Zika virus causes placental pyroptosis and associated adverse fetal outcomes by activating GSDME
Figures
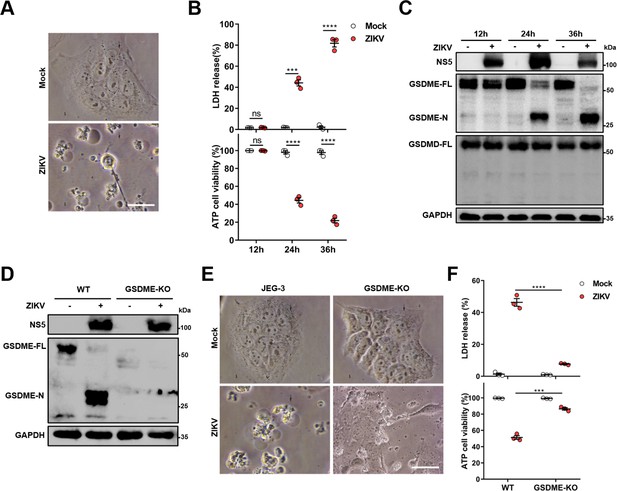
Zika virus (ZIKV) infection induces the gasdermin E (GSDME)-mediated pyroptosis in JEG-3 cells.
JEG-3 or GSDME-KO JEG-3 cells were infected with ZIKV at a multiplicity of infection (MOI) of 1. At indicated time post-infection, cells were subjected to microscopy, cytotoxicity, and Western blot analyses. (A) JEG-3 cells were infected with ZIKV for 24 hr. Representative cell morphology was shown. Scale bar, 50 μm. (B) LDH levels in supernatant and cell viability were measured at indicated time post-infection (n=3). (C) Immunoblot analyses of GSDME-FL, GSDME-N, and GSDMD-FL in ZIKV-infected JEG-3 cells at indicated time post-infection. (D–F) JEG-3 and GSDME-KO JEG-3 cells were infected with ZIKV for 24 hr. Immunoblot analyses of GSDME-FL and GSDME-N by Western blot (D). Representative cell morphology was shown. Scale bar, 50 μm (E). LDH levels in supernatant and cell viability were measured (n=3) (F). LDH release (B, F) is presented as the mean ± SEM of three independent experiments, and the two-tailed unpaired Student’s t-test was used to calculate significance. ***, p<0,001; ****, p<0.0001; ns, no significance.
-
Figure 1—source data 1
Raw data for Figure 1.
- https://cdn.elifesciences.org/articles/73792/elife-73792-fig1-data1-v1.zip
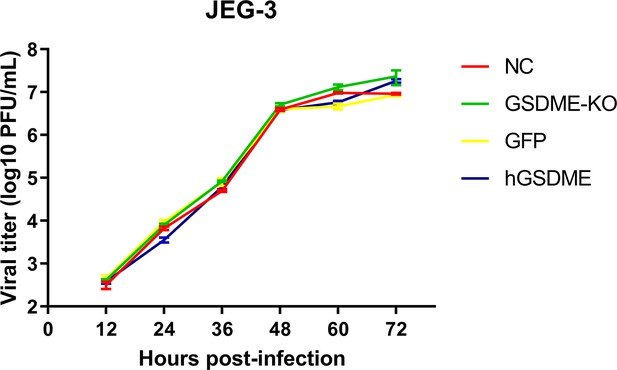
Gasdermin E (GSDME) does not affect Zika virus (ZIKV) replication in JEG-3 cells.
Negative control (NC), GSDME KO, GSDME-overexpressed and GFP-overexpressed JEG-3 cells were infected with ZIKV at a multiplicity of infection (MOI) of 0.1. The supernatants of ZIKV-infected cells were harvested at 12–72 hr post-infection for plaque assay, and the titration was performed on Vero cells (n=3).
-
Figure 1—figure supplement 1—source data 1
Raw data for Figure 1—figure supplement 1.
- https://cdn.elifesciences.org/articles/73792/elife-73792-fig1-figsupp1-data1-v1.xlsx

Both the cellular gasdermin E (GSDME) abundance and the susceptibility to Zika virus (ZIKV) infection determine the occurrence of pyroptosis.
(A–C) Relevant cells were infected with ZIKV at a multiplicity of infection (MOI) of 1. At indicated time post-infection, cells were subjected to microscopy, cytotoxicity, and Western blot analyses. Phase-contrast images of ZIKV-induced pyroptotic cell death in HeLa, HEK 293T, Huh-7, A549, and SH-SY5Y cells at 72 hr post-infection. Scale bar, 50 μm (A). Comparison of ATP cell viability, LDH release-based cell death (n=3) (B), and GSDME cleavage (C) in ZIKV-infected relevant cells. (D) Table summarizing results shown in (A–C), and Figure 2—figure supplement 1 from ZIKV infection experiments with relevant cell lines. The abundance of GSDME, the replication and infection levels of ZIKV in cells, and the ZIKV-induced LDH release were quantified. The cells showing the highest abundance of GSDME (SHSY-5Y), susceptibility to ZIKV (JEG-3), and degree of pyroptosis (JEG-3) were considered as references. Those cells are defined as high (severe) if their corresponding index is higher than the 75% of reference, medium (moderate) if the index is between 75% and 25%, and low (none) when the index is less than the 25% of the reference. (E and F) Analyses of ATP cell viability, LDH release-based cell death (n=3) (E), and GSDME cleavage (F) in JEG-3 cells at 36 hr post-infection. Unpaired t-test versus mock. LDH release (B, E) is presented as the mean ± SEM of three independent experiments, and the two-tailed unpaired Student’s t-test was used to calculate significance. *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001; ns, no significance.
-
Figure 2—source data 1
Raw data for Figure 2.
- https://cdn.elifesciences.org/articles/73792/elife-73792-fig2-data1-v1.zip

The gasdermin E (GSDME) abundance and the susceptibility to Zika virus (ZIKV) infection of different cell lines.
(A) Immunoblot analyses of GSDME-FL in indicated cell lines. (B–C) Relevant cells were infected with ZIKV at a multiplicity of infection (MOI) of 0.1. At indicated time post-infection, cells were subjected to immunofluorescence analysis (scale bar, 400 μm) (B). The supernatants of ZIKV-infected cells were harvested at 12–72 hr post-infection, and the plaque assay was performed on Vero cells (n=3) (C).
-
Figure 2—figure supplement 1—source data 1
Raw data for Figure 2—figure supplement 1.
- https://cdn.elifesciences.org/articles/73792/elife-73792-fig2-figsupp1-data1-v1.zip
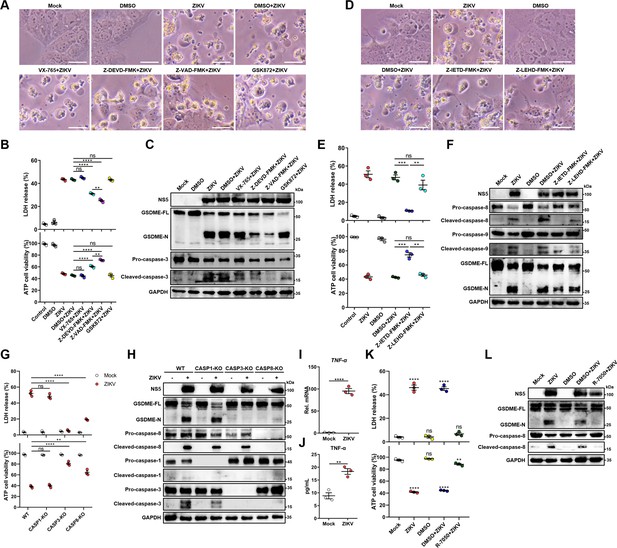
Zika virus (ZIKV) infection activates gasdermin E (GSDME) via extrinsic apoptotic pathway.
(A–C) JEG-3 cells were infected with ZIKV at a multiplicity of infection (MOI) of 1 followed by incubated with either 10 μM VX-765, 25 μM Z-DEVD-FMK, 25 μM Z-VAD-FMK, or 10 μM GSK872. At 24 hr post-infection, the cells were subjected to microscopy (A), cytotoxicity (n=3) (B), and Western blot analyses (C). Scale bar, 50 μm. (D–F) JEG-3 cells were infected with ZIKV at an MOI of 1 followed by incubated with either 25 μM Z-IETD-FMK or 25 μM Z-LEHD-FMK. At 24 hr post-infection, the cells were subjected to microscopy (D), cytotoxicity (n=3) (E), and Western blot analyses (F). Scale bar, 50 μm. (G–H) Caspase-1, caspase-3, and caspase-8 KO JEG-3 cells or wild-type (WT) cells were infected with ZIKV at an MOI of 1. At 24 hr post infection, LDH levels in supernatant, cell viability (n=3) (G) and cleavage of GSDME, caspase-1, caspase-3, and caspase-8 were measured in ZIKV-infected JEG-3 cells. (I–J) JEG-3 cells were mock-infected or infected with ZIKV at an MOI of 1. At 24 hr post-infection, the mRNA level of TNF-α (I) and concentration of TNF-α in the culture supernatant of JEG-3 cells (J) (n=3) were determined by RT-qPCR and enzyme-linked immunosorbent assay (ELISA), respectively. (K–L) JEG-3 cells were infected with ZIKV at an MOI of 1 followed by incubated with 2 μM R-7050. At 24 hr post-infection, the cells were subjected to cytotoxicity (n=3) (K) and Western blot analyses (L). Unpaired t-test versus mock. All data are presented as the mean ± SEM of three independent experiments, and the two-tailed unpaired Student’s t-test was used to calculate significance. **, p<0.01; ***, p<0.001; ****, p<0.0001; ns, no significance.
-
Figure 3—source data 1
Raw data for Figure 3.
- https://cdn.elifesciences.org/articles/73792/elife-73792-fig3-data1-v1.zip
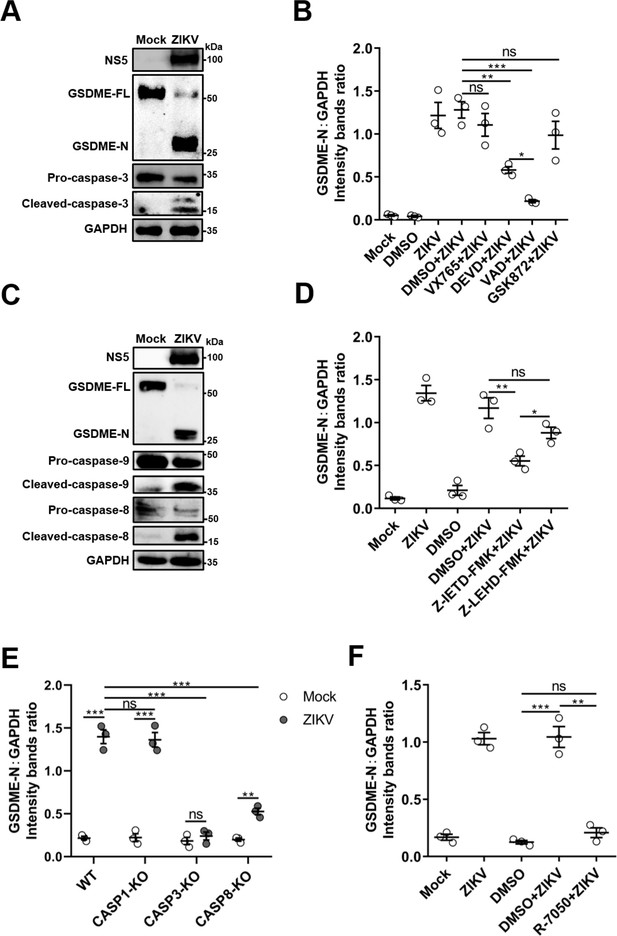
Zika virus (ZIKV) infection activates caspase-3 and caspase-8 in JEG-3 cells.
(A) Immunoblot analyses of GSDME-FL, GSDME-N, and casepase-3 in ZIKV-infected JEG-3 cells. (B) The grayscale analysis of Figure 3C. (C) Immunoblot analyses of GSDME-FL, GSDME-N, caspase-9, and casepase-8 in ZIKV-infected JEG-3 cells. (D) The grayscale analysis of Figure 3F. (E) The grayscale analysis of Figure 3H. (F) The grayscale analysis of Figure 3L. All data are presented as the mean ± SEM of three independent experiments, and the two-tailed unpaired Student’s t-test was used to calculate significance. *, p<0.5; **, p<0.01; ***, p<0.001; ns, no significance.
-
Figure 3—figure supplement 1—source data 1
Raw data for Figure 3—figure supplement 1.
- https://cdn.elifesciences.org/articles/73792/elife-73792-fig3-figsupp1-data1-v1.zip
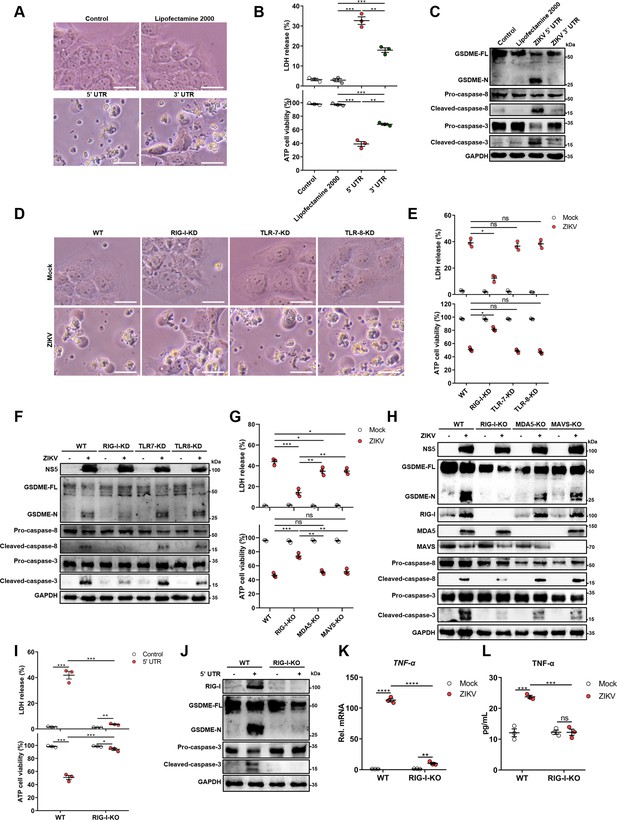
The genomic RNA of Zika virus (ZIKV) activates the gasdermin E (GSDME)-dependent pyroptosis through the RIG-I-caspase-8-caspase-3 pathway.
(A–C) JEG-3 cells were seeded in six-well plates and were transfected with 1 μg ZIKV 5’ untranslated region (UTR) or 3’ UTR. At 24 hr post-transfection, cells were subjected to microscopy (A), cytotoxicity (n=3) (B), and Western blot analyses (C). Scale bar, 50 μm. (D–F) RIG-I, TLR7, or TLR8 knockdown JEG-3 cells or wild-type (WT) JEG-3 cells were infected with ZIKV at a multiplicity of infection (MOI) of 1. At 24 hr post-infection, cells were subjected to microscopy (D), cytotoxicity (n=3) (E), and immunoblot analyses (F). Scale bar, 50 μm. (G–H) ZIKV-infected JEG-3 cells were infected with ZIKV. At 24 hr post-infection, the cells were subjected to cytotoxicity (n=3) (G) and Western blot analyses (H). (I–J) JEG-3 cells and RIG-I KO JEG-3 cells were seeded in six-well plates and were transfected with 1 μg ZIKV 5’ UTR. At 24 hr post-transfection, cells were subjected to cytotoxicity (n=3) (I) and Western blot analyses (J). (K–L) JEG-3 or RIG-I KO JEG-3 cells were mock-infected or infected with ZIKV at an MOI of 1. The mRNA level (K) and concentration of TNF-α in the culture supernatant (L) (n=3) was determined by RT-qPCR and enzyme-linked immunosorbent assay (ELISA), respectively. All data are presented as the mean ± SEM of three independent experiments, and the two-tailed unpaired Student’s t-test was used to calculate significance. **, p<0.01; ***, p<0.001; ****, p<0.0001; ns, no significance. RIG-I, retinoic acid-inducible gene I; TLR, Toll-like receptor.
-
Figure 4—source data 1
Raw data for Figure 4.
- https://cdn.elifesciences.org/articles/73792/elife-73792-fig4-data1-v1.zip
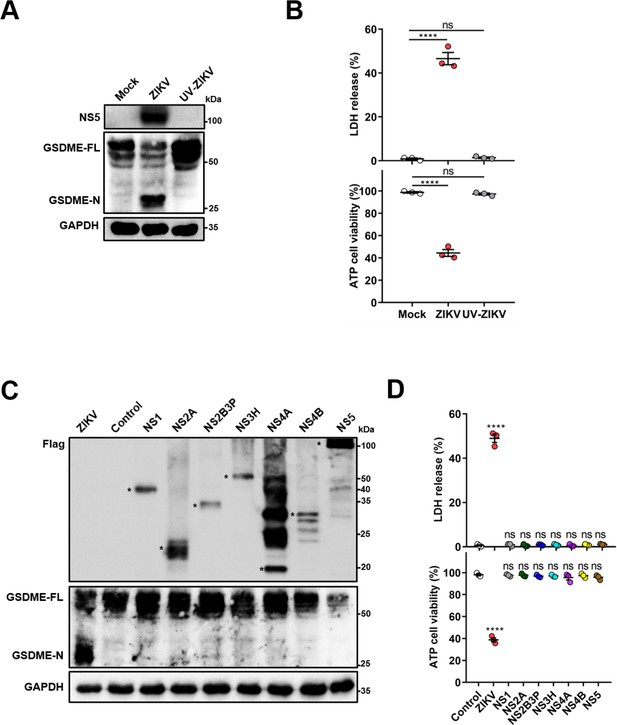
The structural and non-structural proteins of Zika virus (ZIKV) are not capable of inducing pyroptosis in JEG-3 cells.
(A–B) JEG-3 cells were infected with ZIKV or UV-inactivated ZIKV at a multiplicity of infection (MOI) of 1. At 24 hr post-infection, the cleavage of gasdermin E (GSDME) (A), LDH release, and cell viability (n=3) (B) were analyzed. (C–D) JEG-3 cells were seeded in six-well plates followed by transfection with 2 μg of indicated plasmids. At 24 hr post-transfection, the cleavage of GSDME (C), LDH release, and cell viability (n=3) (D) were analyzed. Unpaired t-test versus control. Asterisks indicate specific bands. All data are presented as the mean ± SEM of three independent experiments, and the two-tailed unpaired Student’s t-test was used to calculate significance. ****, p<0.0001; ns, no significance.
-
Figure 4—figure supplement 1—source data 1
Raw data for Figure 4—figure supplement 1.
- https://cdn.elifesciences.org/articles/73792/elife-73792-fig4-figsupp1-data1-v1.zip
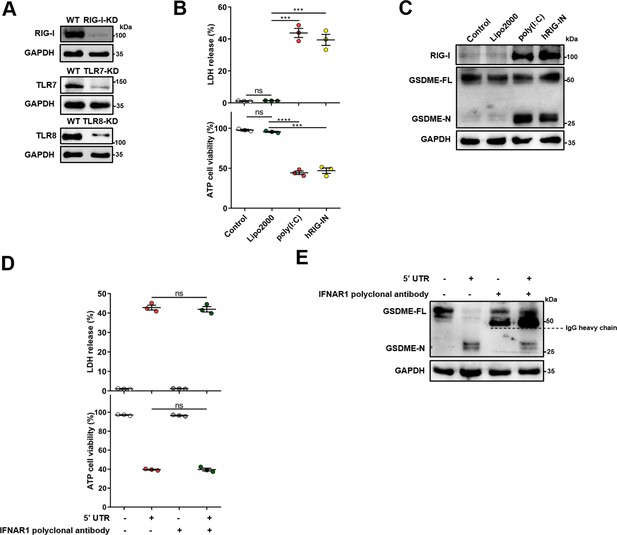
Activation of RIG-I is sufficient to induce gasdermin E (GSDME)-dependent pyroptosis.
(A) Verification of RIG-I, TLR7, and TLR8 knockdown efficiency by immunoblotting. (B–C) JEG-3 cells were seeded in six-well plate and transfected with 7.5 μl Lipofectamine 2000 Transfection Reagent, 3 μg poly (I:C) or 3 μg plasmid encoding human RIG-IN, respectively. At 24 hr post-transfection, the lactate dehydrogenase (LDH) release, cell viability (n=3) (B), and the cleavage of GSDME (C) were analyzed. (D–E) JEG-3 cells were seeded in six-well plate and transfected with 3 μg 5’ untranslated region (UTR), followed by incubation with 5 μg IFNAR1 polyclonal antibody. At 24 hr post-transfection, the LDH release and cell viability (n=3) (D), and the cleavage of GSDME (E) were analyzed. All data are presented as the mean ± SEM of three independent experiments, and the two-tailed unpaired Student’s t-test was used to calculate significance. ***, p<0.001; ns, no significance. RIG-I, retinoic acid-inducible gene I; TLR, Toll-like receptor.
-
Figure 4—figure supplement 2—source data 1
Raw data for Figure 4—figure supplement 2.
- https://cdn.elifesciences.org/articles/73792/elife-73792-fig4-figsupp2-data1-v1.zip
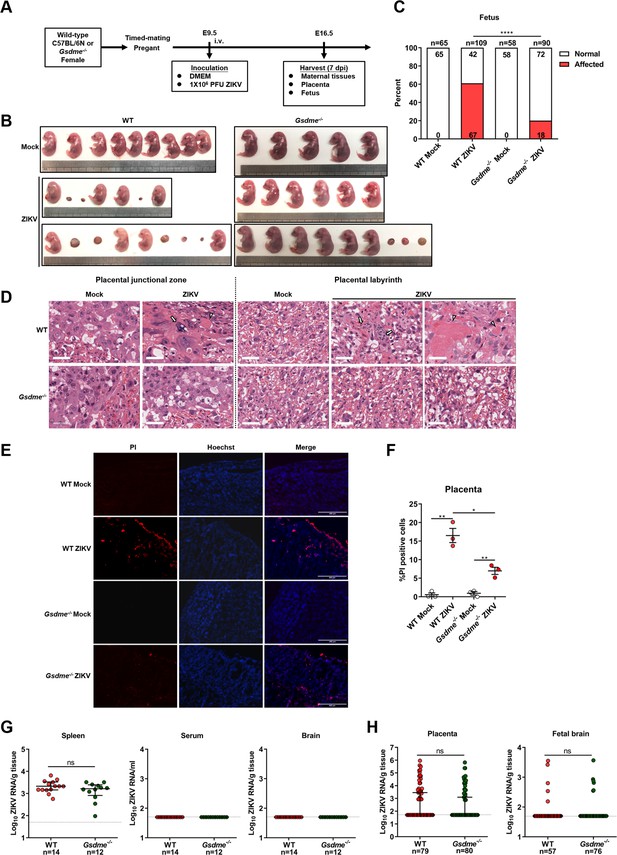
Zika virus (ZIKV) infection of pregnant immunocompetent wild-type (WT) C57BL/6N mice results in gasdermin E (GSDME)-mediated placental pyroptosis and congenital ZIKV syndrome (CZS).
(A) Scheme of infection and the follow-up analyses. The WT or Gsdme-/- female mice were mated with male mice of their respective genotypes, and the pregnant mice were infected intravenously with 1×106 PFU of ZIKV H/PF/2013 strain or with an equal volume of Vero cell culture supernatant at embryonic day 9.5 (E9.5) of pregnancy. The mice were sacrificed at E16.5 and the placentas and individual fetuses were collected for follow-up experiments. (B) Representative images of fetuses from mock- and ZIKV-infected WT and Gsdme-/- dams at E16.5 are shown. (C) Impact of ZIKV infection on fetuses from WT and Gsdme-/- dams at E16.5. The percentage of fetuses that were affected (i.e., had undergone resorption, or exhibited any sign of growth restriction, or malformation) is shown. Numbers on bars indicate normal fetuses (top) or affected fetuses (bottom). (D) Representative hematoxylin and eosin staining showed pathological features of placentas at E16.5. Arrows indicate necrotic trophoblast cells. Arrowheads indicate thrombi. Scale bar, 50 μm. (E–F) Propidium iodide (PI) was intravenously injected into the mice before scarification. Representative placenta section images are shown (E) and PI-positive cells were quantified (n=3) (F). Scale bar, 400 μm. (G) ZIKV RNA levels of maternal spleens, serum, and brains of WT and Gsdme-/- dams infected with ZIKV. (H) ZIKV RNA levels of all placentas and fetal heads carried by WT and Gsdme-/- dams infected with ZIKV. The number of samples in each group is listed. Data for all panels are pooled from three to five independent experiments. For (C), significance was determined by Fisher’s exact test. For (F), significance was determined by a two-tailed Student’s t-test. For (G) and (H), the Mann-Whitney test was used to calculate significance. Data shown are median with interquartile range, and the dotted line depicts the limit of detection. *, p<0.05; **, p<0.01; ****, p<0.0001; ns, no significance.
-
Figure 5—source data 1
Raw data for Figure 5.
- https://cdn.elifesciences.org/articles/73792/elife-73792-fig5-data1-v1.zip
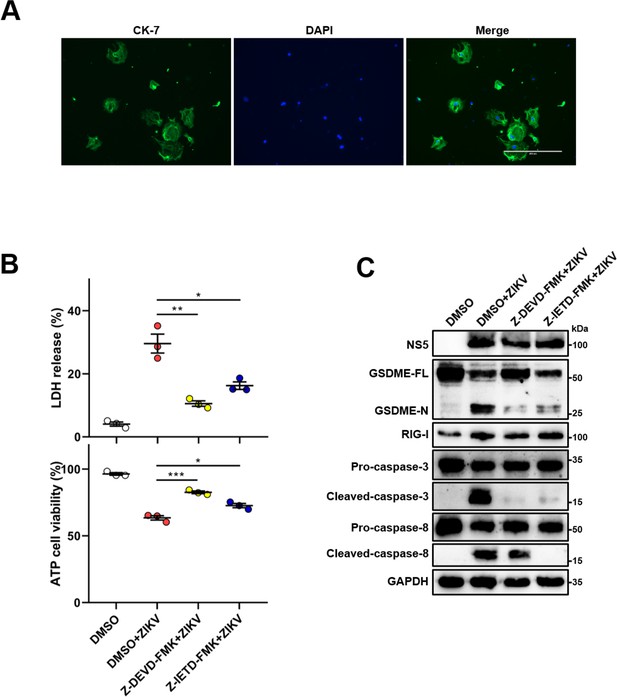
Zika virus (ZIKV) infection induces the gasdermin E (GSDME)-mediated pyroptosis in mouse primary trophoblast cells (MTCs).
(A) Primary MTCs were isolated from mouse placentas, and incubated at 37°C in a 5% CO2 incubator. After 72 hr, immunofluorescence analysis was conducted by using antibody against trophoblast-specific marker CK7 (scale bar, 400 μm). (B–C) MTCs were infected with ZIKV at a multiplicity of infection (MOI) of 5. At 48 hr post-infection, LDH release, cell viability (n=3) (B), and the cleavage of GSDME (C) were analyzed. All data are presented as the mean ± SEM of three independent experiments, and the two-tailed unpaired Student’s t-test was used to calculate significance. *, p<0.05; **, p<0.01; ***, p<0.001; ns, no significance.
-
Figure 5—figure supplement 1—source data 1
Raw data for Figure 5—figure supplement 1.
- https://cdn.elifesciences.org/articles/73792/elife-73792-fig5-figsupp1-data1-v1.zip
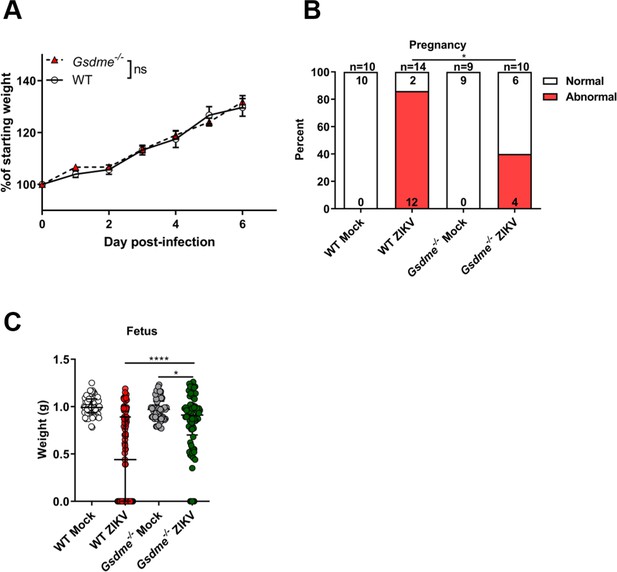
Gasdermin E (GSDME) deletion alleviates Zika virus (ZIKV)-induced abnormal pregnancy and adverse fetal outcomes.
Pregnant wild-type (WT) and Gsdme-/- dams were infected with ZIKV or Vero cell culture supernatant (Mock) as described in Figure 5A. (A) Measurements of daily weight of dams after ZIKV infection are shown as the percentage of maternal starting weight for ZIKV-infected WT mice (n=8) and Gsdme-/- (n=9) mice. Significance was determined by one-way ANOVA corrected using Bonferroni’s test for multiple comparisons. (B) The percentage of dams showing abnormal pregnancy (at least one fetus showed morphological abnormality or suffered demise/resorption). Numbers on bars indicate normal pregnancy (top) or abnormal pregnancy (bottom). Significance was determined by Fisher’s exact test. *, p<0.05. (C) The weight of fetuses (WT Mock, n=65; WT ZIKV, n=109; Gsdme-/- Mock, n=58; Gsdme-/- ZIKV, n=76). Data shown are median with interquartile range, and Mann-Whitney test was used to calculate significance. *, p<0.05; ****, p<0.0001. Data are pooled from three to five independent experiments.
-
Figure 5—figure supplement 2—source data 1
Raw data for Figure 5—figure supplement 2.
- https://cdn.elifesciences.org/articles/73792/elife-73792-fig5-figsupp2-data1-v1.zip
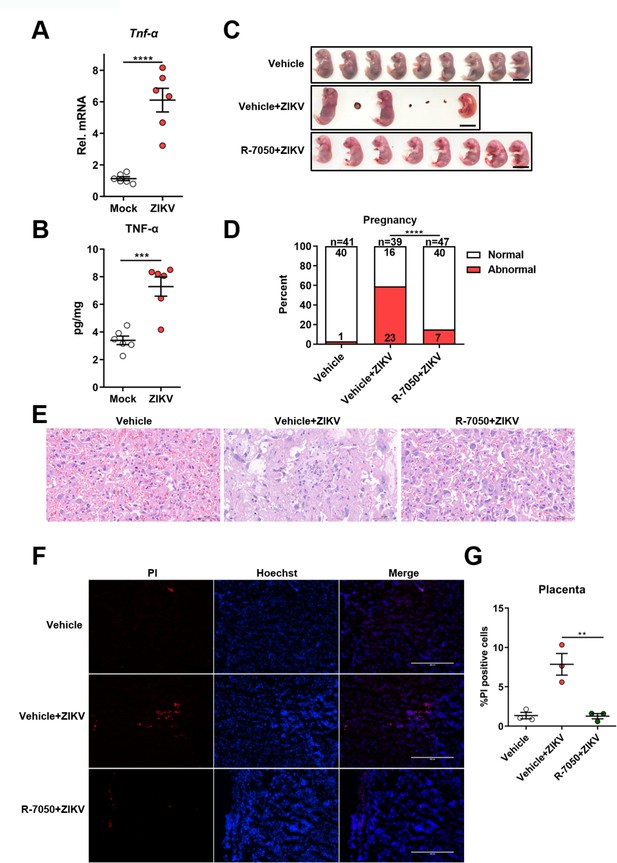
Induction of TNF-α expression contributes to placental damage in Zika virus (ZIKV)-infected pregnant mice.
(A–B) The pregnant C57BL/6N mice were mock-infected or intravenously infected with 1×106 PFU of ZIKV H/PF/2013 strain at embryonic day 9.5 (E9.5). At E16.5, placentas were collected and the mRNA level and concentration of TNF-α in mouse placentas were determined by RT-qPCR (A) and enzyme-linked immunosorbent assay (ELISA) (B), respectively (n=6). (C–G) The pregnant C57BL/6N mice were intravenously infected with 1×106 PFU of ZIKV H/PF/2013 strain at E9.5, followed by treatment with R-7050 (7 mg/kg, i.p.) or DMSO every other day. At E16.5, mice were sacrificed and the placentas and individual fetuses were collected. Representative images of fetuses from mock- and ZIKV-infected dams treated with R-7050 or vehicle at E16.5 are shown. Scale bar, 1 cm (C). The percentages of fetuses that were affected (i.e., had undergone resorption, or exhibited any sign of growth restriction, or malformation) were calculated. Numbers on bars indicate normal fetuses (top) or affected fetuses (bottom) (D). Representative hematoxylin and eosin staining was performed to show the pathological features of placentas at E16.5. The asterisks indicate abnormal spheroid structure. Arrows indicate necrotic trophoblast cells. Arrowheads indicate thrombi. Scale bar, 50 μm (E). Propidium iodide (PI) was intravenously injected into the mice before scarification. Representative placenta section images are shown (F) and PI-positive cells were quantified (n=3) (G). Scale bar, 400 μm. For (A), (B), and (G) significance was determined by a two-tailed unpaired Student’s t-test. For (D), significance was determined by Fisher’s exact test. **, p<0.01; ***, p<0.001; ****, p<0.0001.
-
Figure 6—source data 1
Raw data for Figure 6.
- https://cdn.elifesciences.org/articles/73792/elife-73792-fig6-data1-v1.zip
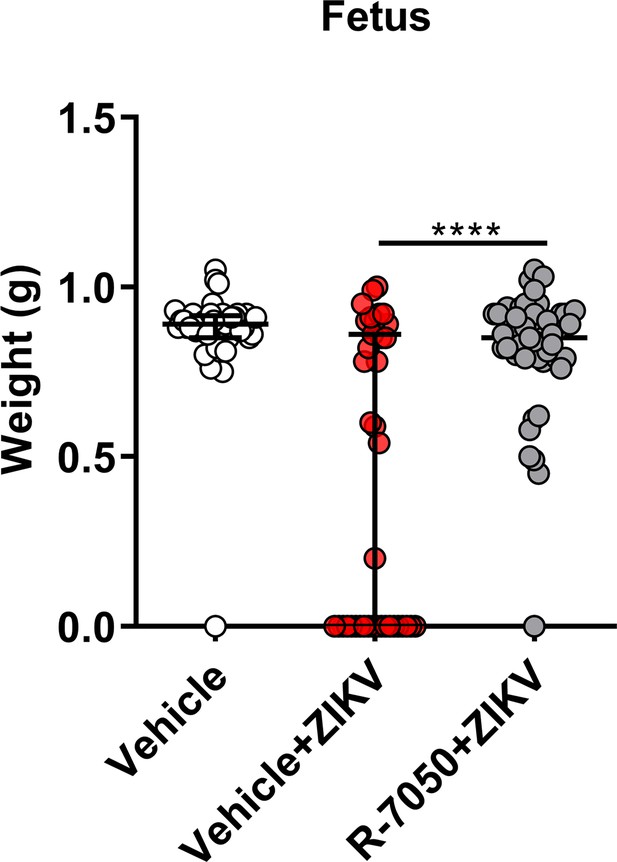
R-7050 treatment attenuates Zika virus (ZIKV)-induced adverse fetal outcomes.
The weight of fetuses from mock- and ZIKV-infected dams treated with R-7050 or vehicle at E16.5 (vehicle, n=41; vehicle + ZIKV, n=39; R-7050 + ZIKV, n=47). Data shown are median with interquartile range, and Mann-Whitney test was used to calculate significance. ****, p<0.0001.
-
Figure 6—figure supplement 1—source data 1
Raw data for Figure 6—figure supplement 1.
- https://cdn.elifesciences.org/articles/73792/elife-73792-fig6-figsupp1-data1-v1.xlsx





