P2RY14 cAMP signaling regulates Schwann cell precursor self-renewal, proliferation, and nerve tumor initiation in a mouse model of neurofibromatosis
Figures
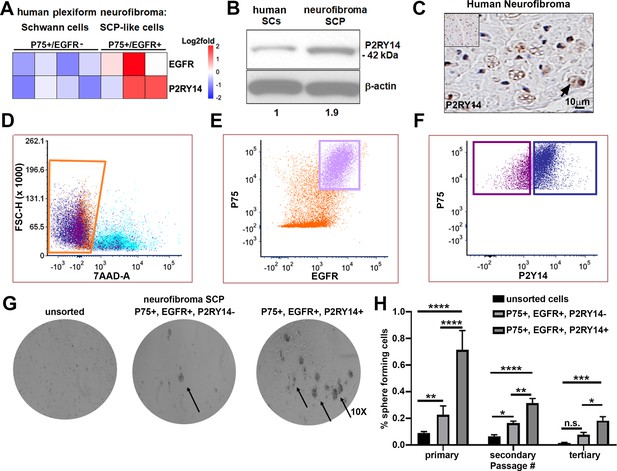
P2RY14 is expressed in human neurofibromas and promotes Schwann cell precursor (SCP) self-renewal in vitro.
(A) Microarray heatmap shows P2ry14 receptor expression in p75+/EGFR+ SCP-like tumor-initiating cells derived from human plexiform neurofibroma tumor cells compared to p75+/EGFR- SCP-like cells. (B) Western blot of human Schwann cells and neurofibroma SCP shows the latter has a 1.9-fold increase in P2ry14 protein expression. (C) Immunohistochemistry of human neurofibroma shows P2ry14 expression (DAB staining: brown [P2ry14 positive cells] blue [cell nuclei]). (D) Representative fluorescence-activated cells sorting (FACS) plot shows live sorted human plexiform neurofibroma tumor cells. (E) Representative FACS plot shows human plexiform neurofibroma tumor cells sorted into p75+/EGFR+ SCP-like tumor-initiating cells (pink square). (F) Representative FACS plot shows p75+/EGFR+ SCP-like tumor-initiating cells further sorted into p75+/EGFR+/P2ry14- (left, purple square) and P75+/EGFR+/P2ry14+ (right, blue square). (G) Photomicrographs of human neurofibromas dissociated using FACS to yield: unsorted, p75+/EGFR+/P2ry14- and P75+/EGFR+/P2ry14+ cells. (H) Quantification of unsorted, p75+/EGFR+/P2ry14- and P75+/EGFR+/P2ry14+ cells plated in sphere medium. (n = 3; two-way ANOVA; primary: **p = 0.0057, ****p < 0.0001; secondary: *p = 0.0487; **p < 0.0024, ****p < 0.0001; tertiary: *p = 0.0321, ***p = 0.0006).
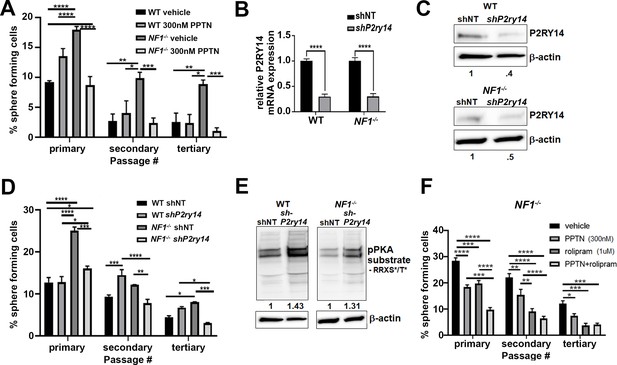
Mouse Nf1 mutant Schwann cell precursors (SCPs) use P2RY14 signaling to regulate self-renewal.
(A) Quantification of percent of sphere forming cells in mouse wild-type (WT) and Nf1-/- SCPs treated with the selective P2ry14 inhibitor (300 nM 4-[4-(4-piperidinyl)phenyl]-7-[4-(trifluoromethyl)phenyl]-2-naphthalenecarboxylic acid [PPTN]) (primary, secondary, tertiary passage) (n = 3; two-way ANOVA; primary: *p = 0.0375, ***p = 0.0001, ****p < 0.0001; secondary: *p = 0.0101, **p = 0.0015, ***p = 0.0009; tertiary: *p = 0.0101, **p = 0.0050, ***p = 0.0005). (B) P2ry14 mRNA expression in WT and Nf1-/- E12.5 mouse SCP treated with sh non-target (shNT) control and shP2ry14 (****p < 0.0001). (C) Western blot of WT and Nf1-/- SCPs treated with shNT and shP2ry14 showing P2ry14 knockdown. WT shP2ry14 show a 0.4-fold decrease of P2ry14 protein compared to WT shNT. Nf1-/- shP2ry14 show a 0.5-fold decrease compared to Nf1-/-. (D) Quantification of percent of sphere forming cells in mouse WT and Nf1-/- SCPs treated with shNT and shP2ry14 (n = 3; two-way ANOVA; primary: *p = 0.0288, ****p < 0.0001; secondary: **p = 0.0029,***p = 0.0005, ****p < 0.0001; tertiary: *p = 0.0154, ***p = 0.0007). (E) Western blot of WT and Nf1-/- Schwann cell (SC) spheres shows changes in pPKA substrate phosphorylation after shP2ry14 knockdown. WT shP2ry14 shows a 1.43-fold increase in pPKA after P2ry14 knockdown. Nf1-/- cells have a 1.31-fold increase in pPKA expression after P2ry14 knockdown. (F) Quantification of percent of sphere forming cells in Nf1-/- mouse SCPs treated with 1 µM rolipram or 300 nM PPTN (n = 3; two-way ANOVA; primary: ***p = 0.0002, ****p < 0.0001; secondary **p = 0.0030, ****p < 0.0001; tertiary: *p = 0.0476, ***p = 0.0004).
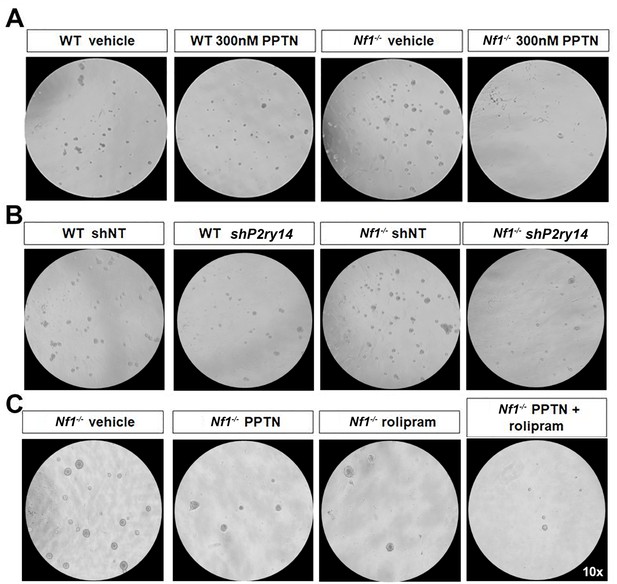
Photomicrographs of three different experiments in which wild-type (WT) and Nf1-/- Schwann cell precursors (SCPs) were treated with 4-[4-(4-piperidinyl)phenyl]-7-[4-(trifluoromethyl)phenyl]-2-naphthalenecarboxylic acid (PPTN), shP2ry14 and rolipram.
(A) Photomicrographs of WT and Nf1-/- mouse Schwann cell (SC) spheres treated with the selective P2ry14 inhibitor (PPTN; 300 nM). (B) Photomicrographs of WT and Nf1-/- mouse SC spheres treated with sh non-target (shNT) and shP2ry14 (09). (C) Photomicrographs of NF1-/- SCPs treated with vehicle and 1 µM of rolipram.
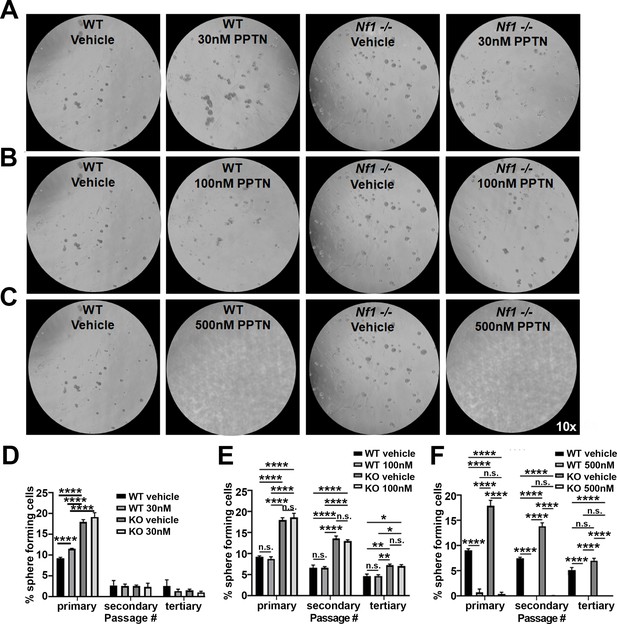
4-[4-(4-Piperidinyl)phenyl]-7-[4-(trifluoromethyl)phenyl]-2-naphthalenecarboxylic acid (PPTN) dose-response analysis in wild-type (WT) and Nf1-/- Schwann cell precursors (SCPs).
(A) Photomicrographs of WT and Nf1-/- mouse Schwann cell (SC) spheres treated with the P2ry14 inhibitor (PPTN; 30 nM). (B) Photomicrographs of WT and Nf1-/- mouse SC spheres treated with the P2ry14 inhibitor (PPTN; 100 nM). (C) Photomicrographs of WT and Nf1-/- mouse SC spheres treated with the P2ry14 inhibitor (PPTN; 500 nM). (Note: for A-C, the same WT and Nf1-/- vehicle controls photomicrographs are used for each dose). (D) Quantification of the percent of sphere forming cells after treatment with the P2ry14 inhibitor (PPTN; 30 nM) in WT and Nf1-/- mouse SC spheres (two-way ANOVA: primary and secondary passage: ****p < 0.0001, tertiary passage: *p = 0.0168, **p = 0.0074). (E) Quantification of percent sphere forming cells after treatment with the P2ry14 inhibitor (PPTN; 100 nM) in WT and Nf1-/- mouse SCP (two-way ANOVA: primary, secondary, and tertiary passage: ****p < 0.0001). (F) Quantification of percent sphere forming cells after treatment with the P2ry14 inhibitor (PPTN; 500 nM) in WT and Nf1-/- mouse SCP; results show that this concentration was toxic to the cells (two-way ANOVA: primary, secondary, and tertiary passage: ****p < 0.0001).
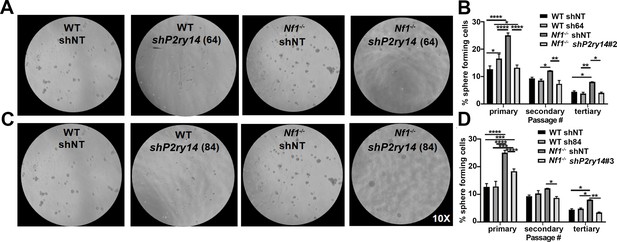
Photomicrographs and quantification analysis of two biological replicates of shP2ry14 in wild-type (WT) and Nf1-/- Schwann cell precursors (SCPs).
(A) Photomicrographs of WT and Nf1-/- SCP treated with shP2ry14 (64). (B) Quantification of the percent of sphere forming cells in WT and Nf1-/- SCP after treatment with shP2ry14 (64) (n = 3; two-way ANOVA: primary: *p = 0.0184, ****p < 0.0001; secondary: *p = 0.0270, **p = 0.0024; tertiary: *p = 0.0270, **p = 0.0078). (C) Photomicrographs of WT and Nf1-/- SCP treated with shP2ry14 (84). (D) Quantification of the percent of sphere forming cells in WT and Nf1-/- SCP after treatment with shP2ry14 (84) (n = 3; two-way ANOVA: primary: ***p = 0.0005, ****p < 0.0001; secondary: *p = 0.0358; tertiary: *p = 0.0245, **p = 0.0030). (Note: for A & C, the same WT and Nf1-/- sh non-target (shNT) control photomicrographs are used). (E) WT and Nf1-/- E12.5 mouse Schwann cells (SCs) cyclic AMP (cAMP) levels measured at baseline and after activation of adenylate cyclase (AC) via forskolin stimulation (n = 3, multiple t-test: vehicle *p = 0.0407, forskolin (5 µM) *p = 0.0045).
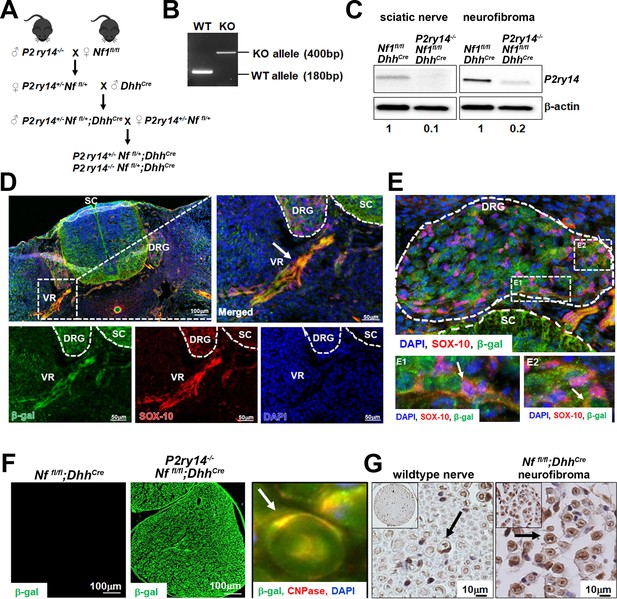
P2RY14 is expressed in Schwann cell precursors (SCPs) and Schwann cells (SCs) in vivo in a mouse model of neurofibromatosis.
(A) Schematic of generation of neurofibroma mice breeding of P2ry14-/- mice with Nffl/fl mice to obtain P2ry14+/-; Nf1fl/fl;DhhCre and P2ry14-/-; Nf1fl/fl;DhhCre littermates after several crosses. (B) Genotyping confirmation of wild-type (WT) and P2ry14 knockout (KO) alleles. (C) Western blot of sciatic nerve and neurofibroma tumors of Nf1fl/fl;DhhCre and P2ry14-/-;Nf1fl/fl;DhhCre mice show decrease in P2ry14 expression upon P2ry14 knockdown (1- to 0.1-fold decrease in sciatic nerve and 1- to 0.2-fold decrease in neurofibroma tissue). (D) Spinal cord (SC) immunofluorescent staining of mouse embryos at embryonic day 12.5 (E12.5) shows P2ry14 expression (β-galactosidase) co-localization with SOX-10 SCs at the dorsal root ganglion (DRG) and ventral roots (VR). (E) Spinal cord (SC) immunofluorescent staining of mouse embryos at E12.5 shows P2ry14 expression (β-galactosidase) co-localization with SOX-10 SCs at DRG. E1 and E2 insets show enlarged sections of the DRG. (F) Immunofluorescent staining of 7-month-old mouse sciatic nerve shows β-galactosidase positive staining as a confirmation of P2ry14 knock-in; co-labeling of β-galactosidase and CNPase shows that P2ry14 co-localizes with SCs (inset). (G) DAB staining of 7-month-old WT nerve and Nf1fl/fl;DhhCre mouse neurofibromas (DAB staining: brown [P2ry14 positive cells] blue [cell nuclei]).
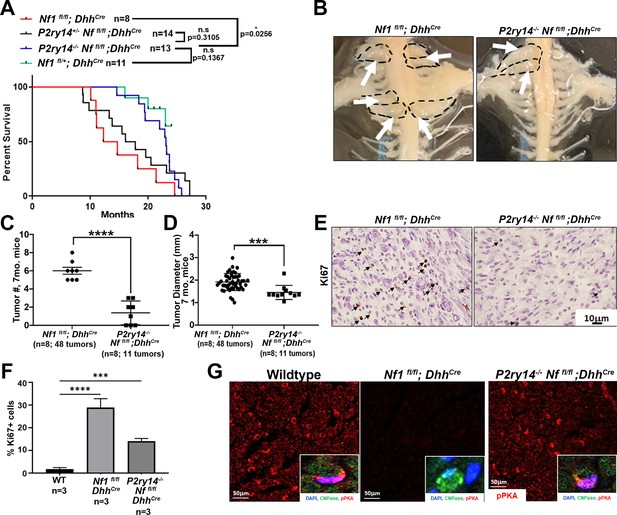
P2RY14 deletion in a mouse model of neurofibroma increases survival and delays neurofibroma initiation.
(A) Kaplan-Meier survival plot of Nf1fl/fl;DhhCre (red line; n = 8); P2ry14+/-; Nf1fl/fl;DhhCre (black line, n = 14); P2ry14-/-; Nf1fl/fl;DhhCre (blue line; n = 13); Nf1fl/+ control (green line, n = 11) (*p = 0.0256) shows P2ry14-/-;Nf1fl/fl;DhhCre have increased survival. (B) Representative image of gross dissection of Nf1fl/fl;DhhCre and P2ry14-/-;Nf1fl/fl;DhhCre mice at 7 months of age. (C) Neurofibroma tumor number quantification at 7 months of age (unpaired t-test ****p < 0.0001). (D) Neurofibroma diameter quantification at 7 months (unpaired t-test ***p = 0.0004) (for C and D: Nf1fl/fl;Dhh+ n = 8 mice, 48 neurofibroma tumors; P2ry14-/-;Nf1fl/fl;Dhh+ n = 8 mice, 11 neurofibroma tumors). (E) Ki67 staining of mouse dorsal root ganglion (DRG) and neurofibroma tumor tissue at 7 months of age. (F) Quantification of Ki67+ cells in mouse DRG and neurofibroma tumor tissue at 7 months of age (one-way ANOVA; multiple comparisons ***p = 0.0008; ****p < 0.0001). (G) Anti-p-PKA substrate staining in wild-type (WT), Nf1fl/fl;DhhCre and P2ry14-/-; Nf1fl/fl;DhhCre mice. p-PKA substrate phosphorylation labeling co-localized with CNPase Schwann cell (SC) marker (insets).
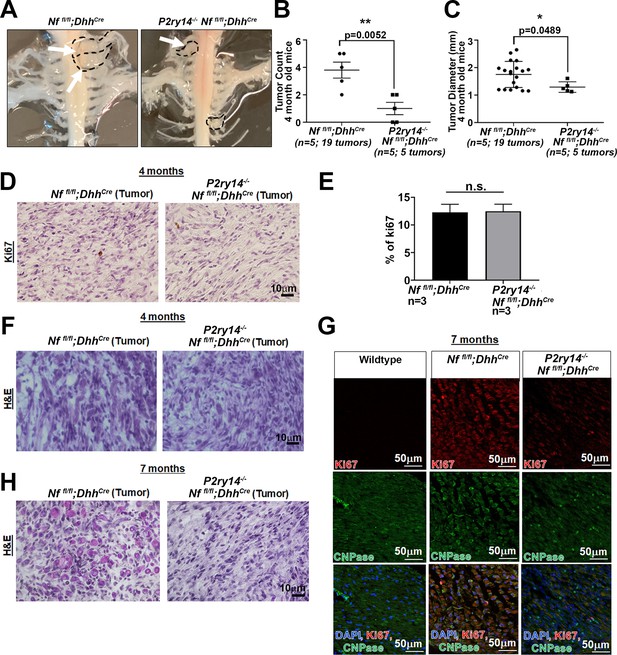
Tumor dissection of Nf1fl/fl;DhhCre and P2ry14-/-;Nf1fl/fl;DhhCre mice at 4 months and immunostaining analysis of 4- and 7-month sciatic nerve and tumors.
(A) Representative image of gross dissection of Nf1fl/fl;DhhCre and P2ry14-/-;Nf1fl/fl;DhhCre mice at 4 months. (B) Neurofibroma tumor number quantification at 4 months of age (unpaired t-test **p=0.0052). (C) Neurofibroma tumor diameter quantification at 4 months (unpaired t-test **p=0.0489) (for figures B and C: Nf1fl/fl;DhhCre n=5 mice, 19 neurofibroma tumors; P2ry14-/-;Nf1fl/fl;DhhCre n=5 mice; 5 neurofibroma tumors). (D) Ki67+ staining of Nf1fl/fl;DhhCre and P2ry14-/-;Nf1fl/fl;DhhCre neurofibromas at 4 months of age. (E) Quantification of percent of Ki67 positive cells in Nf1fl/fl;DhhCre and P2ry14-/-;Nf1fl/fl;DhhCre 4-month-old mice (t-test; n.s.). (F) H&E staining at 4 months in Nf1fl/fl;DhhCre and P2ry14-/-; Nf1fl/fl;DhhCre mice. (G) Ki67 immunofluorescence co-labeling with CNPase (Schwann cell [SC] marker) in 7-month sciatic nerve. (H) H&E tumor staining at 7 months in Nf1fl/fl;DhhCre and P2ry14-/-;Nf1fl/fl;DhhCre mice.
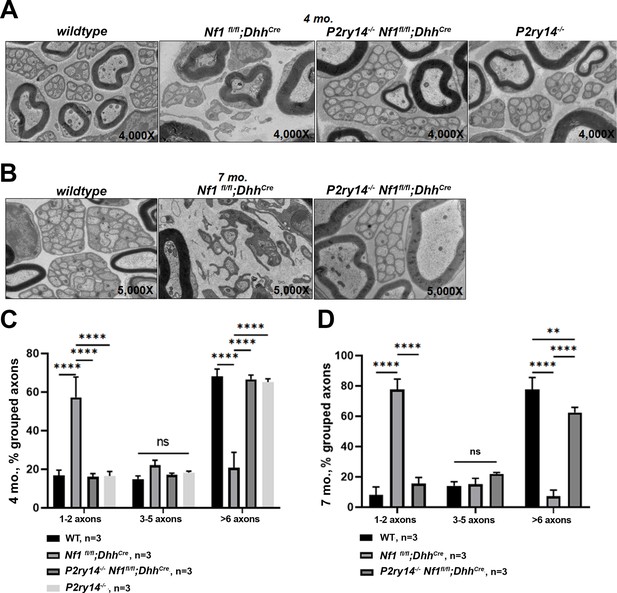
P2RY14 deletion improves nerve ultrastructure.
(A) Electron micrograph of saphenous nerve of 4-month-old wild-type (WT), Nf1fl/fl;DhhCre, P2ry14-/-; Nf1fl/fl;DhhCre and P2ry14-/- mice. (B) Electron micrograph of saphenous nerve of 7-month-old WT, Nf1fl/fl;DhhCre and P2ry14-/-;Nf1fl/fl;DhhCre mice. (C) Remak bundle quantification at 4 months of age (n = 3; two-way ANOVA: ****p < 0.0001). (D) Remak bundle quantification at 7 months of age (n = 3; two-way ANOVA: **p = 0.0027, ****p < 0.0001).
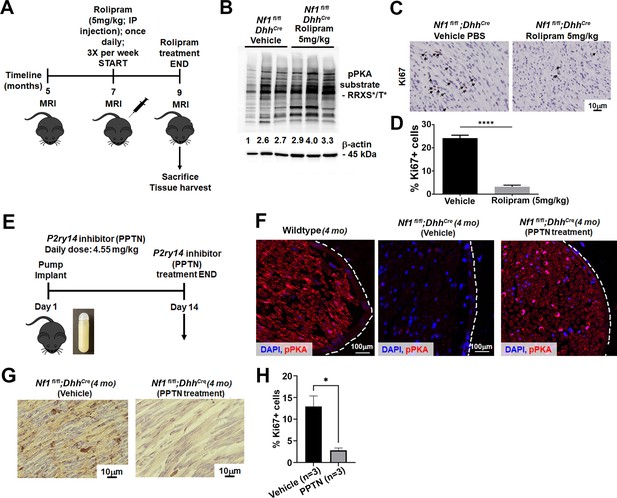
Cyclic AMP (cAMP) increase in a mouse model of neurofibromatosis either by rolipram or P2RY14 inhibitor treatment decreases Schwann cell (SC) proliferation.
(A) Rolipram drug treatment experimental design. (B) Tumor lysates of vehicle and rolipram treated Nf1fl/fl;DhhCre mice show changes in p-PKA substrate. (C) Ki67 staining at 9 months of age in vehicle and rolipram treated mice. (D) Quantification of Ki67+ cells in vehicle treated versus rolipram treated mice (unpaired t-test: ****p < 0.0001; n = 3). (E) P2ry14 inhibitor (4-[4-(4-piperidinyl)phenyl]-7-[4-(trifluoromethyl)phenyl]-2-naphthalenecarboxylic acid [PPTN]) drug treatment experimental design. (F) Immunofluorescent staining of sciatic nerve of 4-month-old wild-type (WT), Nf1fl/fl;DhhCre (vehicle) and Nf1fl/fl;DhhCre (PPTN treated) shows increased p-PKA expression after PPTN treatment. (G) Ki67+ staining of Nf1fl/fl;DhhCre (vehicle) and Nf1fl/fl;DhhCre (PPTN treated) neurofibroma tissue. (H) Quantification of Ki67+ cells after PPTN treatment in neurofibroma tissue.
Additional files
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/73511/elife-73511-transrepform1-v2.pdf
-
Source data 1
Non-annotated original figures.
- https://cdn.elifesciences.org/articles/73511/elife-73511-data1-v2.zip
-
Source data 2
Annotated original figures.
- https://cdn.elifesciences.org/articles/73511/elife-73511-data2-v2.zip





