Reversing chemorefraction in colorectal cancer cells by controlling mucin secretion
Figures
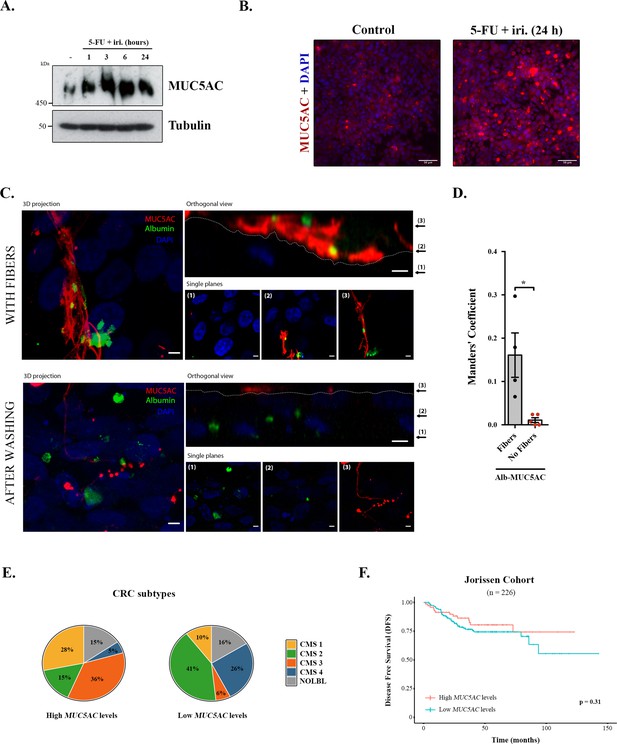
Mucins in colorectal cancer.
(A) Cell lysates from differentiated HT29-M6 cells treated with 5-fluorouracil + irinotecan (5-FU + iri.) (50 µg/mL 5-FU + 20 µg/mL iri.) for 24 hr were analysed by Western blot with an anti-MUC5AC to test expression levels. Tubulin was used as a loading control. (B) Immunofluorescence Z-stack projections of differentiated HT29-M6 cells treated with vehicle (control) or 5-FU + iri. (50 µg/mL 5-FU + 20 µg/mL irinotecan) for 24 hr. Cells were stained with anti-MUC5AC (red) and DAPI (blue). Scale bar = 50 µm. (C) Differentiated HT29 cells were treated with 100 µM ATP for 30 min, washed extensively (upper panel) or smoothly (lower panel), and incubated with Alexa Fluor 488-labelled albumin for 1 hr. Cells were stained with anti-MUC5AC antibody (red) and DAPI (blue). In the orthogonal view, dotted lines across the images demarcate the top surface of the cell. Scale bars correspond to 5 µm. (D) Colocalization between MUC5AC and albumin was calculated from immunofluorescence images by Manders’ coefficient using Fiji. Average values ± SEM are plotted as scatter plot with bar graph. The y-axis represents Manders’ coefficient of the fraction of albumin overlapping with MUC5AC (N ≥ 3). (E) Consensus molecular subtype (CMS) distribution of high and low MUC5AC-expressing colorectal tumours (Jorissen cohort). (F) Disease-free survival (DFS) of colorectal cancer patients with high (n = 73) or low (n = 153) MUC5AC levels (Jorissen cohort). NOLBL: samples without a defined CMS subtype (tumours with no label). *p<0.05, **p<0.01.
-
Figure 1—source data 1
Uncropped gels for Figure 1.
- https://cdn.elifesciences.org/articles/73926/elife-73926-fig1-data1-v2.zip

Experimental design for measuring mucin production after treatment.
(A) Schematic diagram of the experimental set-up to measure mucin production after 5-fluorouracil + irinotecan (5-FU + iri.) treatment.
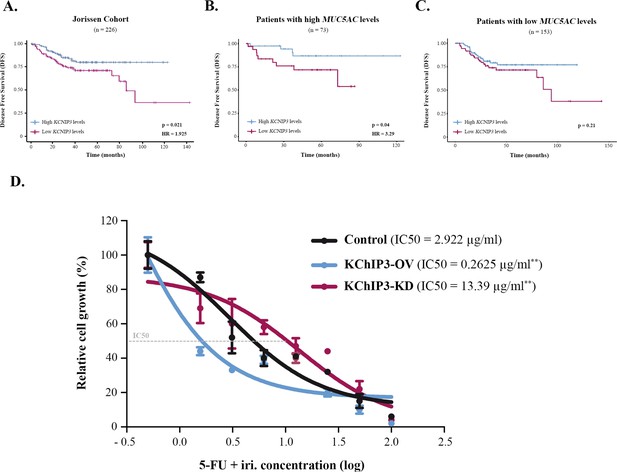
KChIP3 is a prognostic marker of colorectal cancer (CRC).
(A–C) Disease-free survival (DFS) according to KChIP3 levels of CRC patients (low KChIP3 levels, n = 120; high KChIP3 levels, n = 106) (A), CRC patients with high MUC5AC levels (low KChIP3 levels, n = 39; high KChIP3 levels, n = 34) (B), or CRC with low MUC5AC levels (low KChIP3 levels, n = 81; high KChIP3 levels, n = 72) (C). (D) Differentiated control (black), KChIP3-overexpressing cells (KCNIP3-OV, blue) and KChIP3-depleted cells (KCNIP3-KD, red) were treated for 72 hr with increasing concentrations of 5-fluorouracil + irinotecan (5-FU + iri.). Average values ± SEM are plotted as scatter plot (N > 3). The y-axis represents the percentage of cell growth relative to the lowest concentration of 5-FU + iri. The IC50 was calculated from the interpolated curve. HR, hazard ratio. *p<0.05, **p<0.01.
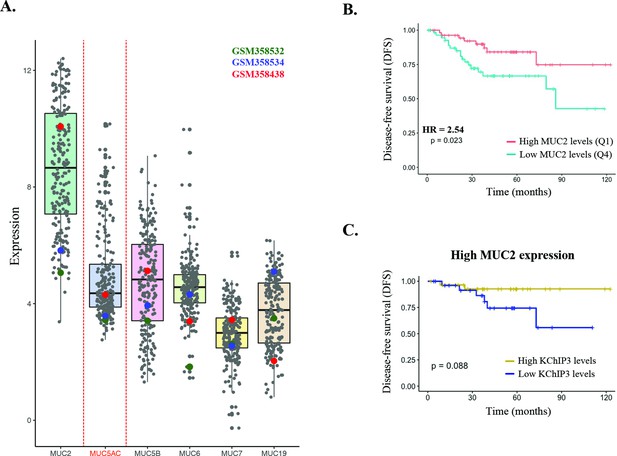
Expression of mucins in patients.
(A) Expression levels of secreted mucins MUC2, MUC5AC, MUC5B, MUC6, MUC7, and MUC19. Each dot represents a different patient. Patients GSM358532, GSM358535, and GSM358538 are highlighted in green, blue, and red, respectively. (B) Disease-free survival (DFS) of colorectal cancer patients with high (n = 73) or low (n = 153) MUC2 levels (Jorissen cohort). (C) DFS according to KChIP3 levels of colorectal cancer patients with high MUC2 expression (low KChIP3 levels, n = 27; high KChIP3 levels, n = 30).
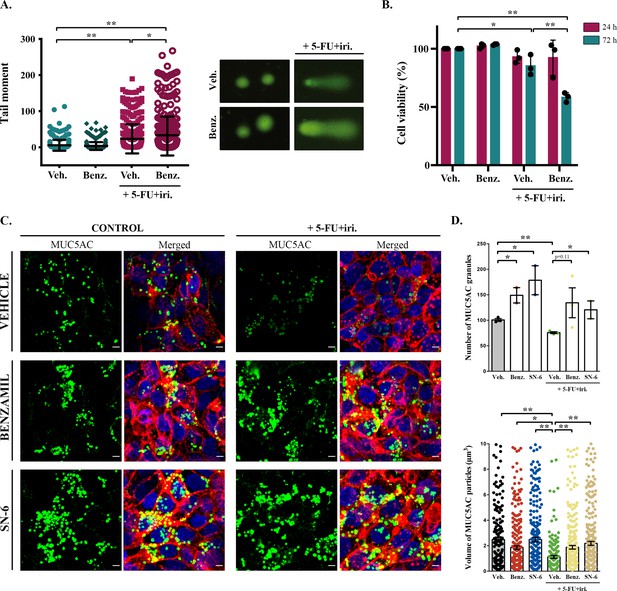
Inhibition of sodium/calcium exchangers (NCXs) enhances cell death by 5-fluorouracil + irinotecan (5-FU+ iri.).
(A) Comet assay of HT29-M6 cell line treated with 5-FU + iri. (25 µg/mL 5-FU + 10 µg/mL irinotecan) and 20 µM benzamil, alone or in combination. The tail moment was measured after 72 hr of treatment (N > 3). (B) Quantification of cell viability in HT29-M6 cell line after treatment (24 or 72 hr) with 5-FU + iri. (10 µg/mL 5-FU + 4 µg/mL Irinotecan) and 20 µM benzamil, alone or in combination (N ≥ 3). (C) Immunofluorescence Z-stack projections of differentiated HT29-M6 cells treated with vehicle, 20 µM benzamil, or 10 µM SN-6 in the presence or absence of 5-FU + iri. Cells were stained with anti-MUC5AC (green), phalloidin (red), and DAPI (blue). Scale bar = 5 µm. (D) Quantification of the number (upper graph) and volume (lower graph) of MUC5AC granules from immunofluorescence images by confocal microscope. Average values ± SEM are plotted as scatter plot with bar graph (N ≥ 3). Veh., vehicle; Benz., benzamil. *p<0.05, **p<0.01.
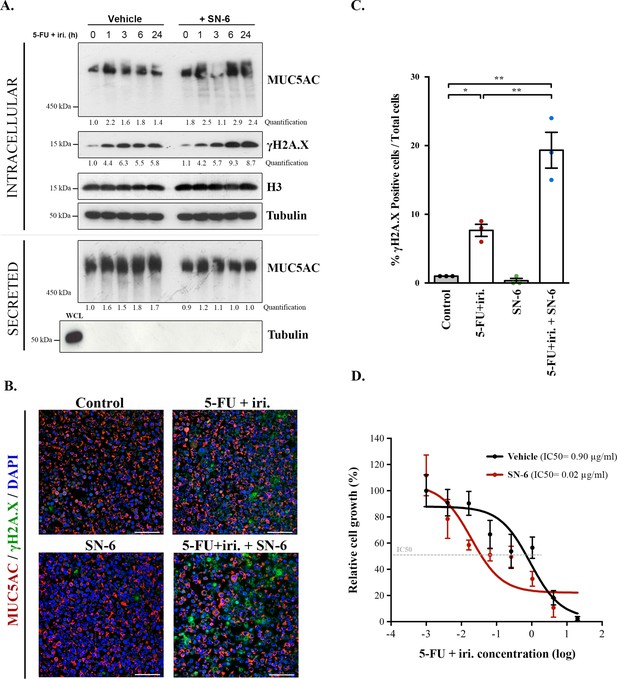
SN-6 treatment increases sensitivity of colorectal cancer (CRC)-derived cells and organoids to 5-fluorouracil + irinotecan (5-FU + iri.).
(A) Cell lysates and secreted medium of differentiated HT29-M6 cells pre-treated with a vehicle or 10 µM SN-6 inhibitor for 24 hr and then exposed to 5-FU + iri. (50 µg/mL 5-FU + 20 µg/mL iri.) for 0, 1, 3, 6, and 24 hr were analysed by Western blot with an anti-MUC5AC, anti-γH2A.X, and H3 to test their levels. Tubulin was used as a loading control. Quantification of MUC5AC and γH2A.X is included (N ≥ 3). (B) Immunofluorescence images of differentiated HT29-M6 cells treated with vehicle (control) or 10 µM SN-6 in the presence or absence of 5-FU + iri. (50 µg/mL 5-FU + 20 µg/mL irinotecan) for 24 hr. Cells were stained with anti-MUC5AC (red), anti-γH2A.X (green), and DAPI (blue). Scale bar = 50 µm. (C) Quantification of the number of γH2A.X-positive cells in the different conditions relative to the total number of cells from the immunofluorescence images (N ≥ 3). (D) CRC patient-derived organoids (PDOs) were treated for 72 hr with increasing concentrations of 5-FU + iri. with vehicle or 10 µM SN-6. Average values ± SEM are plotted as scatter plot. The y-axis represents the percentage of cell growth relative to the lowest concentration of 5-FU + iri. The IC50 was calculated from the interpolated curve (N ≥ 3). *p<0.05, **p<0.01.
-
Figure 4—source data 1
Uncropped gels for Figure 4.
- https://cdn.elifesciences.org/articles/73926/elife-73926-fig4-data1-v2.zip
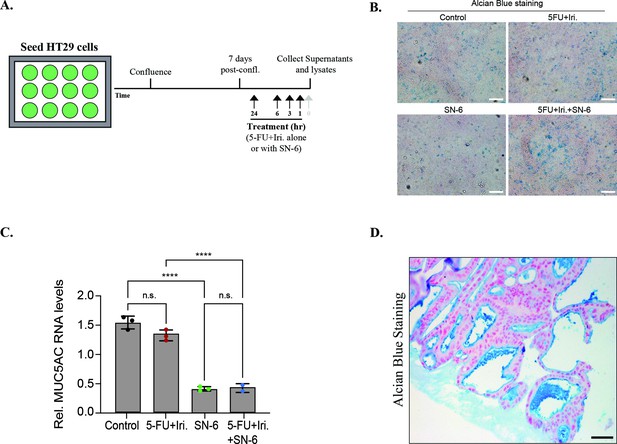
Mucins’ levels in HT29 cells and in a patient-derived organoid.
(A) Schematic diagram of the experimental set-up to measure mucin secretion and production. (B) Representative images of HT29-M6 differentiated cell (vehicle [control], 5-fluorouracil + irinotecan [5-FU+ iri.], SN-6, and 5-FU + iri. + SN-6) stained with Alcian blue. Scale bar = 50 µm. (C) Relative MUC5AC RNA levels in HT29-M6 treated with vehicle (control), 5-FU + iri., SN-6, and 5-FU + iri. + SN-6 (N = 3). (D) Alcian blue-stained patient-derived organoid to visualize external mucins (in blue). Scale bar = 50 µm.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (Homo sapiens) | MUC5AC | Ensembl | ENSG00000215182 | |
| Gene (H. sapiens) | MUC2 | Ensembl | ENSG00000198788 | |
| Gene (H. sapiens) | MUC6 | Ensembl | ENSG00000184956 | |
| Gene (H. sapiens) | MUC5B | Ensembl | ENSG00000117983 | |
| Gene (H. sapiens) | MUC19 | Ensembl | ENSG00000205592 | |
| Gene (H. sapiens) | KCNIP3 | Ensembl | ENSG00000115041 | |
| Cell line (H. sapiens) | HT29-M6 | ATCC | CVCL_G077 | Mycoplasma free |
| Cell line (H. sapiens) | HT29-18N2 | ATCC | CVCL_5942 | Mycoplasma free |
| Antibody | Anti-MUC5AC (mouse monoclonal) | Neomarkers,Waltham, MA | Clone 45M1 | (1:1000) |
| Antibody | Anti-γH2A.X (mouse monoclonal) | Cell Signaling | #2577 | (1:1000) |
| Commercial assay or kit | CometAssay Trevigen Kit | Trevigen | 250-050K | |
| Chemical compound, drug | SN-6 | Sigma-Aldrich | SML1937-5MG | (5 µM) |
| Chemical compound, drug | Benzamil | Sigma-Aldrich | B2417-10MG | 5 µM |
Primer sequences used for detecting mRNA for the respective genes.
| Gene | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|
| MUC5AC | CTGGTGCTGAAGAGGGTCAT | CAACCCCTCCTACTGCTACG |
| TBP | TGCCCGAAACGCCGAATATAATC | GTCTGGACTGTTCTTCACTCTTGG |
| GAPDH | GTCATCCCTGAGCTGAACG | CTCCTTGGAGGCCATGTG |





