Amino acid transporter SLC38A5 regulates developmental and pathological retinal angiogenesis
Figures
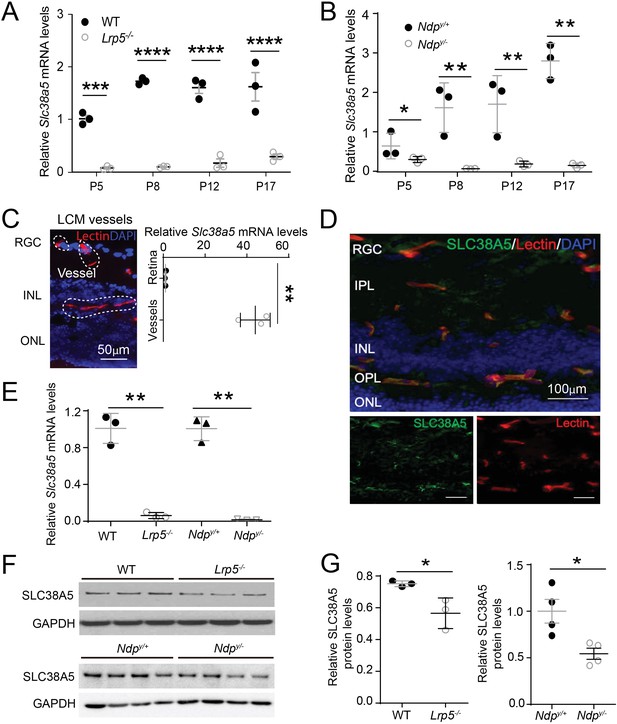
Scl38a5 expression is enriched in retinal blood vessels and down-regulated in the retinas and retinal blood vessels of Wnt signaling deficient Lrp5−/− and Ndpy/− mice.
(A–B) mRNA levels of Slc38a5 were measured by RT-PCR in Lrp5−/− (A) and Ndpy/− (B) retinas compared with their respective wild type (WT) controls during development at postnatal day 5 (P5), P8, P12, and P17. (C) Retinal blood vessels stained with isolectin B4 (red, outlined by the white dashed lines) were isolated by laser-captured microdissection (LCM) from retinal cross-sections. Cell nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole, blue) for illustration purpose only. LCM retinal samples were stained with only isolectin B4 without DAPI. Slc38a5 mRNA levels in LCM isolated retinal blood vessels were compared with the whole retinal levels using RT-qPCR. Scale bars: 50 µm. (D) Immunohistochemistry of retinal cross sections from WT eyes shows colocalization of SLC38A5 antibody (green) in retinal blood vessels stained with isolectin (red), counterstained with nuclear staining DAPI (blue). (E) Slc38a5 mRNA levels in LCM-isolated Lrp5−/− and Ndpy/− retinal blood vessels were quantified with RT-qPCR and compared with their respective WT controls. (F–G) Protein levels of SLC38A5 (52 kDa) in P17 Lrp5−/− and Ndpy/− retinas and their WT controls were quantified with Western blot (F) and normalized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 37 kDa) levels (G). RGC: retinal ganglion cells, IPL: inner plexiform layer, INL: inner nuclear layer, OPL: outer plexiform layer, ONL: outer nuclear layer. Data are expressed as mean ± SEM. n=3–4 per group. *p≤0.05, **p≤0.01, ****p≤0.0001.
-
Figure 1—source data 1
Raw data for Figure 1.
- https://cdn.elifesciences.org/articles/73105/elife-73105-fig1-data1-v1.zip
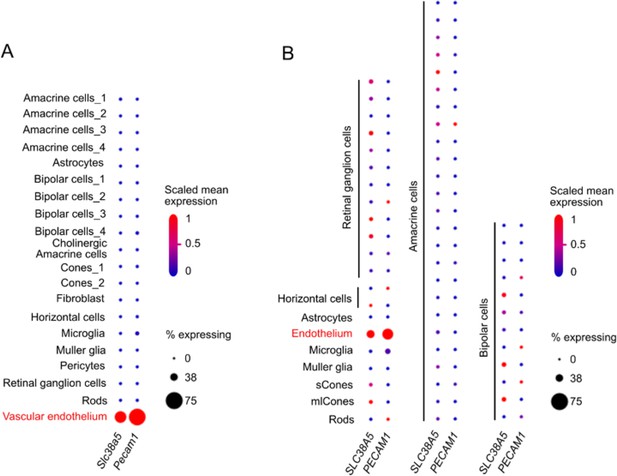
Distinct expression of Slc38a5 in vascular endothelium in mouse and human retina with single-cell transcriptomics.
(A) Dot plot of Slc38a5 and endothelial cell marker Pecam1 gene expression (scaled) for different retinal cell types in P14 C57BL/6 J mouse retinas. Slc38a5 was distinctly expressed in vascular endothelium cluster. Data source: Study - P14 C57BL/6 J mouse retinas (https://singlecell.broadinstitute.org/single_cell/study/SCP301) (Macosko et al., 2015). (B). Dot plot of SLC38A5 and endothelial cell marker PECAM1 gene expression (scaled) for different retinal cell types at human fovea and peripheral retina. Slc38a5 was highly expressed in endothelium cluster. Data source: Study - Cell atlas of the human fovea and peripheral retina (https://singlecell.broadinstitute.org/single_cell/study/SCP839) (Yan et al., 2020). Scaling is relative to each gene’s expression across all cells of a cluster. Gene expression is scaled from 0 to 1 (0.5=the mean across all cells in the cluster file referenced for dot plotting). % expressing is the percent of cells that have one or more transcripts for the gene of interest.
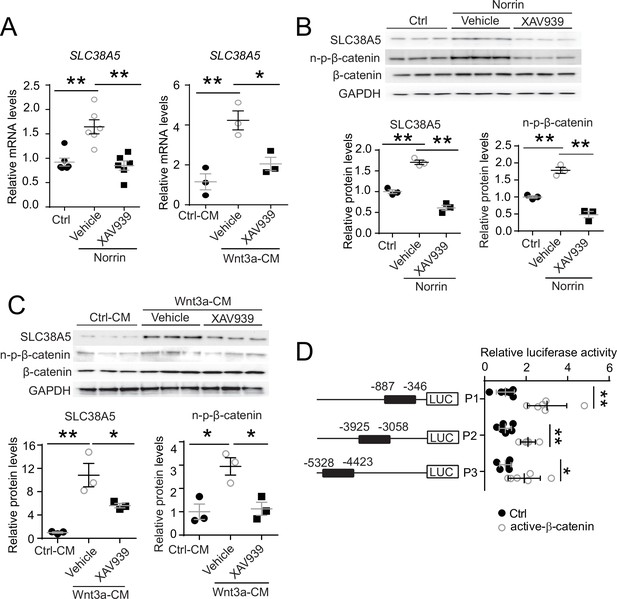
Slc38a5 is a target gene of Wnt signaling in the vascular endothelium.
(A) Slc38a5 mRNA levels were increased in human retinal microvascular endothelial cells (HRMECs) treated with Wnt ligands, recombinant Norrin, and Wnt3a-conditioned medium (Wnt3a-CM), compared with their respective vehicle controls (Ctrl, and Ctrl-CM), and suppressed by a Wnt inhibitor XAV939. (B–C) Protein levels of SLC38A5 in HRMECs were up-regulated by Wnt ligands Norrin (B) and Wnt3a-CM (C), and down-regulated by XAV939. Protein levels of SLC38A5 (52 kDa) and β-catenin (92 kDa) were quantified by Western blotting and normalized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 37 kDa) levels. n-p-β-catenin: non-phosphorylated β-catenin (92 kDa). (D) Three promoter regions upstream of Slc38a5 gene containing potential Wnt-responsive TCF (T-cell factor)-binding motifs (TTCAAAG) was identified based on sequence analysis. Three putative TCF-binding regions: P1 (–887 bp to –346 bp), P2 (–3925 bp to –3058 bp), and P3 (–5328 bp to –4423 bp) were cloned and ligated separately with a luciferase reporter, and co-transfected with an active β-catenin plasmid in HEK 293T cells, followed by measurement of luciferase activity. Data are expressed as mean ± SEM. (A-C) n=3–6 per group. (D) n=5 per construct group. *p≤0.05, **p≤0.01.
-
Figure 2—source data 1
Raw data for Figure 2.
- https://cdn.elifesciences.org/articles/73105/elife-73105-fig2-data1-v1.zip
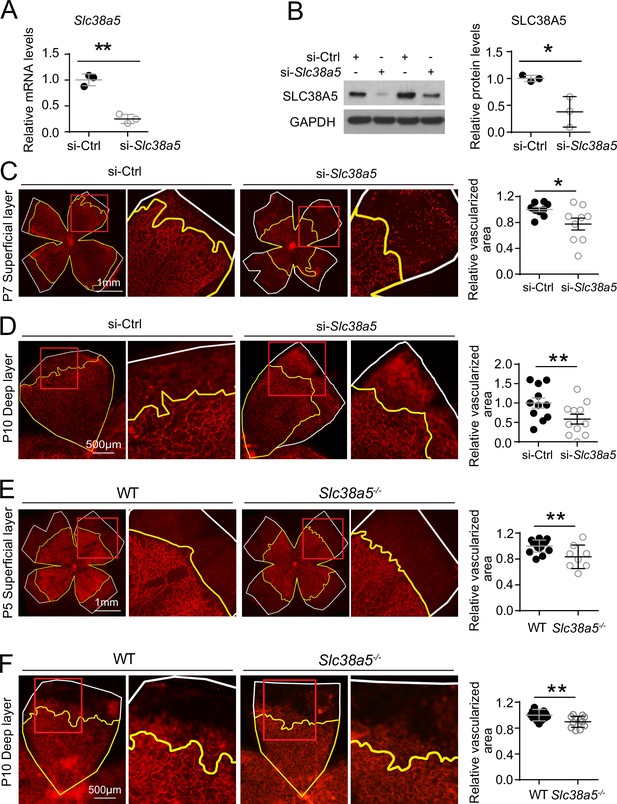
Genetic deficiency of Slc38a5 impairs developmental retinal angiogenesis in vivo.
(A-D) siRNA targeting Slc38a5 (si-Slc38a5) was intravitreally injected in C57BL/6J mice , and the same volume of negative control siRNA (si-Ctrl) was injected into the contralateral eyes. Mice were sacrificed 3 days after injection, and retinas were isolated to detect expression level or to quantify vascular growth. mRNA (A) and protein (B) levels of SLC38A5 (52 kDa) confirm successful knockdown. Proteins were normalized to GAPDH (37 kDa). Each lane represents one retina. Retinal vascular coverage of superficial layer at P7 (C) and deep layer at P10 (D) was analyzed 3 days after intravitreal injection of si-Slc38a5 and compared with their respective controls. Retinas were dissected, stained with isolectin B4 (red), and then flat-mounted to visualize the vasculature. Percentages of vascularized area were quantified in superficial (C, n=9/group) or deep (D, n=11/group) vascular layer. (E&F) Retinal blood vessel development in Slc38a5−/− and WT littermate control mice from the same colony was imaged and quantified at P5 (E, n=8–11/group) and P10 (F, n=12–13/group), with staining of isolectin B4 (red) to visualize the vasculature. In panels C–F, yellow lines outline retinal vascular areas, and white lines indicate total retinal areas. Red boxes indicate location of enlarged insets as shown on the right. Each dot represents one retina. Data are expressed as individual value and mean ± SEM. *p≤0.05, **p≤0.01.
-
Figure 3—source data 1
Raw data for Figure 3.
- https://cdn.elifesciences.org/articles/73105/elife-73105-fig3-data1-v1.zip
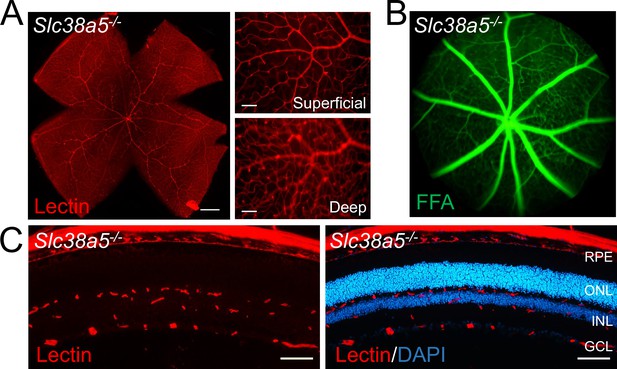
Adult Slc38a5−/− retinas appear normal with intact vascular barrier.
(A) Flat mounts of adult Slc38a5−/− retinas appear to have normal branching and structure of retinal vessels stained with isolectin B4 (red). (B) Fundus fluorescein angiography (FFA) of adult Slc38a5−/− mice shows no sign of vascular leakage of fluorescein (green), indicating intact retinal vessels barrier in Slc38a5−/− eyes. (C) Cross sections of Slc38a5−/− eyes show three normal layers of retinal vessels stained with isolectin B4 (red) and DAPI (blue). RPE: retinal pigment epithelium, ONL: outer nuclear layer, INL: inner nuclear layer, GCL: ganglion cell layer. Scale bars: A, left: 500 µm, right: 100 µm; C, 100 µm.
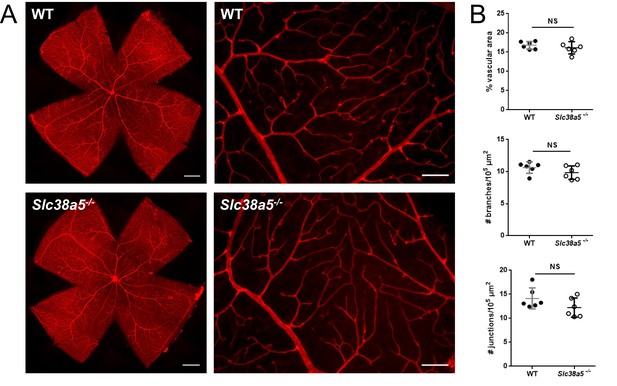
Quantification of adult wild type (WT) and Slc38a5−/− retinas shows comparable retinal vascular density.
(A) Flat mounts of adult WT and Slc38a5−/− retinas and magnified images showing fine branching and structure of retinal vessels stained with isolectin B4 (red). (B) Quantification of superficial vascular layer in coverage of vascular area, number of branches, and number of junctions. n=6/group. NS: not significant. Scale bars: A, left: 500 µm, right: 100 µm.
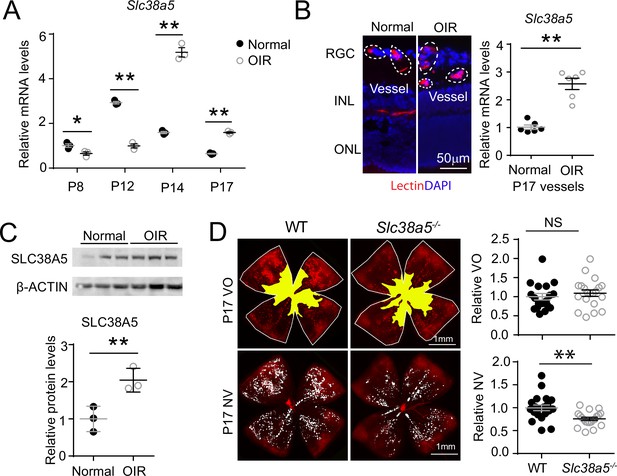
Slc38a5 is enriched in oxygen-induced retinopathy (OIR) pathological neovessels and its deficiency suppresses pathological angiogenesis in OIR.
(A) Slc38a5 mRNA expression was measured by RT-qPCR at P8, P12, P14, and P17 in C57BL/6J OIR retinas compared with age-matched normoxic control mice. Slc38a5 mRNA levels were decreased during hyperoxia stage (P8 and P12) and increased in hypoxia stage (P14 and P17). (B) Slc38a5 mRNA expression was analyzed using RT-qPCR in laser capture micro-dissected pathological neovessels from P17 unfixed C57BL/6J OIR retinas compared with normal vessels isolated from P17 normoxic retinas. Images on the left are representative retinal cross-sections from normal and OIR retinas stained with isolectin B4 (red) and DAPI (blue), with dotted lines highlighting micro-dissected retinal vessels. GCL: ganglion cell layer, INL: inner nuclear layer, ONL: outer nuclear layer. (C) Protein levels of SLC38A5 (52 kDa) were increased in C57BL/6J OIR retinas at P17 compared with normoxic controls using Western blot and quantified with densitometry. Proteins were normalized to β-ACTIN (42 kDa). (D) Slc38a5−/− exposed to OIR had decreased levels of pathological NV (neovascularization) compared with WT OIR controls bred in the same colony at P17. There was no significant difference in VO (vaso-obliteration) between the two groups. Scale bar: 50 µm (B), 1 mm (D). Each dot represents one retina. Data are expressed as mean ± SEM. n=3–6 per group (A–C), n=20 per group (D). *p≤0.05; **p≤0.01; n.s.: not significant.
-
Figure 4—source data 1
Raw data for Figure 4.
- https://cdn.elifesciences.org/articles/73105/elife-73105-fig4-data1-v1.zip
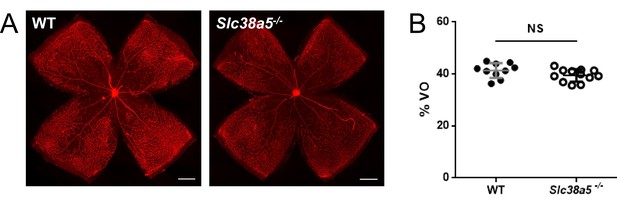
Quantification of vaso-obliteration of P12 wild type (WT) and Slc38a5−/− retinas in oxygen-induced retinopathy shows comparable levels.
(A) Flat mounts of P12 WT and Slc38a5−/− retinas showing central avascular area of vaso-obliteration (VO) absence of isolectin B4 staining (red). (B) Quantification of VO. n=10–12/group. NS: not significant. Scale bars: 500 µm.
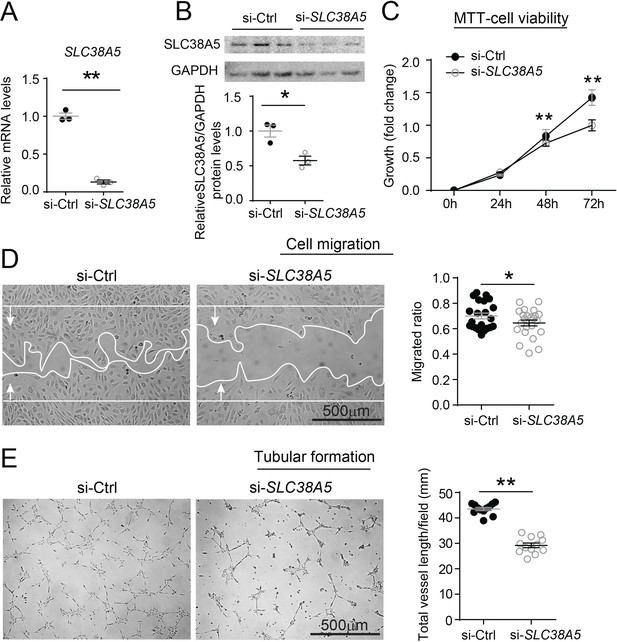
Inhibition of SLC38A5 dampens endothelial cell viability, migration, and tubular formation in vitro.
Human retinal microvascular endothelial cells (HRMECs) were transfected with siRNA targeting SLC38A5 (si- SLC38A5) or control siRNA (si-Ctrl). (A–B) mRNA (A) and protein (B) levels of SLC38A5 (52 kDa) confirm successful knock down by si-SLC38A5. Protein levels were normalized to GAPDH (37 kDa). (C) HRMEC cell viability was measured with MTT assay. Cell growth rate was calculated as fold change normalized to the values at 0 hr. (D) HRMECs were grown to confluence and treated with si-SLC38A5 or si-Ctrl for 48 hr. Cells were then treated with mitomycin to stop cell proliferation. A scratch was performed to the cells to generate a wound. Migrated areas (new cell growth areas normalized by original wound areas) of HRMECs were measured after 16 hr. (E) Tubular formation assay was conducted by collecting cells after 48 hr of si-SLC38A5 transfection and seeding cells onto Matrigel-coated wells to grow for additional 9 hr. Representative images show formation of endothelial cell tubular network, and total vessel length per field was analyzed by Image J. Scale bar: 500 µm (D&E). Data are shown as mean ± SEM; n=3–6/group. *p≤0.05; **p≤0.01.
-
Figure 5—source data 1
Raw data for Figure 5.
- https://cdn.elifesciences.org/articles/73105/elife-73105-fig5-data1-v1.zip
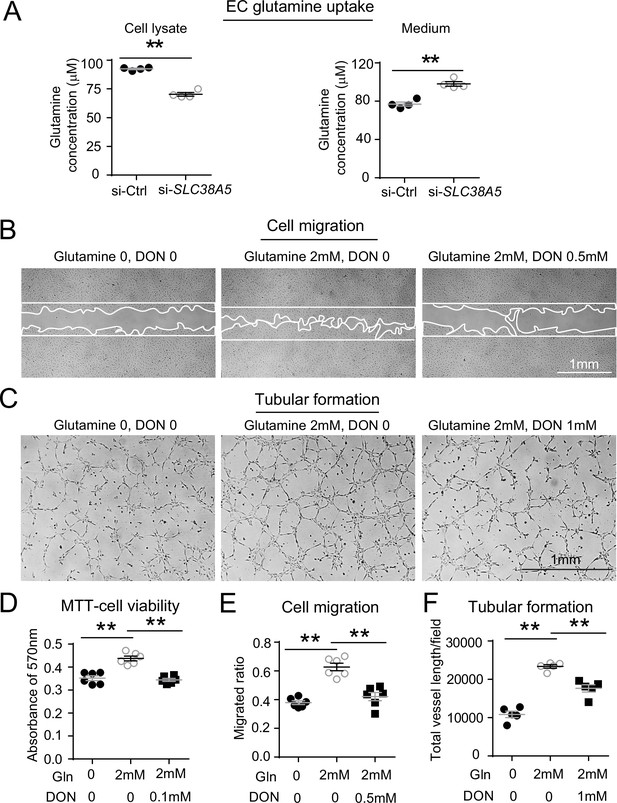
SLC38A5 facilitates endothelial cell (EC) uptake of glutamine, which is essential for EC viability, migration, and tubular formation.
(A) SLC38A5 knockdown with si-SLC38A5 suppressed glutamine uptake by human retinal microvascular endothelial cells (HRMECs), with decreased glutamine levels in HRMEC cell lysates and increased culture medium levels, measured with a glutamine/glutamate-Glo bioluminescent assay. Levels of glutamine/glutamate in HRMECs and culture medium samples were determined from bioluminescence readings by comparison to a standard titration curve. (B & E) HRMECs were grown to confluence, and a scratch was applied to generate a wound. Mitomycin was used to stop cell proliferation. A glutamine antagonist, 6-diazo-5-oxo-norleucine (DON), was used to broadly inhibit glutamine uptake. 16 hr were given to the cells to migrate. Representative images are shown in (B), and the quantification of migrated areas is shown in (E). (C, F) HRMECs treated were seeded onto Matrigel for 9 hr and treated with glutamine and DON for tubular formation. Representative images are shown in (C), and the quantification of total vessel length per field is shown in (F). (D) HRMEC cell viability was measured at 24 hr by MTT assay and normalized to the levels at 0 hr to quantify the cell growth rate. Scale bars: 1 mm (B&C). Data are expressed as means ± SEM. n=4–6 per group. *p≤0.05; **p≤0.01.
-
Figure 6—source data 1
Raw data for Figure 6.
- https://cdn.elifesciences.org/articles/73105/elife-73105-fig6-data1-v1.zip
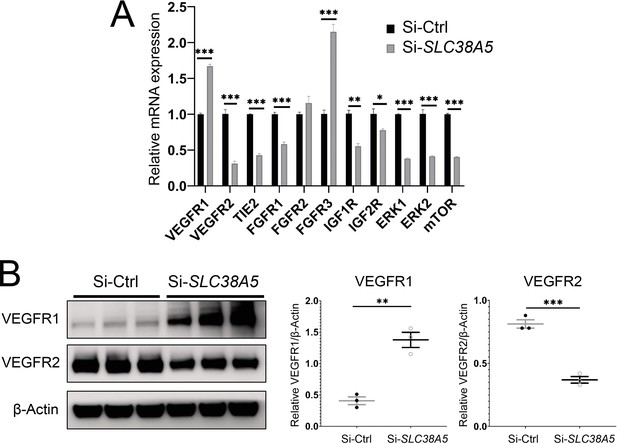
Suppression of Slc38a5 modulates growth factor receptors including vascular endothelial growth factor receptor 1 (VEGFR1) and VEGFR2.
Human retinal microvascular endothelial cells were transfected with siRNA targeting SLC38A5 (si-SLC38A5) or control siRNA (si-Ctrl) for 72 hr and collected for RT-qPCR or Western blots. (A) mRNA levels of growth factor receptors and signaling molecules were normalized by expression of 18 S (n=3–6/group). (B) Western Blots show protein levels of VEGFR1 and VEGFR2 with Si-SLC38A5 or si-Ctrl treatment. Data are shown as mean ± SEM; n=3/group. **p≤0.01; ***p≤0.001.
-
Figure 7—source data 1
Raw data for Figure 7.
- https://cdn.elifesciences.org/articles/73105/elife-73105-fig7-data1-v1.zip
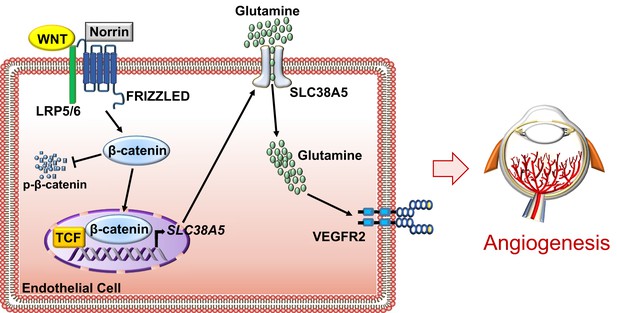
Schematic illustration of a pro-angiogenic role of amino acid (AA) transporter SLC38A5 in retinal angiogenesis.
In vascular endothelial cells (ECs), Wnt ligands (Wnts and Norrin) activate Wnt/β-catenin signaling, which controls the transcription of EC-enriched SLC38A5 by potentially binding to a TCF-binding site on SLC38A5 promoter or through an indirect transcriptional mechanism. Endothelial SLC38A5 facilitates EC uptake of AAs such as glutamine as energy fuel and source of protein synthesis. Altered glutamine and nutrient availability in EC subsequent affects VEGFR2 levels and signaling, and thus retinal angiogenesis. In retinopathy, expression of both Wnt receptors and endothelial SLC38A5 is enriched in pathological neovessels, promoting glutamine availability and thereby contributing to regulation of VEGFR2 mRNA transcription and protein signaling, resulting in formation of pathologic retinal neovascularization. Inhibition of SLC38A5 may suppress pathologic neovessels and alleviate pathologic neovascularization in retinopathy.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (Mus musculus) | Slc38a5−/− | J.Kim et al., Cell Metab 25, (2017). PMID:28591637 | ||
| Genetic reagent (M. musculus) | Lrp5−/− | Jackson Laboratory | Stock no. 005823 | |
| Genetic reagent (M. musculus) | Ndpy/− | Jackson Laboratory | Stock no. 012287 | |
| Genetic reagent (M. musculus) | C57BL/6J | Jackson Laboratory | Stock no: 000664 | |
| Antibody | SLC38A5 (Rabbit polyclonal) | Biorbyt | orb317962 | Dilution Western: 1:1000 IHC: 1:200 |
| Antibody | Non-phosphorylated β-catenin (Rabbit polyclonal) | Cell Signaling Technology | 8814 S | Dilution 1:1000 |
| Antibody | β-Catenin (Rabbit polyclonal) | Santa Cruz Biotechnology | sc-7199 | Dilution 1:1000 |
| Antibody | Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Mouse monoclonal) | Santa Cruz Biotechnology | sc-32233 | Dilution 1:2000 |
| Antibody | β-ACTIN (Mouse monoclonal) | Sigma-Aldrich | A1978 | Dilution 1:1000 |
| Antibody | VEGFR1 (Rabbit polyclonal) | Cell Signaling Technology | 2893 S | Dilution 1:2000 |
| Antibody | VEGFR2 (Rabbit monoclonal) | Cell Signaling Technology | 2479 | Dilution 1:2000 |
| Antibody | Anti-mouse IgG, HRP-conjugated (Sheep polyclonal) | Sigma-Aldrich | NA9310 | Dilution 1:10000 |
| Antibody | Anti-rabbit IgG, HRP-conjugated (Donkey polyclonal) | Sigma-Aldrich | SAB3700934 | Dilution 1:10000 |





