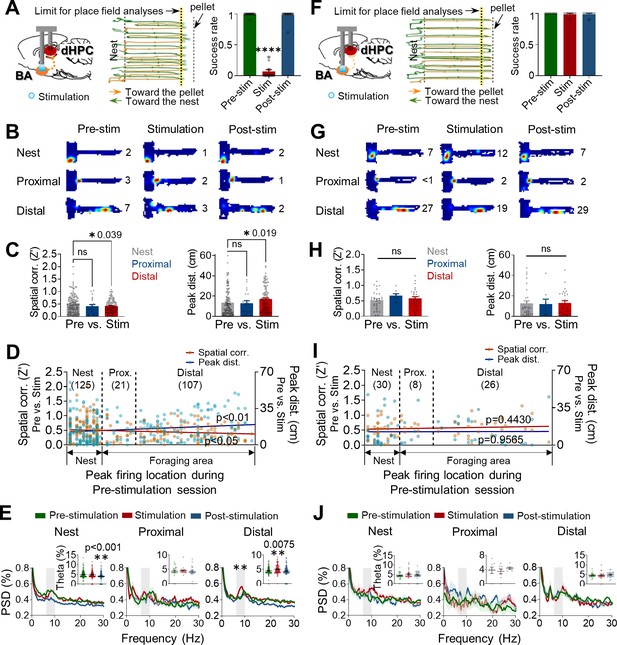
Optogenetic stimulations of the amygdala alter the stability of place cells.
(A–E) Data were collected from rats exhibiting defensive behaviors responding to the photostimulation. (A) An illustration showing that optogenetic stimulation of the basal amygdala (BA) presumably sends strong (primarily polysynaptic) inputs to the dorsal hippocampus (dHPC) when the stimulations elicited behavioral effects (left). A representative tracking plot during the stimulation session (middle). Orange lines and blue circles indicate the outbound trails and stimulation locations, respectively, that evoked escape responses (green lines). The yellow-tinted dotted line represents the limit of place field analyses. The success rate of pellet retrieval during the photostimulation session significantly decreased (right, n = 17 recording days, ****p<0.0001). (B) Examples of place fields from the nest, proximal, and distal cells during each session (the numerical value represents the peak firing rate). (C) Differences in the pixel-by-pixel spatial correlations (Z’) value (left, *p=0.039) and peak distances between the pre-stimulation and stimulation sessions (right, *p=0.019). (D) Spatial correlations (individual data: orange squares, regression line: dark red, r = −0.1320, *p=0.0359) and peak distances (individual data: blue circles, regression line: dark blue, r = 0.1625, **p=0.0096) of all place cells between pre-stimulation and stimulation sessions are plotted in the order of X position of rats. (E) Power spectral densities (PSD, shown as % of total PSD) of different frequency bands from each cell type during the three sessions. The dark lines and shaded bands represent the mean and SEM, respectively. The gray band represents the theta range (6–10 Hz), and the percentage of theta power during the three sessions is shown in the inserted bar graphs. (F–J) The same analyses as in (A–E), and data were collected from rats not exhibiting defensive behaviors to the photostimulations. (F) When the stimulation of the BA was too weak (left) to elicit defensive behaviors (middle), the success rates across the three sessions were not different (right, n = 6 recording days, p>0.999). (I) Spatial correlations: r = 0.0.09758, p=0.4430; peak distances r = 0.0.006948, p=0.9565. Data are presented as mean ± SEM.
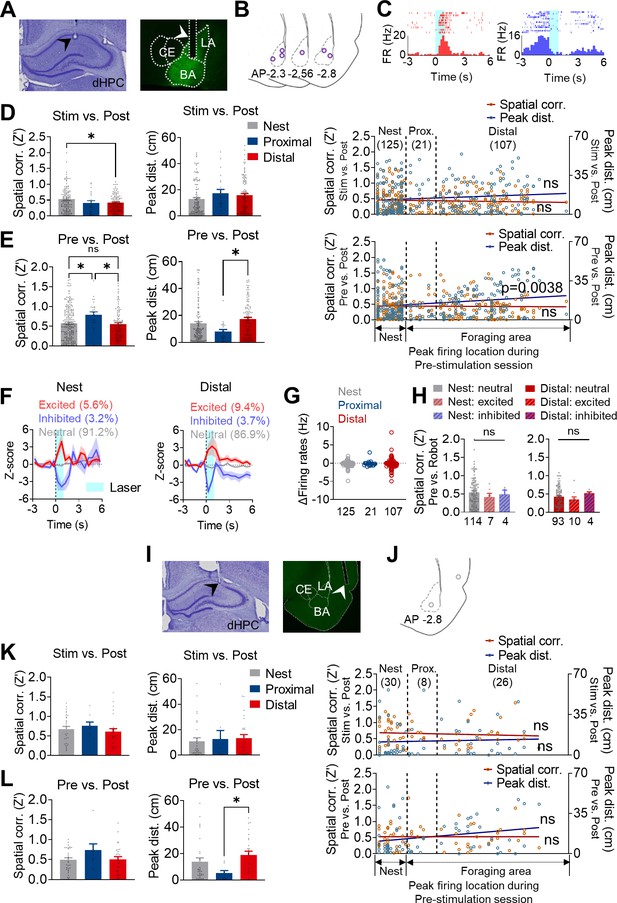
Basal amygdala (BA) photostimulation effects on place cell stability.
(A–H) Data from the rats with behavioral effects in response to the photostimulations. (A) A photomicrograph of a tetrode tip in the dorsal hippocampus (dHPC; left) and viral expressions with an optic fiber track in the BA (right). (B) Histological reconstruction of the optic fiber tips from rats that showed behavioral effects to the photostimulations (purple circles). (C) Rastor plots and peri-event histograms (PETHs) of representative photostimulation-excited dHPC cell (left) and photostimulation-inhibited dHPC cell (right). (D) Differences in the spatial correlations (left, *p=0.0312) and peak distance (middle, p=0.1635) between the stimulation and post-stimulation sessions are plotted as the order of X position of rats (spatial correlations: individual data, orange squares; regression line, dark red; r = 0.1153, p=0.3432; peak distances: individual data, blue circles; regression line, dark blue; r = −0.05983, p=0.0671). (E) The same analyses as in (D) from the data between pre- and post-stimulation sessions spatial correlation, left, nest vs. proximal: *p=0.021, proximal vs. distal: *p=0.026; peak distance, middle, proximal vs. distal: *p=0.0110; plotted as the order of X position of rats, right, spatial correlation, r = −0.001710, p=0.7867, peak distance, r = 0.1815, **p=0.0038. (F) The mean Z-scored firing rates of each response type to the stimulations (excited, inhibited, or neutral) in the nest, proximal, and distal cells. The dark lines and shaded bands represent the mean and SEM. (G) The firing rate changes to the stimulations (Δ firing rates: firing rate1s stimulation ─ firing rate1s before stimulation) of nest, proximal, and distal cells. (H) Spatial correlations between pre-stimulation and stimulation sessions of nest and distal cells that were responsive or non-responsive to the photostimulations. (I–L) Data from the rats without behavioral effects to the photostimulations. (K) Spatial correlations: individual data, orange squares; regression line, dark red, r = −0.08056, p=0.5269; peak distances: individual data, blue circles; regression line, dark blue; r = 0.04943, p=0.6981. (L) Spatial correlations: individual data, orange squares; regression line, dark red, r = −0.0004102, p=0.9974; peak distances: individual data, blue circles; regression line, dark blue; r = 0.2326, p=0.0644.
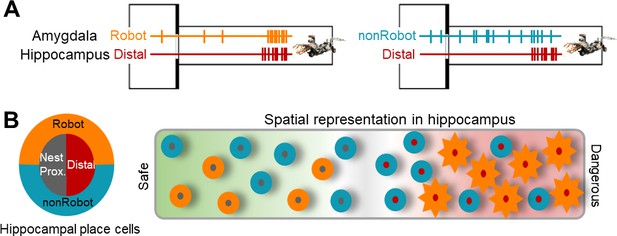
A hypothetical coding model of the safe-danger boundary by the amygdala-hippocampus network.
(A) Illustrations of amygdala and hippocampus cell pairs that showed synchronized firings when the animal is confronted with the robot-predator: Robot-Distal (left) and non-Robot-Distal (right) pairs. (B) An illustration of spatial representation of the safe-danger boundary (the gray area) in the hippocampus as an outcome of interaction with the amygdala. The concentric circles, outer amygdala cells (Robot or nonRobot), and inner hippocampal place cells (nest/proximal or distal) represent safe vs. dangerous environments based on the information from/to the amygdala. Specifically, distal cells synced with Robot cells show a greater extent of remapping (represented as sun-shape), which is presumably due to the eminent fear information received by the amygdala.
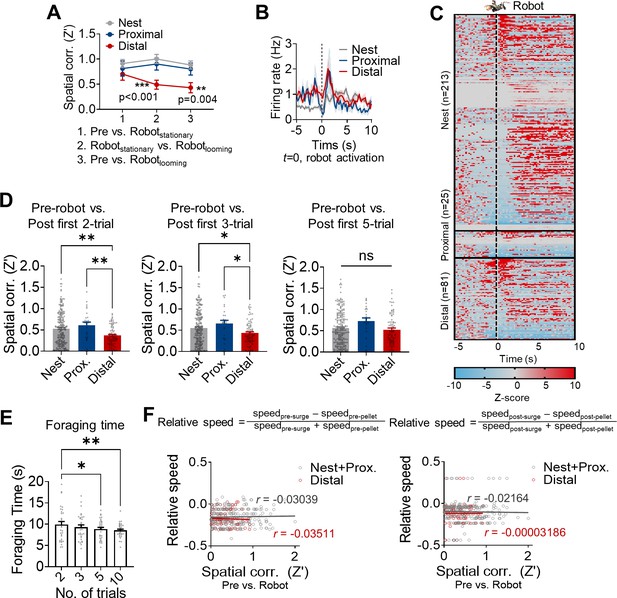
The dorsal hippocampus and the risky foraging behaviors.
(A) Spatial correlations between pre-robot vs. stationary robot (x-axis 1), stationary robot vs. looming robot (x-axis 2), and pre-robot vs. looming robot (x-axis 3) sessions in nest (n = 32), proximal (n = 11) and distal (n = 16) place cells from a total of 6 rats. (B) The mean firing rates of the nest, proximal, and distal cells aligned to the robot activation. (C) Z-scored activities of all individual dHPC units during –5 s to 10 s after the robot activation (200 ms bin). (D) Spatial correlations between the pre-robot vs. first two (left), three (middle), and five (right) trials of post-robot sessions. (E) The mean foraging time during the first 2, 3, 5, and 10 trials. (F) The correlations between the relative outward (left, pre-pellet vs. pre-surge epochs) or inward (right, post-pellet vs. post-surge epochs) speed of the animals and spatial correlations (pre-robot vs. robot sessions) of the place cells.
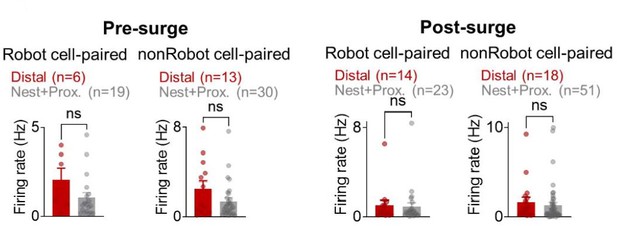
The mean firing rates during the pre-surge (left) and post-surge (right) epochs of Robot/nonRobot cell-paired distal/nest+proximal cells.
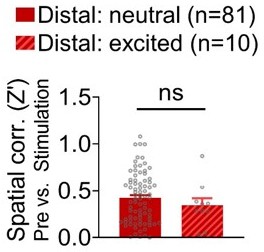
Spatial correlations (pre-stimulation vs. stimulation sessions) of stimulation-neutral distal cells and stimulation-excited distal cells.
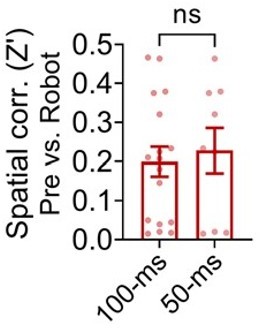
Spatial correlations between the pre-robot and robot sessions from the distal cells that showed significant spike synchrony with the robot cells within ±100 ms or ±50 ms.
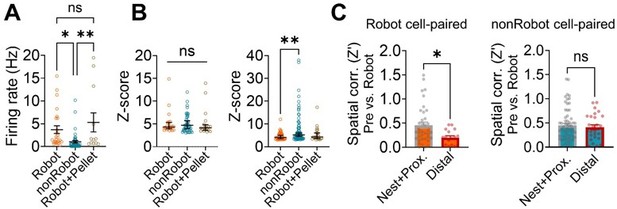
Spike synchrony between the dorsal hippocampus and basal amygdala and its impact on spatial correlations of place cells.
(A) Firing rate differences between Robot, nonRobot and Robot+Pellet cells. (B) The dHPC spikes’ Z-score from significant Robot cell, nonRobot cell, and Robot+Pellet cell-paired CCs during the pre-surge (left) and post-surge (right). (C) The spatial correlations of nest vs. distal cells that were paired with Robot cells (left) or nonRobot cells (right).
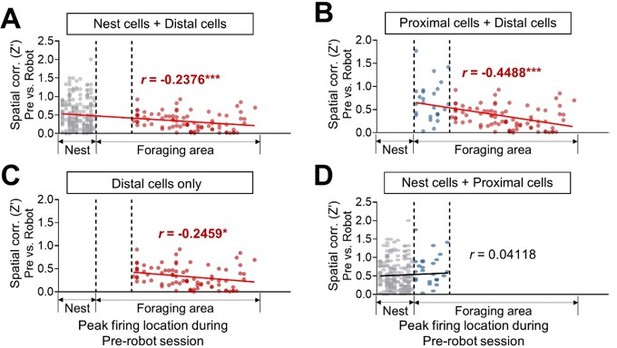
Spatial correlations in nest + distal cells.
(A), proximal + distal cells (B), distal cells only (C), and nest + proximal cells (D) between pre-robot and robot sessions are plotted as a function of the peak firing location during the pre-robot session (left, nest; right, end of the foraging apparatus).

The comparisons of the peak firing locations of all distal cells during the pre-robot and robot sessions.
(A) The peak firing locations of all distal cells (n=81) during the pre-robot (top) and robot (bottom) sessions. The x-axis denotes the distance from the nest. (B) The distance differences of the peak firing locations between the pre-robot and robot sessions in all distal cells (peak firing locationrobot – peak firing locationpre-robot). Below zero indicates that the firing location moved toward the nest.
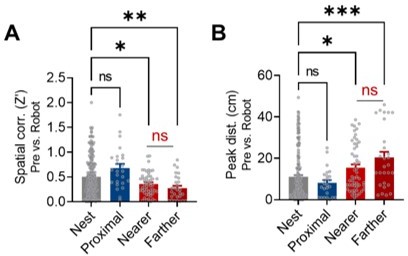
Spatial correlations and peak distances between the pre-robot vs. robot sessions from the nest, proximal, nearer distal, and farther distal cells.
(A) Spatial correlations between the pre-robot and robot sessions from the nest, proximal, and distal cells (Nearer distal cells: fired relatively nearer to the nest; Farther distal cells: fired farther from the nest). (B) Peak distances between the pre-robot and robot sessions from the nest, proximal, and distal cells (Nearer distal cells: fired relatively nearer to the nest; Farther distal cells: fired farther from the nest).
Additional files
-
Supplementary file 1
Supplementary file 1A.
The percentage of the basal amygdala (BA) cell types. A total of 250 pyramidal cells were recorded. The bottom table shows the percentage of the cells showing the same, different, or no responses between the pre-robot and the robot sessions. Supplementary file 1B. Firing properties of dHPC place cells during the pre-robot, robot, and post-robot sessions. Kruskal–Wallis test (average firing rate, peak rate, spatial info, and running speed) and one-way ANOVA (field size); *p<0.05, **p<0.01, and ***p<0.001 compared to the nest cells; #p<0.05 compared to the proximal cells. Supplementary file 1C. Firing properties of place cells during the pre-stimulation, stimulation, and post-stimulation sessions. Data are from the rats with behavioral effects to the photostimulations. Kruskal–Wallis test; **p<0.01 and ***p<0.001 compared to the nest cells; #p<0.05, ##p<0.01, and ###p<0.001 compared to the proximal cells. Supplementary file 1D. Firing properties of place cells during the pre-stimulation, stimulation, and post-stimulation sessions. Data are from the rats without behavioral effects to the photostimulations. Kruskal–Wallis test; *p<0.05 and ***p<0.001 compared to the nest cells; #p<0.05 compared to the proximal cells. Supplementary file 1E. Descriptions and coordinates for each experiment. Supplementary file 1F. Summary of statistical tests performed.
- https://cdn.elifesciences.org/articles/72040/elife-72040-supp1-v2.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/72040/elife-72040-transrepform1-v2.pdf





