KIF2C regulates synaptic plasticity and cognition in mice through dynamic microtubule depolymerization
Figures
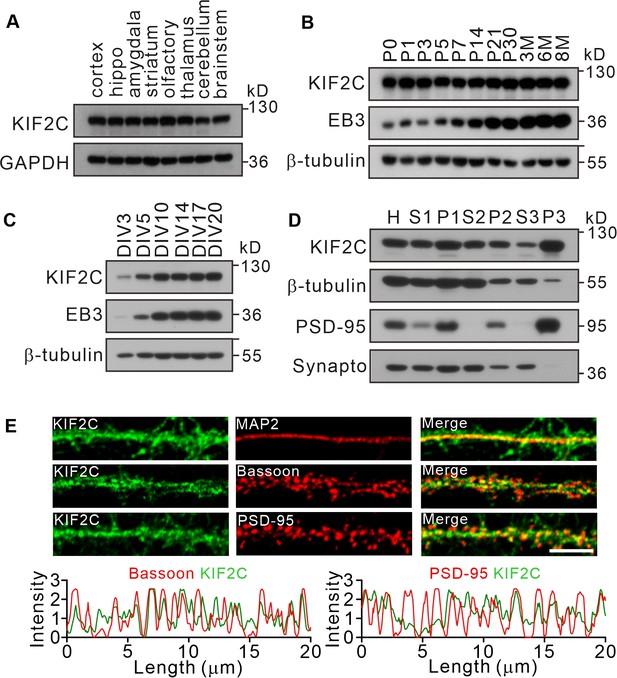
Expression of KIF2C in the central nervous system.
(A) Expression of KIF2C in different mouse brain regions by immunoblotting. (B) Expression of KIF2C and EB3 in mouse hippocampus at different day in vitro. (C) Developmental expression patterns of KIF2C and EB3 in cultured hippocampal neurons. (D) Subcellular distribution of KIF2C in mouse hippocampus. H, homogenate; S1, low-speed supernatant; P1, nuclei; S2, microsomal fraction; P2, synaptosomal fraction; S3, presynaptosomal fraction; P3, postsynaptic density and synapto, synaptophysin. (E) Representative images of hippocampal neurons double-labeled with antibodies against KIF2C, PSD-95 (postsynaptic marker) and Bassoon (presynaptic marker). Scale bar, 5 μm.
-
Figure 1—source data 1
Original files of blots with the relevant bands.
- https://cdn.elifesciences.org/articles/72483/elife-72483-fig1-data1-v1.zip
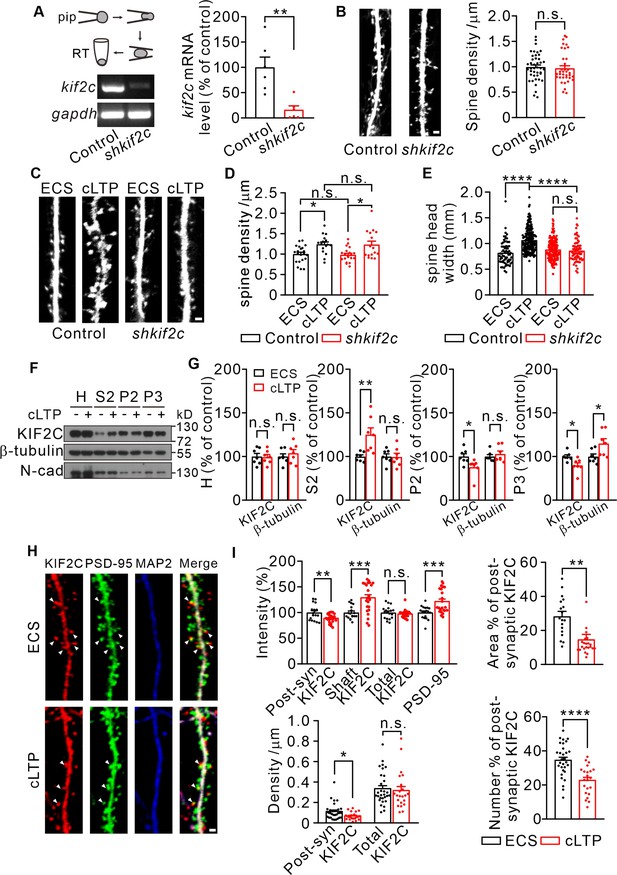
KIF2C displays translocation after cLTP and is required for synaptic structure plasticity.
(A) Electrophoresis of kif2c and gapdh amplicons from individual control and shkif2c hippocampal neurons. Histograms show percentage changes of kif2c mRNA levels (% of control, **p < 0.01, n = 6, Student’s t-test). (B) Cultured hippocampal neurons were infected by control shRNA and shkif2c lentivirus at DIV 7–10, and captured at DIV 18–20. GFP was used to label neuron volume. Scale bar, 1 μm. Spine number per 1 μm (right), n = 20 neurons from three independent culture. Student’s t-test. (C–E) Cultured hippocampal neurons were infected by control shRNA and shkif2c lentivirus at DIV 7–10, and incubated with ECS or glycine (200 μM) for 10 min at DIV 18–20. Scale bar, 1 μm. Spine density (D) and spine head width (E) are calculated. Spine density, n = 17 neurons from three independent culture. Spine head width, n = 24 neurons from three independent culture. n.s., p > 0.05, *p < 0.05, ****p < 0.0001; One way-ANOVA for spine density and head width (post hoc comparison).(F) Subcellular fraction of KIF2C in cultured hippocampus neurons after 10 min of glycine treatment. N-cadherin (N-cad) was served as negative control. H, Homogenate; S2, microsomal fraction; P2, synaptosomal fraction and P3, postsynaptic density from ECS and cLTP treated neurons. (G) Quantification of KIF2C and β-tubulin accumulation with or without cLTP treatment in each fraction. *p < 0.05, **p < 0.01; Student’s t-test, n = 7 experimental repeats. (H) Representative images of co-localization of KIF2C (red), PSD-95 (green), and Microtubule associated protein 2 (MAP2) (blue). Scale bar, 1 μm. (I) The percentage of KIF2C puncta that were colocalized with PSD-95 puncta decreased after cLTP treatment. n = 20 neurons from three cultures. n.s., p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; Student’s t-test.
-
Figure 2—source data 1
Values for kif2c mRNA levels; Values for spine density and spine head width before or after cLTP stimulation in control and shkif2c neurons; Values for KIF2C synaptic expression before or after cLTP stimulation.
- https://cdn.elifesciences.org/articles/72483/elife-72483-fig2-data1-v1.xlsx
-
Figure 2—source data 2
Original files of gels and blots with the relevant bands.
- https://cdn.elifesciences.org/articles/72483/elife-72483-fig2-data2-v1.zip
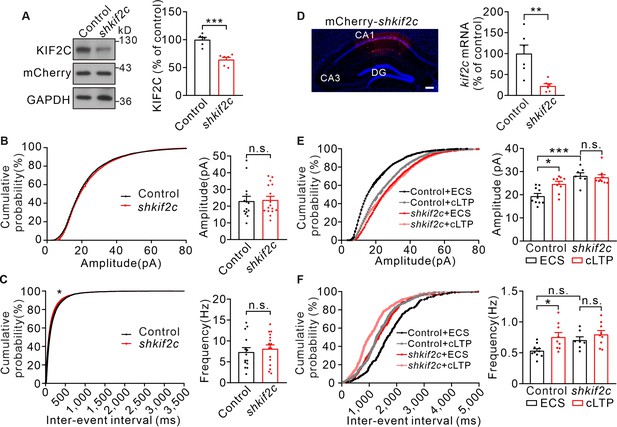
Miniature excitatory synaptic currents (mEPSCs) recordings of control and shkif2c neurons.
(A) Total fractions from mCherry-scramble shRNA (control) and mCherry- shkif2c (shkif2c) hippocampus neurons were probed with antibodies to KIF2C, mCherry, and GAPDH. GAPDH was internal controls. Histograms show percentage changes of KIF2C protein normalized to control. n = 6 experiment repeats, ***p < 0.001, Student’s t-test. (B) Amplitude of mEPSCs recorded from control (n = 14) and shkif2c (n = 16) cultured hippocampus neuron at DIV 18–20. p = 0.7123, Student’s t-test; n.s., p > 0.05, Kolmogorov-Smirnov test. (C) Frequency of mEPSCs recorded from control (n = 14) and shkif2c (n = 16) cultured hippocampus neuron at DIV 18–20. p = 0.5828, Student’s t-test. *p < 0.05, Kolmogorov-Smirnov test. (D) Diagram of mCherry- shkif2c injection, and coronal section image of the CA1 region. Scale bar, 100 μm. Histograms show percentage changes of kif2c mRNA levels (% of control, **p < 0.01, n = 6). Student’s t-test. (E) Control and shkif2c CA1 slices were incubated with ACSF or glycine (200 μM, induce cLTP) for 10 min before mEPSCs recording. Histograms show mEPSCs amplitude of control+ ACSF (n = 10 neurons), control+ cLTP (n = 9 neurons), shkif2c + ACSF (n = 7 neurons), shkif2c + cLTP (n = 9 neurons), n.s., p > 0.05, *p < 0.05, *** p < 0.001, One-way ANOVA (post hoc comparison). (F) mEPSCs frequency of control+ ACSF (n = 10 neurons), control+ cLTP (n = 9 neurons), shkif2c + ACSF (n = 7 neurons), shkif2c + cLTP (n = 9 neurons), n.s., p > 0.05, *p < 0.05, One-way ANOVA (post hoc comparison).
-
Figure 2—figure supplement 1—source data 1
Values for KIF2C protein levels.
Values for mEPSCs of control and shkif2c neurons; Values for kif2c mRNA levels; Values for mEPSCs of control and shkif2c neurons before and after cLTP.
- https://cdn.elifesciences.org/articles/72483/elife-72483-fig2-figsupp1-data1-v1.xlsx
-
Figure 2—figure supplement 1—source data 2
Original files of blots with the relevant bands.
- https://cdn.elifesciences.org/articles/72483/elife-72483-fig2-figsupp1-data2-v1.zip
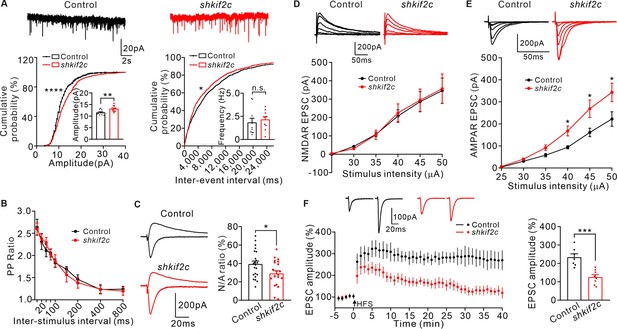
Knockdown KIF2C impairs synaptic transmission and plasticity.
(A) mEPSCs recorded from control and shkif2c CA1 pyramidal neurons. n = 10 per group, n.s., p > 0.05, **p < 0.01, Student’s t-test; For the cumulative probability curves, *p < 0.05, ****p < 0.0001, Kolmogorov-Smirnov test. (B) Paired-pulse stimuli evoked EPSCs with different interval. n = 9 neurons per group. The data were analyzed in separate t-tests at each stimulus intensity; n.s., p > 0.05. (C) AMPAR and NMDAR EPSCs in CA1 pyramidal neurons. Sample sweeps illustrating NMDAR-EPSCs recorded at +40 mV and AMPAR-EPSCs recorded at –70 mV (left). Histograms show ratio of NMDAR-EPSCs amplitude / AMPAR-EPSCs amplitude (right). n = 22 neurons per group; *p < 0.05, Student’s t-test. (D) Sample traces and input-output curve of NMDA receptor-mediated EPSCs of control and shkif2c neurons (n = 6, p > 0.05). The data were analyzed in separate t-tests at each stimulus intensity. (E) Sample traces and input-output curve of AMPA receptor-mediated EPSCs between control and shkif2c neurons (n = 6, *p < 0.05). The data were analyzed in separate t-tests at each stimulus intensity. (F) Example EPSCs before (baseline) and after (t = 40 min) 2× HFS stimulation in control and shkif2c CA1 pyramidal neurons. Time course of percentage changes of EPSCs amplitudes in control and shkif2c CA1 pyramidal neurons(left). Histogram shows EPSC amplitude of control and shkif2c neurons (35–40 min) (right). ***p < 0.001; Student’s t-test.
-
Figure 3—source data 1
Values for mEPSCs of control and shkif2c neurons.
Values for PPR ratio; Values for input-output curve of AMPAR and NMDAR mediated EPSCs between control and shkif2c neurons; Values for percentage changes of EPSCs amplitudes in control and shkif2c CA1 pyramidal neurons.
- https://cdn.elifesciences.org/articles/72483/elife-72483-fig3-data1-v1.xlsx
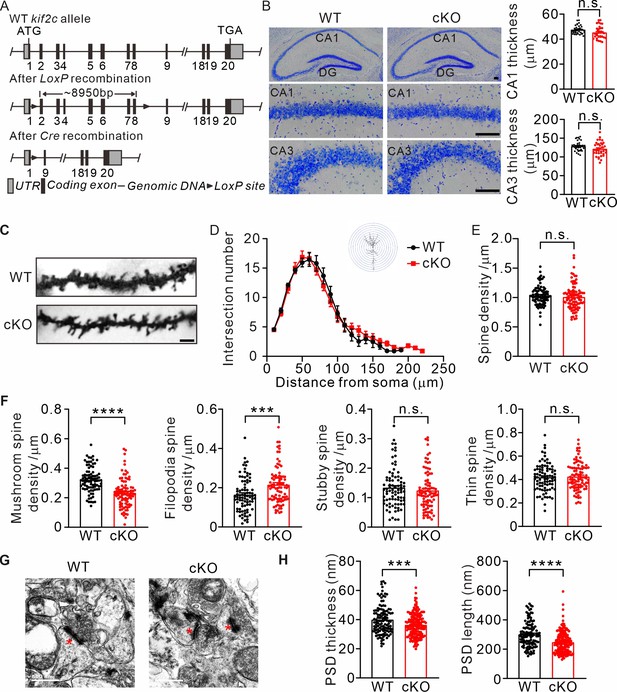
Abnormal spine formation in KIF2C conditional knockout mice.
(A) Targeting strategy to generate kif2cflox/flox mice. Two LoxP sites were inserted into intron 1–2 and 8–9. (B) Nissl staining of adult mice brain coronal sections and magnified images of hippocampal CA1 and CA3 regions. Scale bar, 50 μm. n = 4 mice per genotypes; n.s., p > 0.05, Student’s t-test. (C–F) Golgi staining of WT and cKO hippocampus CA1 pyramidal neuron. n = 4 mice per genotypes; Scale bar, 2 μm. (D) Sholl analysis of dendritic arborization. The number of intersections related to distance from soma was quantified. p > 0.05, Two-way ANOVA RM and post hoc comparisons. (E) Histograms show spine number per 1 μm of WT and cKO CA1 pyramidal neuron. p = 0.3809. Student’s t-test. (F) The density of four types of spines in WT and cKO CA1 pyramidal neuron. Mushroom spine density was 0.32 ± 0.009 μm–1 (WT) and 0.23 ± 0.01 μm–1 (cKO) (****p < 0.0001). Filopodia spine density was 0.16 ± 0.009 μm–1 (WT) and 0.22 ± 0.01 μm–1 (cKO) (***p < 0.001). Stubby spine density was 0.12 ± 0.009 μm–1 (WT) and 0.13 ± 0.01 μm–1 (cKO) (p = 0.3104). Thin spine density was 0.41 ± 0.009 μm–1 (WT) and 0.43 ± 0.01 μm–1 (cKO) (p = 0.5511). Student’s t-test. (G) Transmission EM of synapse in hippocampal CA1 region from WT and KIF2C cKO mice (n = 3 mice per genotype) at 6–8 weeks. Scale bar, 500 nm. (H) Quantification of PSD thickness and length. PSD thickness: 40.02 ± 0.93 nm (WT) and 36.37 ± 0.58 nm (cKO). PSD length: 301.4 ± 6.8 nm (WT) and 247.7 ± 5.4 nm (cKO). ***p < 0.001; ****p < 0.0001; Student’s t-test.
-
Figure 4—source data 1
Values for CA1 and CA3 thickness in WT and cKO mice; Values for Sholl analysis and spine density; Values for PSD thickness and length.
- https://cdn.elifesciences.org/articles/72483/elife-72483-fig4-data1-v1.xlsx
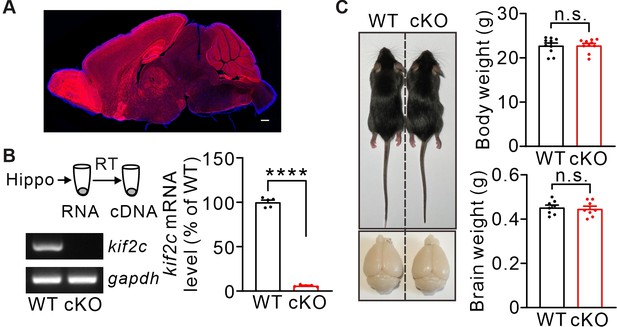
kif2cflox/flox;NestinCre mice.
(A) kif2cflox/flox;NestinCre mouse was crossed with Ai9 reporter mouse lines and the expression of Cre-recombinase was characterized by observing tdTomato reporter in Ai9;NestinCre mice (1 month). We found that tdTomato fluorescence was present in the cortex, hippocampus, cerebellum and many other brain regions. Scale bar: 500 μm. (B) Electrophoresis of kif2c (387 bp), and gapdh (220 bp) amplicons from individual WT and cKO hippocampus tissues. Right histograms show percentage changes of kif2c mRNA levels. n = 5, ****p < 0.0001, Student’s t-test. (C) cKO mice (2 month) displayed normal body weight and brain size. Body weight: WT, 22.77 ± 0.6 g; cKO, 22.8 ± 0.5 g, n = 10 per genotype. n.s., p > 0.05, Student’s t-test. Brain weight: WT, 0.45 ± 0.01 g; cKO, 0.44 ± 0.01 g, n = 8 per genotype. n.s., p > 0.05, Student’s t-test.
-
Figure 4—figure supplement 1—source data 1
Values for kif2c mRNA levels; Values for brain weight and body weight.
- https://cdn.elifesciences.org/articles/72483/elife-72483-fig4-figsupp1-data1-v1.xlsx
-
Figure 4—figure supplement 1—source data 2
Original files of gels with the relevant bands.
- https://cdn.elifesciences.org/articles/72483/elife-72483-fig4-figsupp1-data2-v1.zip
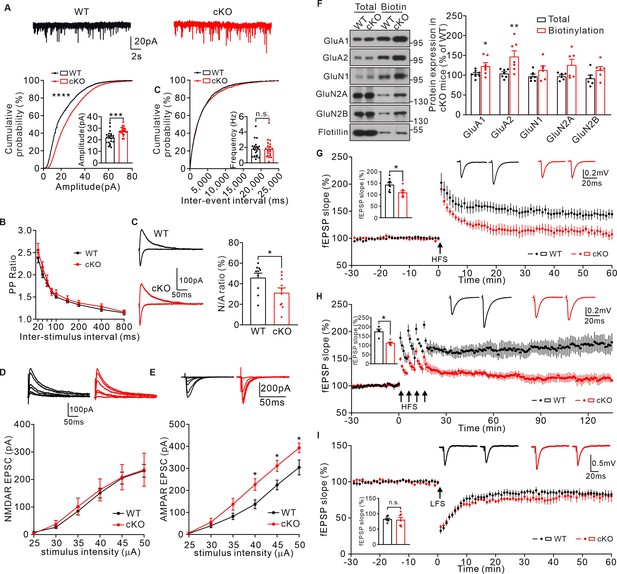
KIF2C deficiency impairs synaptic transmission and plasticity.
(A) mEPSCs recorded from WT and cKO CA1 pyramidal neurons. n = 21 neurons per group; ***p < 0.001, Student’s t-test; For the cumulative probability curves, n.s. p > 0.05, ****p < 0.0001, Kolmogorov-Smirnov test. (B) Paired-pulse stimuli evoked EPSPs with different interval. n = 9 neurons per group, p > 0.05. The data were analyzed in separate t-tests at each stimulus intensity. (C) AMPAR and NMDAR EPSCs in CA1 pyramidal neurons of WT and cKO mice. Sample sweeps illustrating NMDAR-EPSCs recorded at +40 mV and AMPAR-EPSCs recorded at −70 mV. Histograms show ratio of NMDAR-EPSCs amplitude / AMPAR-EPSCs amplitude (right). n = 10 neurons, *p < 0.05. Student’s t-test. (D) Sample traces and input-output curve of NMDA receptor-mediated EPSCs between WT and cKO mice (n = 6 neurons, p > 0.05). The data were analyzed in separate t-tests at each stimulus intensity. (E) Sample traces and input-output curve of AMPA receptor-mediated EPSCs between WT and cKO mice (n = 6 neurons, *p < 0.05). The data were analyzed in separate t-tests at each stimulus intensity. (F) Cell-surface biotinylation shows a significant increase in GluA1 and GluA2 subunit surface expression in cKO hippocampus compared with WT. Histograms show percent change of GluA1, GluA2, GluN1, GluN2A, GluN2B subunits in total and biotinylation fraction. Flotillin was the internal control. n = 7 experiment repeats, *p < 0.05, **p < 0.01, Student’s t-test. (G) Example fEPSP before (baseline) and after (t = 60 min) 1× HFS stimulation in WT and cKO CA1 slices. Time course of percentage changes of fEPSP slope in WT and cKO CA1 slices. WT, n = 9 slices from 7 mice; cKO, n = 7 slices from 6 mice. Histogram shows fEPSP slope of WT and cKO neurons (55–60 min). *p < 0.05, Student’s t-test. (H) Example fEPSP before (baseline) and after (t = 135 min) 4× HFS stimulation in WT and cKO CA1 slices. Time course of percentage changes of fEPSP slope in WT and cKO CA1 slices. WT, n = 4 slices from 4 mice; cKO, n = 5 slices from 4 mice. Histogram shows fEPSP slope of WT and cKO neurons (130–135 min). *p < 0.05, Student’s t-test. (I) Time course of percentage changes of fEPSP slope before (baseline) and after low frequency stimulus (LFS) in control and cKO CA1 slices. WT, n = 7 slices from 5 mice; cKO, n = 7 slices from 6 mice. Histogram shows fEPSP slope of WT and cKO neurons (55–60 min). p = 0.7642, Student’s t-test.
-
Figure 5—source data 1
Values for mEPSCs of WT and cKO neurons.
Values for PPR ratio; Values for input-output curve of AMPAR and NMDAR mediated EPSCs between WT and cKO neurons; Values for NMDAR and AMPAR subunit surface expression; Values for percentage changes of fEPSCs amplitudes in WT and cKO neurons.
- https://cdn.elifesciences.org/articles/72483/elife-72483-fig5-data1-v1.xlsx
-
Figure 5—source data 2
Original files of blots with the relevant bands.
- https://cdn.elifesciences.org/articles/72483/elife-72483-fig5-data2-v1.zip
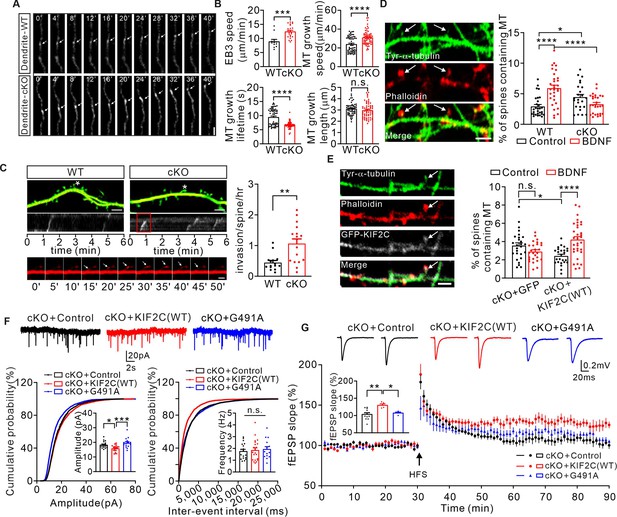
Abnormal MT dynamics causes deficit in synaptic transmission and plasticity.
(A) Time-lapse recordings of EB3-tdtomato-infected neurons showing EB3-tdtomato comets in dendrite of WT and cKO hippocampus neuron. Subsequent images show low-pass-filtered time series in row. Scale bar is 1 μm, time in seconds. (B) Analysis of the EB3 comet dynamics in dendrites of WT and cKO hippocampus neuron. n = 58 per group from seven independent experiments. Histograms show EB3 speed velocity (***p < 0.001), MT growth speed (****p < 0.0001), MT growth lifetime (****p < 0.0001) and MT growth length (p = 0.2850). Student’s t-test. (C) Images of dendrites from WT and cKO cultured hippocampal neurons transfected with EB3-tdtomato (red) and GFP (green). Scale bar, 5 μm. Spines labeled with ‘‘*’’ were depicted in the kymograph. The invasion shown in the red boxed region of kymograph is depicted below. Sequential frames showed an MT entering the labeled spine of cKO neuron. Scale bar,1 μm. Histograms show MT-spine invasion frequency (invasions/spine/hour). (WT: 0.45 ± 0.07, n = 15; cKO: 1.07 ± 0.15, n = 17. **p < 0.01). Student’s t-test. (D) Tyrosinated α-tubulin antibody-labeled MTs (white arrows) were detected in a small percentage of phalloidin (F-actin) -labeled spines under basal conditions (DIV 18–20). Quantification of the number of spines containing MTs before and after BDNF treatment. Scale bar, 5 μm. 2.9% ± 0.32% (WT, n = 29), 5.9% ± 0.5% (WT+ BDNF, n = 31), 4.5% ± 0.46% (cKO, n = 24), 3.3% ± 0.29% (cKO+ BDNF, n = 30). *p < 0.05, ****p < 0.0001, One-way ANOVA (post hoc comparison). (E) DIV 18–20 cKO hippocampus neurons transfected with GFP vector or GFP-KIF2C labeled with GFP (white), tyrosinated α-tubulin (green) and phalloidin (red). Quantification of the number of spines containing MTs before and after BDNF treatment. Scale bar, 2 μm. 3.6% ± 0.31% (cKO+ GFP + control, n = 27), 2.9% ± 0.20% (cKO+ GFP + BDNF, n = 27), 2.4% ± 0.19% (cKO+ KIF2 C(WT)+ control, n = 25), 4.2% ± 0.34% (cKO+ KIF2 C(WT)+ BDNF, n = 33). *p < 0.05, ****p < 0.0001, One-way ANOVA (post hoc comparison). (F) mEPSCs recorded from control (n = 18 neurons), KIF2C(WT) (n = 21 neurons) and KIF2C(G491A) (n = 15 neurons) expressed in cKO CA1 pyramidal neurons. n.s. p > 0.05, *p < 0.05, ***p < 0.001, One-way ANOVA (post hoc comparison). (G) Example fEPSP before (baseline) and after (t = 60 min) 1× HFS stimulation in cKO+ control, cKO+ KIF2 C(WT) and cKO+ KIF2 C(G491A) CA1 slices. Time course of percentage changes of fEPSP slope in cKO+ control, cKO+ KIF2 C(WT) and cKO+ KIF2 C(G491A) CA1 pyramidal neurons. cKO+ control, n = 13 slices from 10 mice; cKO+ KIF2 C(WT) and cKO+ KIF2 C(G491A), n = 5 slices from 4 mice. Histogram shows fEPSP slope of cKO+ control, cKO+ KIF2 C(WT) neurons and cKO+ KIF2 C(G491A) (55–60 min). *p < 0.05, **p < 0.01; One-way ANOVA (post hoc comparison).
-
Figure 6—source data 1
Values for EB3 speed velocity, MT growth speed, MT growth lifetime, and MT growth length.
Values for MT invasion frequency; Values for number of spines containing MTs before and after BDNF treatment; Values for mEPSCs of cKO+ control, cKO+ KIF2 C(WT), and cKO+ KIF2 C(G491A); Values for percentage changes of fEPSCs amplitudes of cKO+ control, cKO+ KIF2 C(WT), and cKO+ KIF2 C(G491A).
- https://cdn.elifesciences.org/articles/72483/elife-72483-fig6-data1-v1.xlsx
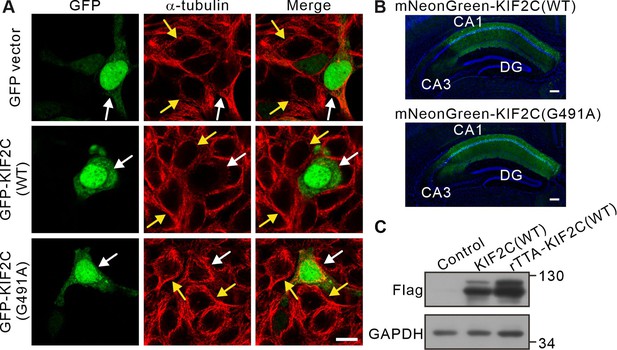
KIF2C(WT) and KIF2C(G491A) expression.
(A) GFP vector, GFP-KIF2C(WT), and GFP-KIF2C(G491A) mutant were transfected into HEK 293T cells and α-tubulin was stained. White arrow points to transfected cells, yellow arrows point to intact cells. Scale bar: 10 μm. (B) Coronal section of the CA1 region image after mNeonGreen-KIF2C(WT) and mNeonGreen-KIF2C(G491A) virus injection. Scale bar: 200 μm. (C) Expression of Flag-KIF2C(WT) in CA1 region total protein, GAPDH was the internal control.
-
Figure 6—figure supplement 1—source data 1
Original files of blots with the relevant bands.
- https://cdn.elifesciences.org/articles/72483/elife-72483-fig6-figsupp1-data1-v1.zip
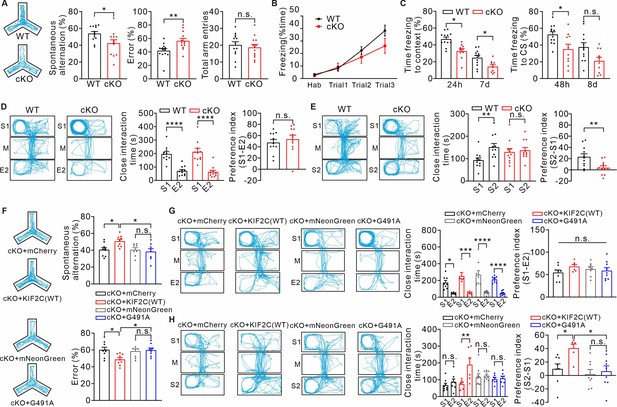
KIF2C cKO mice exhibit abnormal cognitive behaviors.
(A) Y maze test from WT and cKO. n = 13 per genotype. n.s., p > 0.05, *p < 0.05, **p < 0.01, Student’s t-test. (B–C) Cued fear conditioning test. (B), WT and cKO mice showed no significant differences of freezing response to the tone across three conditional stimulus (CS)-unconditional stimulus (US) pairings. p > 0.05; Two-way ANOVA. (C), Expression of conditioned freezing response to the background context or CS at indicated time. WT: n = 12; cKO: n = 10. *p < 0.05, Two-way ANOVA RM comparisons. (D–E) Three-chamber test. (D), WT and cKO mice spent more time with stranger one in sociability test. n.s., p > 0.05, ****p < 0.0001. Student’s t-test. (E), cKO mice did not display a preference for stranger two in social novelty test. n = 11 per genotype. n.s., p > 0.05, **p < 0.01, Student’s t-test. (F) cKO mice with KIF2C(WT) re-expression displayed a normal alternation in the Y-maze test. cKO mice with KIF2C(G491A) re-expression displayed similar deficiency in the Y-maze test. cKO+ mCherry, n = 9; cKO+ KIF2 C(WT), n = 9; cKO+ mNeonGreen, n = 8; cKO+ KIF2 C(G491A), n = 8. n.s., p > 0.05, *p < 0.05, One-way ANOVA (post hoc comparison). (G–H) KIF2C(WT) re-expression in CA1 can restore social novelty in cKO mice. cKO mice with KIF2C(G491A) expression displayed similar deficiency in social novelty test. cKO+ mCherry: n = 8; cKO+ KIF2 C(WT): n = 6; cKO+ mNeonGreen: n = 8; cKO+ KIF2 C(G491A): n = 9. n.s., p > 0.05, *p < 0.05, ***p < 0.001. One-way ANOVA (post hoc comparison).
-
Figure 7—source data 1
Values for Y-maze test of WT and cKO mice.
Values for freezing levels during fear conditioning test of WT and cKO mice; Values for close interaction time during three-chamber test of WT and cKO mice; Values for Y-maze test of cKO+ mCherry, cKO+ KIF2 C(WT), cKO+ mNeonGreen, and cKO+ KIF2 C(G491A) mice; Values for close interaction time during three-chamber test of cKO+ mCherry, cKO+ KIF2 C(WT), cKO+ mNeonGreen, and cKO+ KIF2 C(G491A) mice.
- https://cdn.elifesciences.org/articles/72483/elife-72483-fig7-data1-v1.xlsx
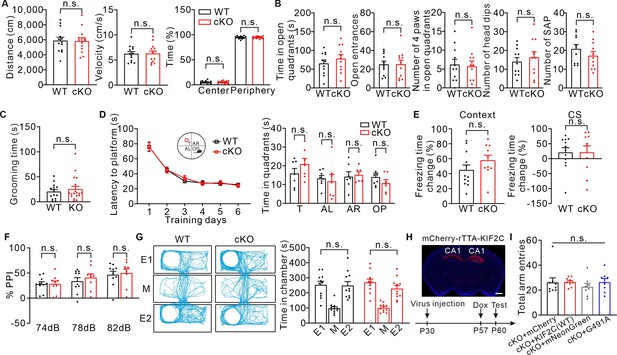
WT and KIF2C cKO mice behavioral tests.
(A) Open field test from WT and cKO mice. Quantification of velocity, distance of activities and exploration time. n = 13 per genotype. Student’s t-test. (B) Elevated-zero maze test. Time in open quadrants, open entrances, number of 4 paws in open quadrants, number of head dips and number of stretch attendance postures (SAP) were calculated. n = 11 per genotype. Student’s t-test. (C) Self-grooming test. WT: n = 15; cKO: n = 18. Student’s t-test. (D) WT and cKO mice displayed similar latency to platform over training days in Morris water maze test. T, target; AL, adjacent left; AR, adjacent right; OP, opposite. n.s., p > 0.05; Two-way ANOVA. (E) The percentage change in freezing time in two consecutive times ((Freezing time24h- Freezing time7d)/ Freezing time24h to contextual cue and (Freezing time48h- Freezing time8d)/ Freezing time48h to conditioned stimulus) showed no significant difference between WT (n = 12) and cKO (n = 10) mice. n.s., p > 0.05. Student’s t-test. (F) Prepulse inhibition (PPI) test. Pulse intensity was set to 120 dB. WT: n = 10; cKO: n = 9. Data are represented as means ± SEMs. n.s., p > 0.05, Student’s t-test. (G) Both WT and KIF2C cKO mice show no position preference in habituation stage. n.s., p > 0.05. Student’s t-test. (H) Time line of virus injection, doxycycline (Dox) administration and behavior test. Coronal image of rAAV-TRE3G- mcherry-KIF2C re-expression in CA1. Scale bar: 800 μm. (I) cKO mice with KIF2C(WT) or KIF2C(G491A) re-expression displayed a normal arm entrance in Y-maze test. cKO+ mCherry, n = 9; cKO+ KIF2 C(WT), n = 9; cKO+ mNeonGreen, n = 8; cKO+ KIF2 C(G491A), n = 8. n.s., p > 0.05, One-way ANOVA (post hoc comparison).
-
Figure 7—figure supplement 1—source data 1
Values for moving distance, velocity and center zone exploration time in the open-field test.
Values for time in open quadrants, open entrances, number of 4 paws in open quadrants, number of head dips and number of stretch attendance postures (SAP) during elevated-zero maze test; Values for grooming time; Values for latency to platform and time in quadrants during Morris water maze; Values for freezing time change during fear conditioning test; Values for PPI; Values for time in each chamber during three-chamber test; Values for arm entries during Y-maze test.
- https://cdn.elifesciences.org/articles/72483/elife-72483-fig7-figsupp1-data1-v1.xlsx
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Mus musculus, male/female) | C57BL/6 | Shanghai SLAC Laboratory Animal C.,Ltd | ||
| Strain, strain background (Mus musculus, male/female) | kif2cflox/flox mouse | Model Animal Research Center of Nanjing University | ||
| Strain, strain background (Mus musculus, male/female) | NestinCre mouse | Zhejiang University | Wang et al., 2019 | |
| Cell line (Homo-sapiens) | HEK 293T | ATCC | CRL-3216 | |
| Genetic reagent (virus) | pAKD-CMV-bGlobin-mcherry-H1-shRNA | ObioTechnology | ||
| Genetic reagent (virus) | pAAV-CMV-KIF2C-3FLAG-P2A-mNeonGreen-CW3SL | ObioTechnology | ||
| Genetic reagent (virus) | pAAV-CMV-KIF2C(G491A)–3FLAG-P2A-mNeonGreen-CW3SL | ObioTechnology | ||
| Genetic reagent (virus) | rAAV-TRE3G- KIF2C-3*Flag –2A-mCherry-WPRE-pA | BrainVTA | ||
| Antibody | Anti-KIF2C, (rabbit polyclonal) | Proteintech | Cat# 12139–1-AP, RRID:AB_2877829 | IF (1:500), WB (1:500) |
| Antibody | Anti-PSD-95, (mouse monoclonal) | Abcam | Cat# ab2723, RRID:AB_303248 | IF (1:1000) WB (1:1000) |
| Antibody | Anti-MAP2, (chicken polyclonal) | Abcam | Cat# ab5392, RRID:AB_2138153 | IF (1:2000) |
| Antibody | anti-GFP (rabbit polyclonal) | Abcam | Cat# ab6556, RRID:AB_305564 | IF (1:2000) |
| Antibody | Anti-α-tubulin, (rabbit monoclonal) | Abcam | Cat# ab52866, RRID:AB_869989 | IF (1:1000) |
| Antibody | Anti-mCherry, (rabbit polyclonal) | Abcam | Cat# ab167453, RRID:AB_2571870 | WB (1:2000) |
| Antibody | Anti-EB3, (rabbit monoclonal) | Abcam | Cat# ab157217, RRID:AB_2890656 | WB (1:10,000) |
| Antibody | Anti-α-tubulin, tyrosinated, (rat monoclonal) | Millipore | Cat# MAB1864-I, RRID:AB_2890657 | IF (1:1000) |
| Antibody | Anti-synaptophysin, (mouse monoclonal) | Millipore | Cat# MAB368, RRID:AB_94947 | IF (1:1000) WB (1:2000) |
| Antibody | Anti-GAPDH, (mouse monoclonal) | Millipore | Cat# MAB374, RRID:AB_2107445 | WB (1:10,000) |
| Antibody | Anti-GluA1, (rabbit monoclonal) | Millipore | Cat# 04–855, RRID:AB_1977216 | WB (1:1000) |
| Antibody | Anti-GluA2, (mouse monoclonal) | Millipore | Cat# MAB397, RRID:AB_2113875 | WB (1:2000) |
| Antibody | Anti-GluN2A, (rabbit polyclonal) | Cell Signaling Technology | Cat# 4205, RRID:AB_2112295 | WB (1:2000) |
| Antibody | Anti- GluN2B, (rabbit polyclonal) | Cell Signaling Technology | Cat# 4207, RRID:AB_1264223 | WB (1:2000) |
| Antibody | Anti-GluN1, (rabbit polyclonal) | Cell Signaling Technology | Cat# 5704, RRID:AB_1904067 | WB (1:2000) |
| Antibody | Anti-flotillin, (mouse monoclonal) | BD Bioscience | Cat# 610820, RRID:AB_398139 | WB (1:1000) |
| Antibody | Anti-β-tubulin, a (mouse monoclonal) | Santa Cruz | Cat# sc-5274, RRID:AB_2288090 | WB (1:2000) |
| Antibody | Anti- bassoon, (mouse monoclonal) | Enzo Life Sciences | Cat# ADI-VAM-PS003-F, RRID:AB_11181058 | IF (1:1000) |
| Antibody | Anti- N-cadherin, (rabbit polyclonal) | Bioworld Technology | Cat# BS2224, RRID:AB_1664028 | WB (1:1000) |
| Chemical compound, drug | CNQX | Sigma-Aldrich | Cat# C239; CAS: 115066-14-3 | 20 μM |
| Chemical compound, drug | AP-V | Sigma-Aldrich | Cat# A8054; CAS: 79055-68-8 | 50 μM |
| Chemical compound, drug | Doxycycline Hyclate | Selleck | Cat# S4163; CAS:24390-14-5 | 40 μg/ml |
| Chemical compound, drug | Glycine | Sigma-Aldrich | Cat# G8898; CAS:56-40-6 | 200 μM |
| Chemical compound, drug | bicuculline | Abcam | Cat# ab120108; CAS: 40709-69-1 | 20 μM |
| Chemical compound, drug | Picrotoxin | Sigma-Aldrich | Cat# P1675; CAS: 124-87-8 | 100 μM |
| Commercial assay or kit | SYBR Green qPCR Master Mix | QIAGEN | Cat# 204,057 | |
| Commercial assay or kit | Nissl staining Kit | Beyotime | Cat# C0117 | |
| Commercial assay or kit | FD Rapid GolgiStain Kit | FD NeuroTechnologies | Cat# PK401 | |
| Commercial assay or kit | BCA Protein Assay Kit | Thermo Fisher | Cat# 23,227 | |
| Software, algorithm | GraphPad Prism (8.0) | GraphPad Software | https://www.graphpad.com/scientific-software/prism/ | RRID: SCR_002798 |
| Software, algorithm | ImageJ | National Institutes of Health | https://imagej.nih.gov/ij/ | RRID:SCR_003070 |
| Software, algorithm | ANY-maze tracking software | Stoelting Co | https://www.stoeltingco.com/anymaze.html | RRID:SCR_014289 |
| Software, algorithm | PlusTipTracker | UTSouthwestern Medical Center Danuser Lab | https://www.utsouthwestern.edu/labs/danuser/software/ | |
| Software, algorithm | Sigmaplot | systatsoftware | http://www.systatsoftware.cn/ | RRID:SCR_003210 |
| Software, algorithm | MATLAB R2017b | MathWorks | https://se.mathworks.com/products/matlab.html | RRID: SCR_001622 |
| Sequence-based reagent | KIF2C floxP _F | This paper | PCR primers | GGT CCA GCT CTT TAC TGA TGT GTT C |
| Sequence-based reagent | KIF2C floxP _R | This paper | PCR primers | ACA AAG CAA GTC CAG GTC CAA G |
| Sequence-based reagent | Nestin-Cre_F | This paper | PCR primers | TGC AAC GAG TGA TGA GGT TC |
| Sequence-based reagent | Nestin-Cre_R | This paper | PCR primers | GCT TGC ATG ATC TCC GGT AT |
| Sequence-based reagent | kif2c_F | This paper | PCR primers | TGC CGT TGT TGA TGG TCA GTG |
| Sequence-based reagent | kif2c_R | This paper | PCR primers | GGA GAC ACT TGC TGG GAA CAG |
| Sequence-based reagent | shkif2c_F | This paper | PCR primers | TGG ATC GAA GGA GGT ACC AC |
| Sequence-based reagent | shkif2c_R | This paper | PCR primers | CAC TGA CCA TCA ACA ACG GCA |
| Sequence-based reagent | gapdh_F | This paper | PCR primers | AGG TCG GTG TGA ACG GAT TTG |
| Sequence-based reagent | gapdh_R | This paper | PCR primers | TGT AGA CCA TGT AGT TGA GGT CA |
| Peptide, recombinant protein | BDNF | Sigma-Aldrich | Cat# GF301 | 50 ng/mL |
Additional files
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/72483/elife-72483-transrepform1-v1.docx
-
Source data 1
All original files of blots and gels with the relevant bands.
- https://cdn.elifesciences.org/articles/72483/elife-72483-data1-v1.zip





