Ecological and social pressures interfere with homeostatic sleep regulation in the wild
Figures
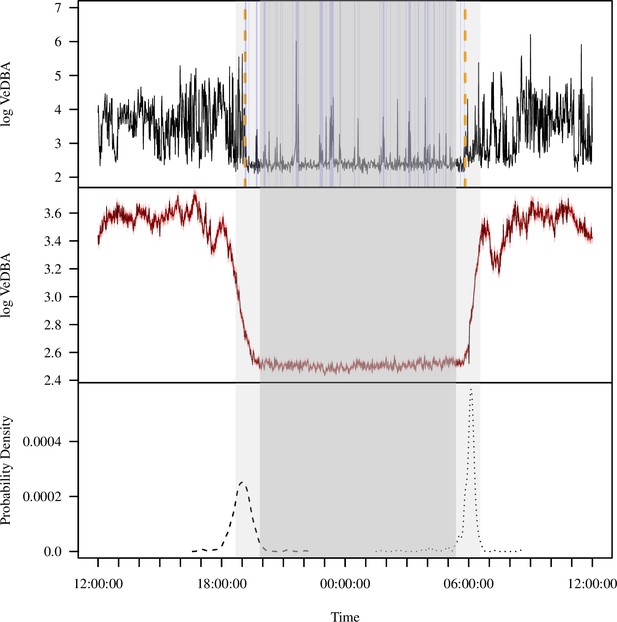
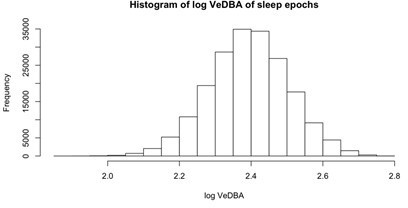
Extracting activity and sleep from accelerometry in a group of wild olive baboons.
Adapting algorithms developed by van Hees et al., 2015 and van Hees et al., 2018, we used the vectorial dynamic body acceleration (VeDBA), a measure of overall activity, to determine the sleep onset and awakening times (A; orange dashed lines), as well as periods of wake after sleep onset (A; blue shading) for each individual baboon on each day. These metrics allowed us to calculate the total sleep time, sleep period duration, sleep efficiency, and sleep fragmentation. The plot (A) shows the data of one individual within a single noon-to-noon period as an example. Averaged across all individuals on all nights (N = 354 baboon-nights), the log VeDBA shows that baboons exhibit activity patterns typical of a diurnal animal with monophasic sleep (B), with a consolidated period of very low levels of activity during the night. Although the timing of waking (C; dotted line) was more consistent across the group and across the study period than the timing of sleep onset (C; dashed line), both sleep onset and waking typically occurred within astronomical twilight. The red shading in (B) indicates ±1 SE. In all subplots, the gray shaded region depicts the period between sunset and sunrise, with double shading from the end of evening astronomical twilight to the beginning of morning astronomical twilight.
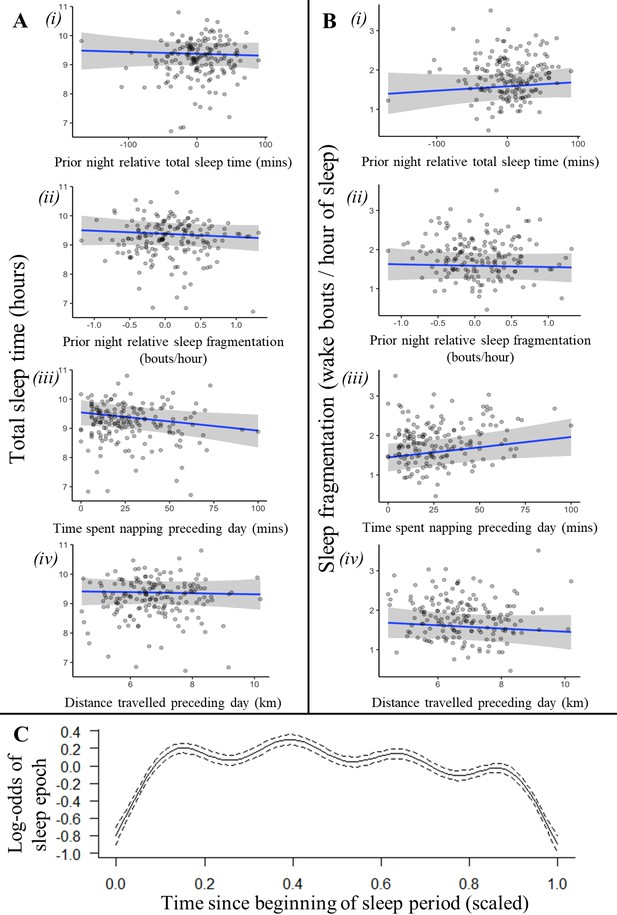
Recent history of sleep and activity has a weak influence on baboon sleep patterns.
Neither the relative sleep time on the previous night, the relative sleep fragmentation on the previous night, nor the distance traveled on the preceding day influenced sleep duration (Ai, ii, iv) or sleep fragmentation (Bi, ii, iv), although baboons did sleep less (Aiii) and experience more fragmented sleep (Biii) following days with more napping. Additionally, the likelihood of a baboon being asleep did not substantially decrease as the night progressed and the baboon payed off its sleep debt (C). In (C), time since the beginning of the sleep period is scaled from 0 (beginning) to 1 (end of the sleep period). Subplots depict the conditional effect of each variable from models of the data, with raw data points overlaid.
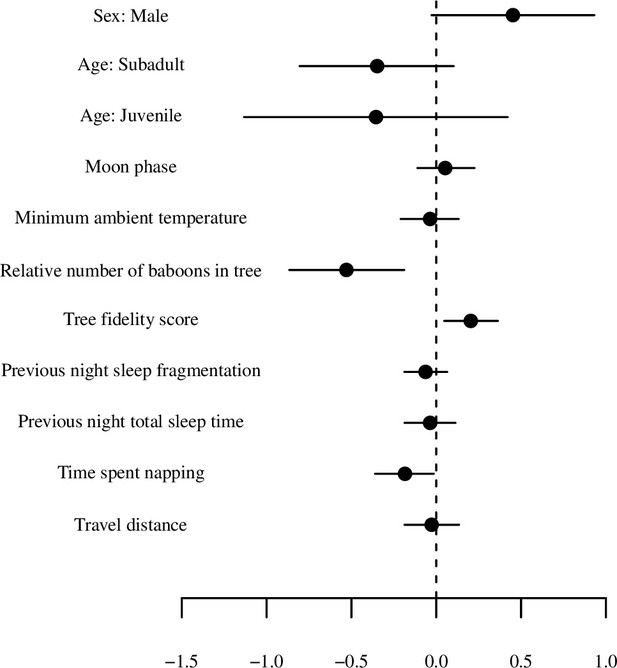
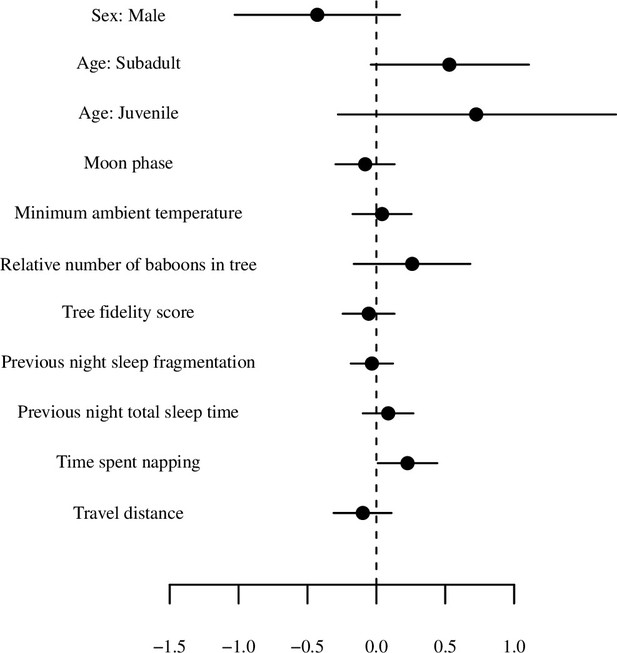
Model output plot of model of total sleep time (for the first 20 days) with all numerical variables standardized.
Points represent posterior means and line segments represent 95% credible intervals. The categorical variable tree is not plotted.
Model output plot of model of sleep fragmentation (for the first 20 days) with all numerical variables standardized.
Points represent posterior means and line segments represent 95% credible intervals. The categorical variable tree is not plotted.
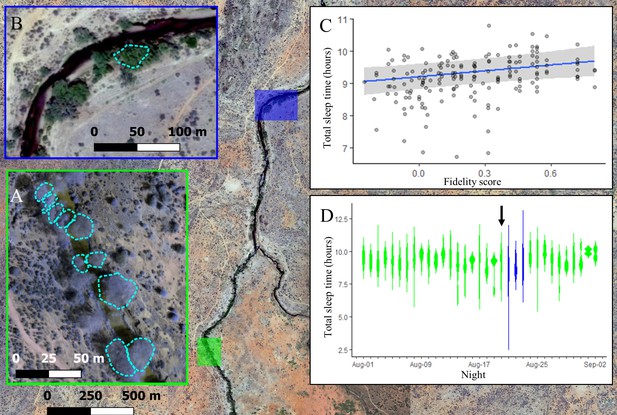
The location where baboons sleep has consequences for sleep duration.
Group members spent the majority of the study (32/35 nights) sleeping in 10 yellow fever (V. xanthophloea) trees in a grove along the Ewaso Ng’iro river (A). Within this sleep site, baboons slept longer when sleeping in trees to which they showed high fidelity (C). At 20:55 on the 21st night of the study, a leopard mounted an unsuccessful attack on the group in their sleep site. The following day, the baboons moved to a new sleep site 1.5 km away from their main sleep site (B). Baboons slept substantially less following this change in sleep site, but this effect did not persist beyond the first night in the new location (D). (C) Depicts the conditional effect from a model of the data, with raw data points overlaid, and (D) depicts a violin plot of the data, with color corresponding to the sleep site (A, B). The arrow in (D) indicates the night on which a leopard launched a failed attack on the group.
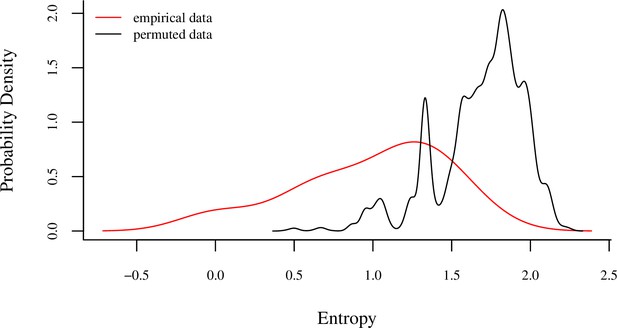
Comparison of the Shannon entropies of individuals’ sleep tree occupancy within their main sleep site to a null distribution produced by 1000 identity permutations.
The analysis revealed lower entropy in tree occupancy than expected by random chance (one-tailed two-sample Kolmogorov–Smirnov test: p<1.0 × 10–9), indicating that individuals exhibited high fidelity to particular trees. The red line represents the distribution of Shannon entropies of individuals’ sleep tree occupancy calculated from the empirical data, and the black line represents the distribution of entropy of sleep tree occupancy derived from the permuted dataset.
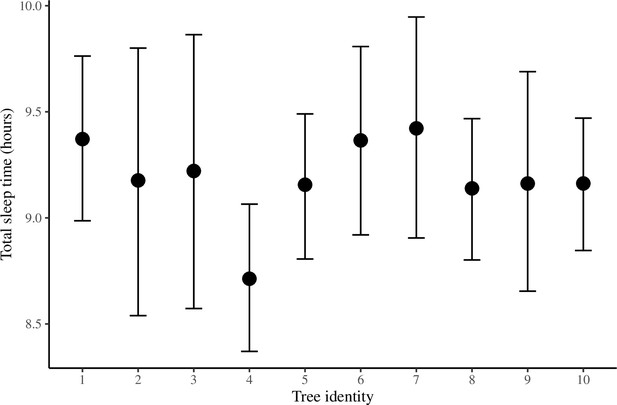
The conditional effect of tree identity on total sleep time.
The conditional effects plotted here are from the unstandardized Bayesian linear mixed model (LMM) of total sleep time.
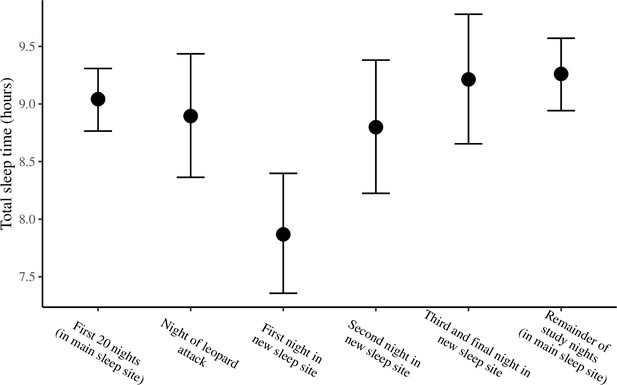
The conditional effect of night condition on total sleep time.
The conditional effects presented here are from the unstandardized model of total sleep time.
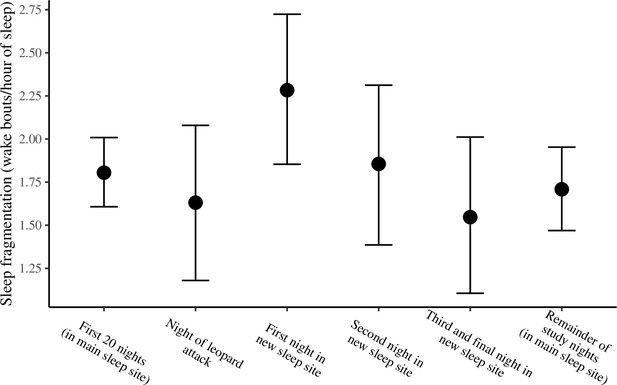
The conditional effect of night condition on sleep fragmentation.
The conditional effects presented here are from the unstandardized model of sleep fragmentation.
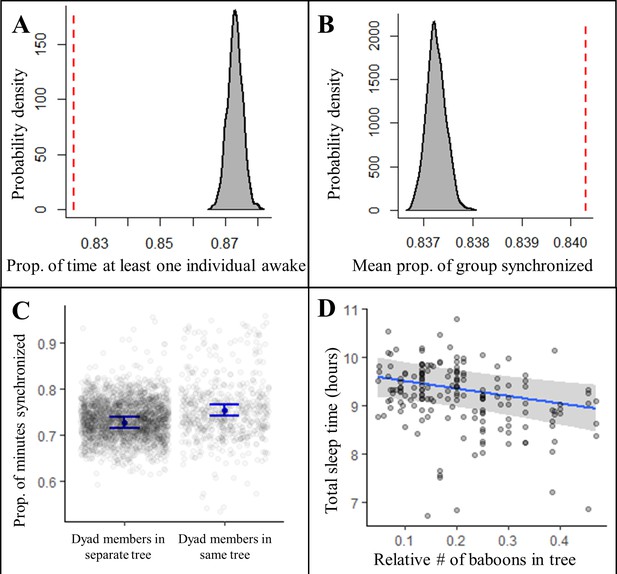
Collective dynamics within the sleep site influence sleep patterns.
Group-mates’ periods of nocturnal wakefulness were not staggered, but rather synchronized, as indicated by a significantly lower proportion of time with at least one individual awake (A, dotted red line; Fisher’s exact test: p<0.0001) and a significantly greater proportion of the group exhibiting synchronized behaviors (B, dotted red line; Fisher’s exact test: p<0.0001) than expected based on 1000 time-shifted datasets (gray distribution). Synchronized sleep patterns likely result from individuals waking in response to the nighttime activity of nearby group-mates as dyads show greater synchronization when dyad members sleep in the same tree compared to when they sleep in different trees (C). As a consequence of these local social perturbations, baboons sleep less when sleeping in trees with more group-mates (D). Subplots (C) and (D) depict the conditional effects from models of the data, with raw data points overlaid.
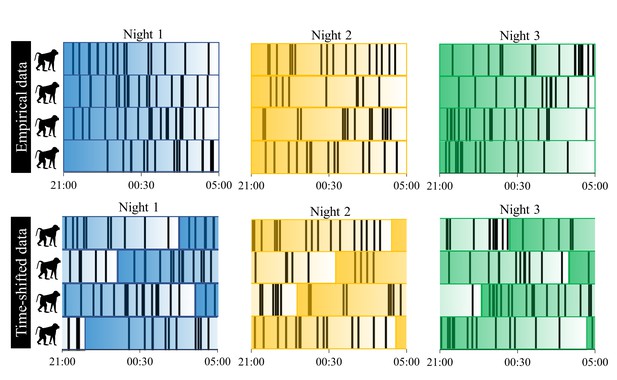
A toy example of the procedure we used to test for sentinel behavior and synchronization of nighttime sleep-wake schedules.
Each row represents a baboon’s time series of sleep and wake activity during the night, with black vertical lines indicating periods of nocturnal waking behavior. Colors correspond to different nights, and the transparency of the color indicates the time within the night, with reference to the empirical, unshifted data. The time-shifting procedure was repeated 1000 times to generate a null distribution for the proportion of minutes in which at least one individual is awake during the night and the mean proportion of the group exhibiting synchronized behavior.
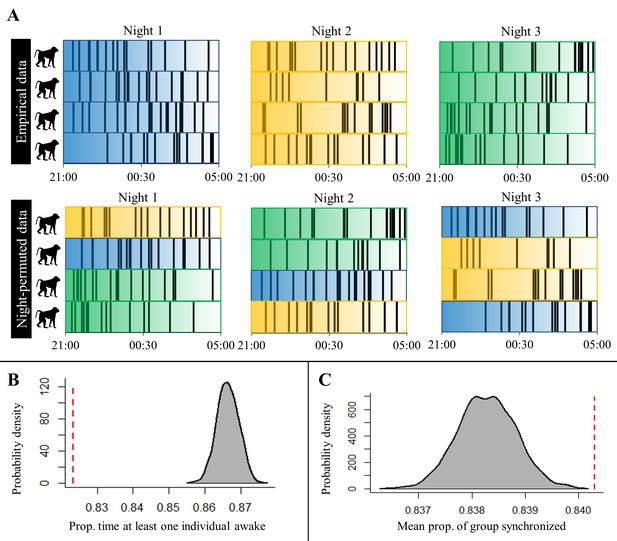
An alternative permutation procedure used to test for sentinel behavior and synchronization of nighttime sleep-wake schedules, and the results produced by this approach.
(A) A toy example of the procedure alternative to the one presented in the main text (and represented in Figure 4—figure supplement 1) that we used to confirm findings concerning sentinel behavior and synchronization of nighttime behavior that we derived from the procedure presented in the main text. Each row represents a baboon’s time series of sleep and wake activity during the night, with black vertical lines indicating periods of nocturnal waking behavior. Colors correspond to different nights, with reference to the empirical, unpermuted data, and the transparency of the color indicates the time within the night. The night permutation procedure was repeated 1000 times to generate a null distribution for the proportion of minutes in which at least one individual is awake during the night and the mean proportion of the group exhibiting synchronized behavior. (B) Comparison of the empirical proportion of minutes in which at least one individual is awake (red dotted line) to its null distribution (gray density plot; Fisher’s exact test: p<0.0001). (C) Comparison of the empirical mean of the proportion of the group exhibiting synchronized behavior (red dotted line) to its null distribution (gray density plot; Fisher’s exact test: p<0.0001). This method of permutation controls for the possibility that baboons are synchronized in their behavior simply as a result of species-typical nocturnal waking patterns that are consistent across baboons and across nights.
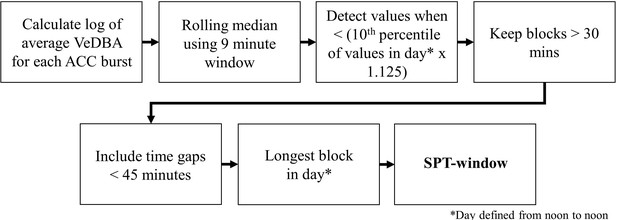
SPT-window detection algorithm adapted from Figure 1 in van Hees et al., 2018.

Examples of the three different behaviors, ‘wakefulness,’ ‘resting wakefulness,’ and ‘sleep,’ that were scored during the validation study.
Images presented here are extracted from the thermal imaging that was used for the behavioral scoring.
Additional files
-
Supplementary file 1
Study metadata, detailed outputs from statistical analyses, and results of validation study.
(a) Individual metadata. Table depicts the sex, age, weight, capture date, as well as data collection start and end dates for each study individual. F, female; M, male; A, adult; SA, subadult; J, juvenile; ACC, accelerometry. (b) Pearson correlation coefficient between the metrics of sleep extracted from the accelerometry data. Total sleep time is correlated with all sleep metrics, and sleep fragmentation is correlated with sleep efficiency. (c) Model output table of model of total sleep time (for the first 20 days) with all numerical variables standardized. (d) Model output table of model of total sleep time (for the first 20 days) with no standardization of variables. (e) Model output table of model of total sleep time (for the first 20 days) with all numerical variables standardized (daytime vectorial dynamic body acceleration [VeDBA] included instead of travel distance). (f) Model output table of model of time spent napping during the day (for the first 20 days) with all numerical variables standardized. (g) Model output table of model of time spent napping during the day (for the first 20 days) without standardization of the variables. (h) Model output table of model of total sleep time using data from entire study duration (including after the leopard attack) with all variables standardized. (i) Model output table of model of total sleep time using data from entire study duration (including after the leopard attack) without standardization of variables. (j) Model output table of model of sleep fragmentation (for the first 20 days) with all numerical variables standardized. (k) Model output table of model of sleep fragmentation (for the first 20 days) with no standardization of variables. (l) Model output table of model of sleep fragmentation (for the first 20 days) with all numerical variables standardized (daytime VeDBA included instead of travel distance). (m) Model output table of model of sleep fragmentation using data from entire study duration (including after the presumed leopard attack) with all variables standardized. (n) Model output table of model of sleep fragmentation using data from entire study duration (including after the presumed leopard attack) without standardization of variables. (o) Model output table of model of synchronization (i.e., the proportion of minutes during a night that both dyad members exhibit the same behavior, either sleep or wakefulness) with response variable standardized. (p) Model output table of model of synchronization (i.e., the proportion of minutes during a night that both dyad members exhibit the same behavior, either sleep or wakefulness) without standardization of the response variable. (q) Model output table of model of an individual being awake in a given epoch. The previous night relative total sleep time and previous night relative sleep efficiency variables are standardized. Data that was modeled here was a subset of the full dataset, in which the focal baboon was not awake in the previous epoch, and the current epoch occurred between 21:00 and 05:00 and prior to the 15th day of the study, when several of the baboons’ collars ceased collecting data. (r) Confusion matrix reporting the results of the validation study. Table entries represent the number of minute epochs classified according to the accelerometer-based technique and direct behavioral observation.
- https://cdn.elifesciences.org/articles/73695/elife-73695-supp1-v1.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/73695/elife-73695-transrepform1-v1.pdf