Allosteric modulation of the adenosine A2A receptor by cholesterol
Figures
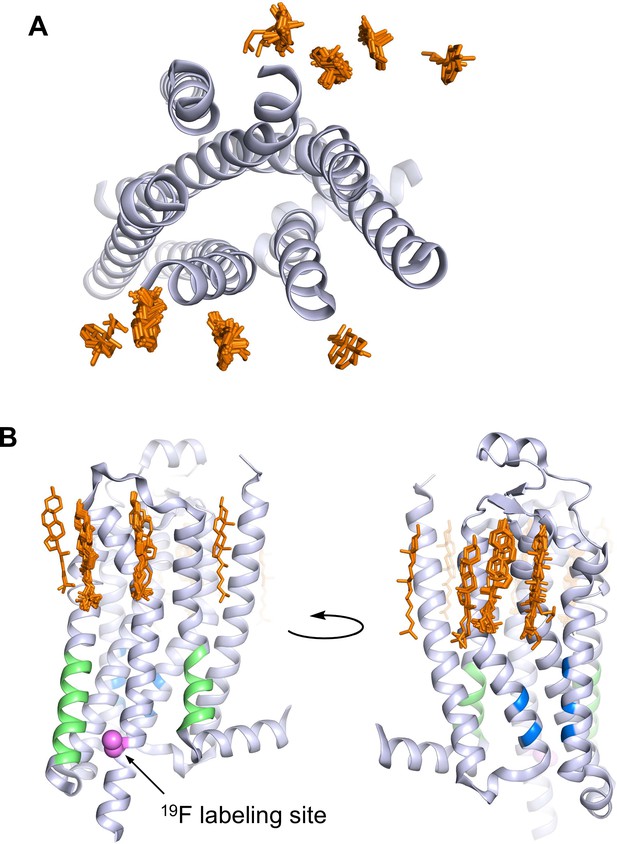
A2AR crystal structures reveal many cholesterol interaction sites.
(A) Overlay of 38 currently published A2AR crystal structures containing co-crystallized cholesterol (extracellular view, with cholesterols shown as orange sticks). For simplicity, extracellular loops and fusion proteins are removed and only one receptor structure is shown (PDB: 4EIY). (B) Side views of (A) highlighting the CCM (blue), the CRAC motifs (green), and V229C 19F labeling site (violet).
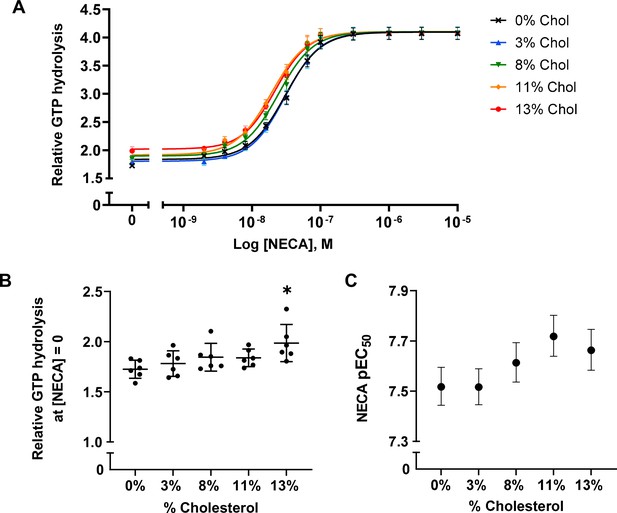
A2AR agonist potency and basal activity are weakly enhanced by cholesterol.
(A) Agonist (NECA) dose-response curves for A2AR-nanodiscs containing varying concentrations of cholesterol. The vertical axis represents GTP hydrolysis by purified Gαβγ (cumulative over 90 min) in the presence of A2AR and agonist, relative to GTP hydrolysis by Gαβγ alone in the absence of A2AR. Each data point represents the mean ± SEM (n = 6, technical triplicates). (B) Relative GTP hydrolysis in the presence of apo A2AR (no agonist) in nanodiscs containing varying concentrations of cholesterol. Data represents mean ± SD (n = 6, averages from each technical triplicate presented as individual points) and the asterisk represent statistical significance relative to the 0% cholesterol condition. Statistical significance was determined by one-way ANOVA followed by the Tukey’s multiple comparison test. * p ≤ 0.05. (C) pEC50 values of the NECA dose-response curves in (A). Error bars represent 95% (asymmetrical profile likelihood) confidence intervals.
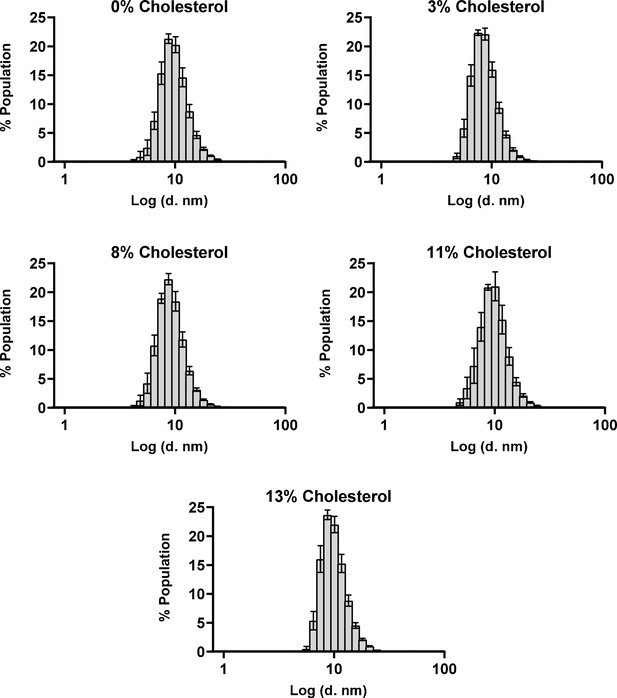
The size distribution of nanodiscs are minimally affected by cholesterol incorporation.
Hydrodynamic diameters of A2AR-nanodiscs containing varying levels of cholesterol, measured through dynamic light scattering. Data represent mean ± SD (n ≥ 3, technical triplicates).
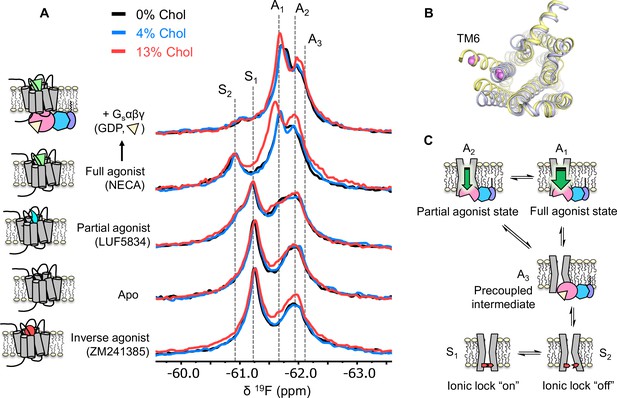
Cholesterol induces a small population increase in the active state conformers of A2AR.
(A) 19F NMR spectra of A2AR in nanodiscs containing 0, 4, and 13% cholesterol, as a function of ligand and G protein. Two inactive states (S1-2) and three active states (A1-3), previously identified, are indicated by gray dashed lines. For each ligand condition, spectra from the three cholesterol concentrations are normalized via their inactive state intensity. (B) Intracellular view of an inactive (gray, PDB: 4EIY) and an active (yellow, PDB: 5G53) crystal structure of A2AR highlighting the movement of TM6 upon activation. The 19F-labeling site is shown in violet. (C) Cartoon representations of the key functional states of A2AR indicated in (A). At the bottom are two inactive states (S1 and S2) where a conserved salt bridge is either intact or broken. A3 is an intermediate state that facilitates G-protein recognition and precoupling. A1 and A2 are active states that drive nucleotide exchange. While A1 is more efficacious and preferentially stabilized by the full agonist, A2 is less efficacious and reinforced by a partial agonist.
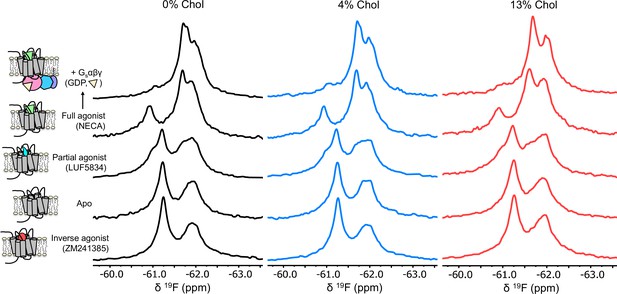
A2AR exhibits similar activation signatures in the absence and presence of cholesterol.
Non-overlapped (relative to Figure 3) 19F NMR spectra of A2AR-V229C in nanodiscs containing 0, 4, and 13% cholesterol.
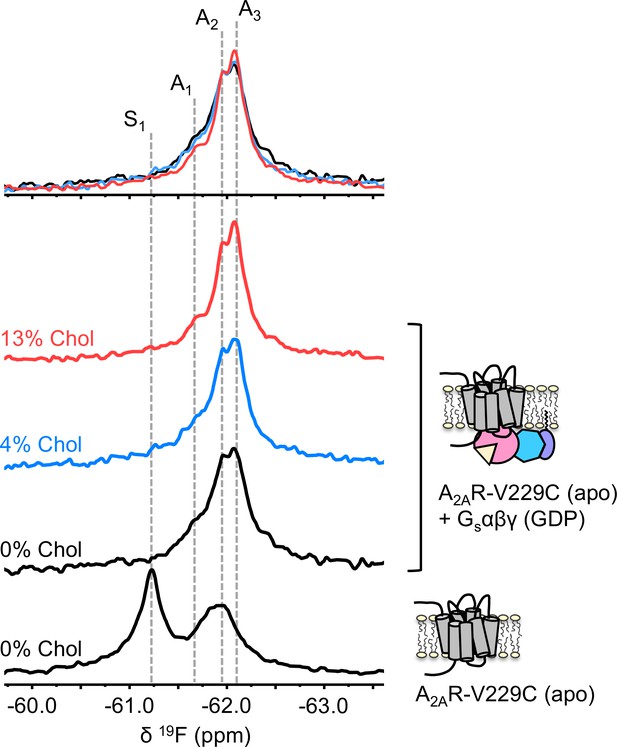
The precoupled complex of A2AR-Gsαβγ is stabilized by cholesterol.
19F NMR spectra of apo A2AR in the presence of 0, 4, and 13% cholesterol, and as a function of Gαβγ. The key functional states are indicated by gray dashed lines and the three spectra in the presence of G protein are normalized via the A2 state.
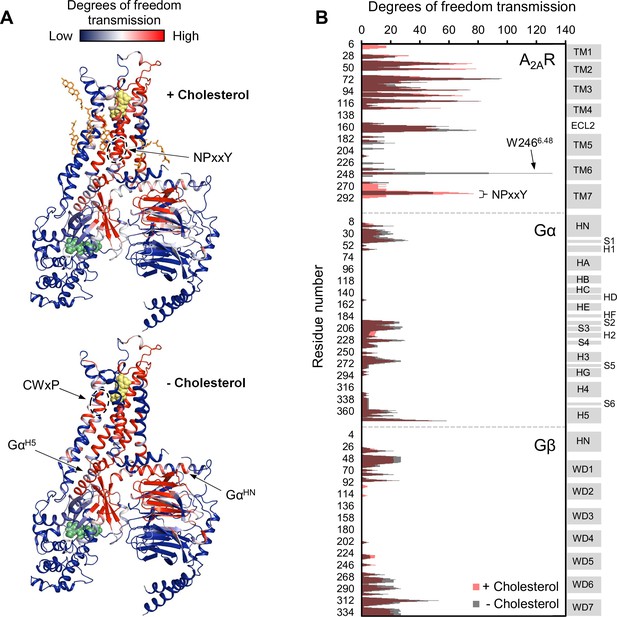
Removal of cholesterol leads to a small overall enhancement in allosteric transmission from the agonist binding site.
(A) Allosteric networks within the A2AR-Gαβγ complex in the presence and absence of cholesterol, revealed through RTA analysis via rigidification of the agonist NECA (yellow spheres). The intensity of allosteric transmission is measured by the resulting regiospecific changes in degrees of freedom and is mapped in color (red/blue gradient bar). Cholesterol molecules are shown as orange sticks while green spheres represent GDP. (B) The intensity of allosteric transmission is plotted for each residue in A2AR, Gα, and Gβ. Secondary structural elements are indicated on the right. Gray blocks denote α-helices and β-strands, while white gaps represent loops. For the Gα subunit, the common Gα numbering system is used (Flock et al., 2015).
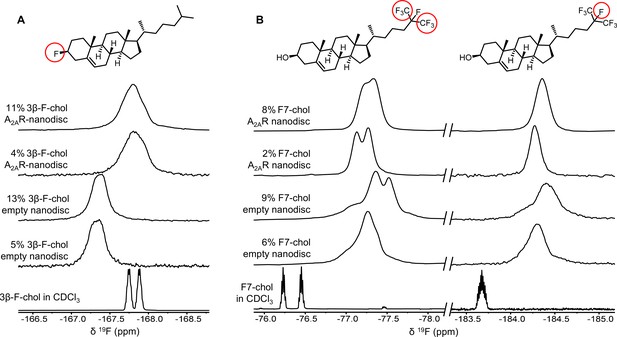
19F-cholesterol analogs interact with A2AR but show no clear evidence of a long-lived bound state.
Non-decoupled 19F NMR spectra of 3β-F-chol (A) and F7-chol (B) in chloroform, empty nanodisc, and A2AR (apo)-embedded nanodisc. The fluorine groups contributing to each of the resonances are circled and shown above the corresponding peak.
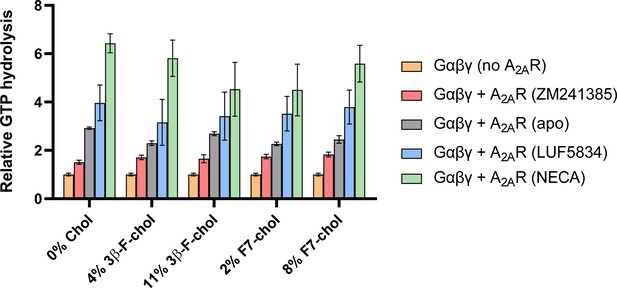
Incorporation of 19F-cholesterol analogs into nanodiscs did not affect A2AR ligand sensitivity and G-protein activation.
Cumulative hydrolysis of GTP by Gαβγ in the presence of A2AR-nanodiscs with and without 19F-cholesterol analog, relative to GTP hydrolysis by Gαβγ alone in the absence of A2AR. To assess ligand sensitivity, samples were saturated with either inverse agonist (ZM241385), no ligand, partial agonist (LUF5834), or full agonist (NECA). Data represent mean ± SEM (n = 3, technical triplicates).
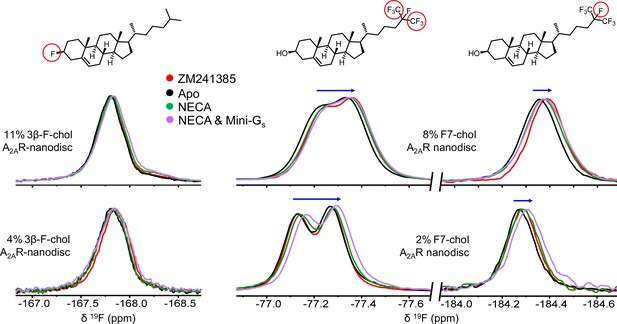
19F-cholesterol analogs behave similarly in the presence of agonist- and inverse agonist-bound A2AR.
19F NMR spectra of 3β-F-chol (A) and F7-chol (B) in A2AR-embedded nanodiscs in the presence of inverse agonist (ZM241385), no ligand, full agonist (NECA), or NECA+ mini Gs. The fluorine groups giving rise to each resonance are shown above their corresponding peaks and the blue arrows indicate the direction of chemical shift change in response to the addition of ligand and mini-Gs.
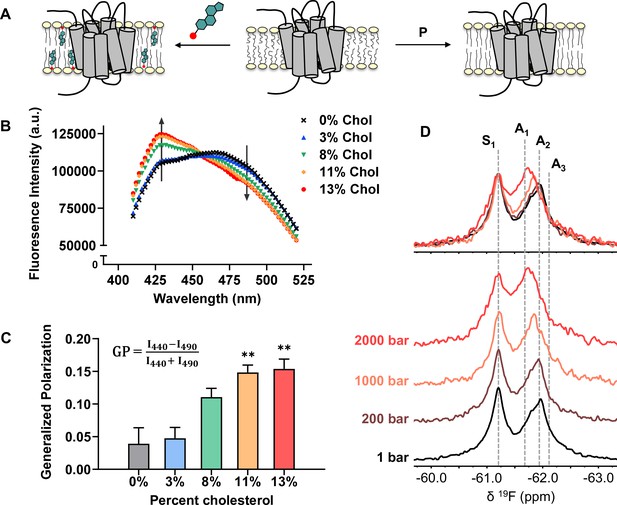
Cholesterol allostery in A2AR is likely a result of indirect membrane effects.
(A) The lipid bilayer can be rigidified and thickened upon addition of cholesterol or an increase in lateral pressure. (B) Averaged fluorescence spectra (n = 4) of Laurdan in A2AR-embedded nanodiscs containing varying concentrations of cholesterol. (C) The emission intensity of Laurdan at 440 nm and 490 nm were used to calculate the generalized polarization values. Data represent mean ± SEM (n = 4, technical triplicates). Astrisks represent statistical significance over both the 0% and the 3% conditions. Statistical significance was determined via one-way ANOVA followed by Tukey’s multiple comparisons test. ** p ≤ 0.01. (D) 19F NMR spectra of A2AR, in the absence of ligand or cholesterol, acquired at 1, 200, 1000, and 2000 bar pressures. The key functional states are indicated by gray dashed lines.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Pichia pastoris) | SMD 1163 | Invitrogen | A2AR expression host | |
| Strain, strain background (Escherichia coli) | BL21 (DE3) | Invitrogen | Cat#: C600003 | Gα expression host |
| Strain, strain background (Spodoptera frugiperda) | Sf9 | ATCC | ATCC: CRL-1711 | Gβγ expression host |
| Recombinant DNA reagent | Plasmid: pET15b containing wild type Gsα | This paper | The plasmid is available upon request to the corresponding author | |
| Commercial assay or kit | GTPase-Glo | Promega | Cat#: V7681 | For GTP hydrolysis assay |
| Commercial assay or kit | Cholesterol quantification kit | R-Biopharm and Roche Diagnostics | Cat#: 10139050035 | |
| Chemical compound, drug | 2-Bromo-N-(4-(trifluoromethyl)phenyl)acetamide (BTFMA) | Apollo Scientific | Cat#: PC8478 | |
| Chemical compound, drug | 5'-N-Ethylcarboxamidoadenosine (NECA) | Tocris | Cat#: 1,691 | |
| Chemical compound, drug | LUF 5834 | Tocris | Cat#: 4,603 | |
| Chemical compound, drug | ZM 241385 | Tocris | Cat#: 1,036 | |
| Chemical compound, drug | Methyl-β-cyclodextrin | Millipore Sigma | Cat#: 332,615 | |
| Chemical compound, drug | Guanosine 5'-diphosphate (GDP) | Millipore Sigma | Cat#: 51,060 | |
| Chemical compound, drug | 1-Palmitoyl-2-oleoyl-glycero-3-phosphocholine (POPC) | Avanti Polar Lipids | Cat#: 850457 C | |
| Chemical compound, drug | 1-Palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1'-rac-glycerol) (POPG) | Avanti Polar Lipids | Cat#: 840457 C | |
| Chemical compound, drug | Cholesterol | Millipore Sigma | Cat#: C8667 | |
| Chemical compound, drug | F7-Cholesterol (25,26,26,26,27,27,27-heptafluorocholesterol) | Avanti Polar Lipids | Cat#: 700,002 | |
| Chemical compound, drug | 3β-Fluoro-cholest-5-ene | This paper | Synthetic methods are described in this paper. The compound is available upon request to the corresponding author | |
| Chemical compound, drug | Laurdan | Millipore Sigma | Cat#: 850582 P | |
| Software, algorithm | MestReNova version 12.0.4 or higher | Mestrelab Research | https://mestrelab.com/ |





