Cryo-EM structures reveal high-resolution mechanism of a DNA polymerase sliding clamp loader
Figures
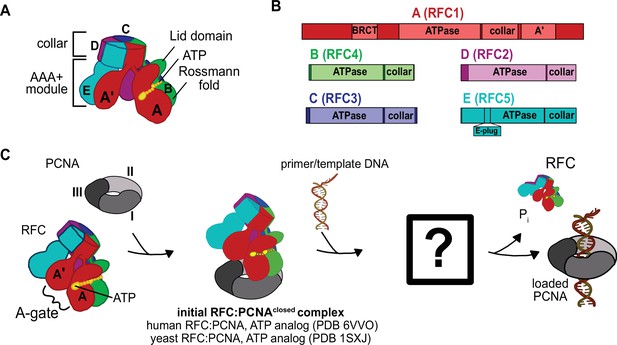
Architecture of the eukaryotic clamp loader (RFC) and clamp (PCNA).
(A) RFC is composed of five different subunits (named A–E) that each consist of the AAA+ ATPase module and a collar domain. The nucleotide-binding site is sandwiched between the N-terminal Rossmann fold domain and the Lid domain of the ATPase module at the subunit interface. The ATPase module and a C-terminal extension of the A subunit called the A′-domain form the A-gate. (B) Domain organization of RFC subunits. (C) Clamp loading begins with binding of ATP to RFC, followed by PCNA binding. How PCNA is opened and DNA binds to the open RFC:PCNA complex is not known. DNA-binding triggers ATP hydrolysis, PCNA closure, and RFC ejection. Structures obtained prior for RFC:PCNA complexes are indicated (Bowman et al., 2004; Gaubitz et al., 2020).
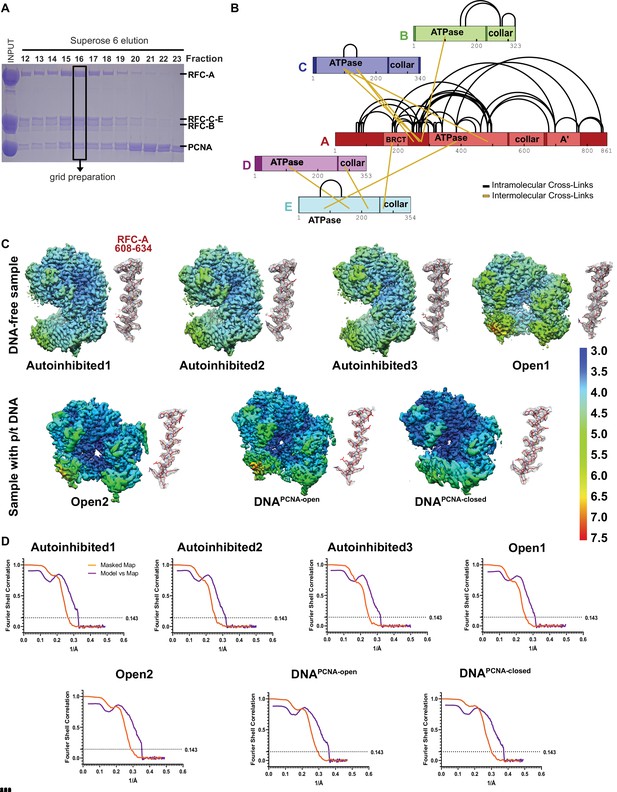
Characterization and cryo-EM of full-length RFC:PCNA.
(A) Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) gel of purified RFC and PCNA after gel filtration. A fraction with stoichiometric amounts of RFC and PCNA was used for grid preparation. (B) Crosslinking of the RFC:PCNA at a concentration of 1 mM bis(sulfosuccinimidyl)suberate (BS3) led to the identification of intermolecular and intramolecular crosslinks in RFC, and are shown in schematic representation. 88% of the crosslinks mapped to RFC-A and no crosslinks in PCNA were detected, although PCNA was detected in the sample. (C) Local resolution of reconstructions (center) and a representative section of each complex subunit for each reconstruction. (D) Fourier shell correlation (FSC) curves for the two halves of the reconstructions as well as model vs map curves.
-
Figure 1—figure supplement 1—source data 1
Image of SDS-PAGE gel of fractions from gel filtration of RFC and PCNA.
- https://cdn.elifesciences.org/articles/74175/elife-74175-fig1-figsupp1-data1-v2.zip
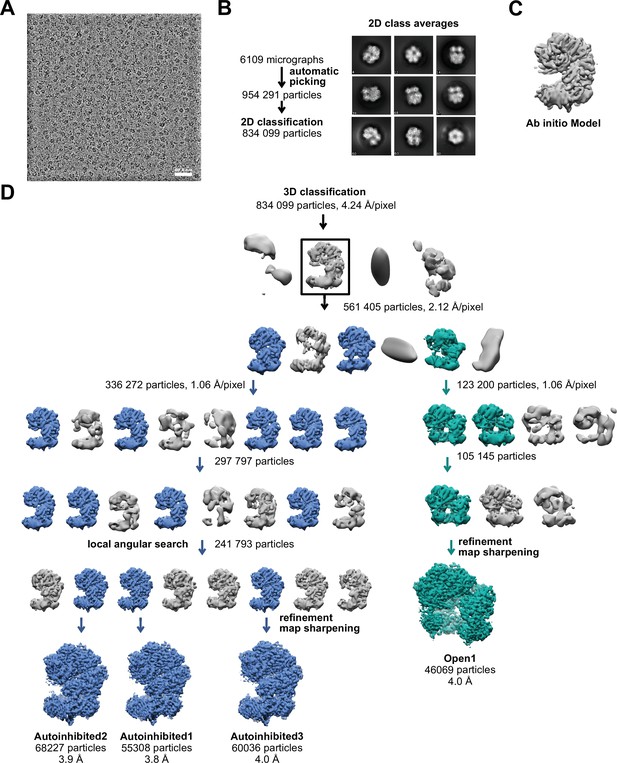
Schematic of yRFC:PCNA cryo-EM processing.
(A) A downfiltered micrograph taken on a Thermo Fisher Scientific Titan Krios with a Gatan K2 detector is displayed. (B) 2D class averages show different side views. (C) The 3D reference for refinement was generated ab initio with cisTEM (Grant et al., 2018). (D) The ab initio model was downfiltered to 50 Å and used as reference for 3D classification. The first round of classification was performed with the 2× binned particle stack. Further rounds of classification with the unbinned stack improved the resolution. 3D classification with local angular search further helped to improve the resolution of the reconstructions representing complexes with closed PCNA (blue).
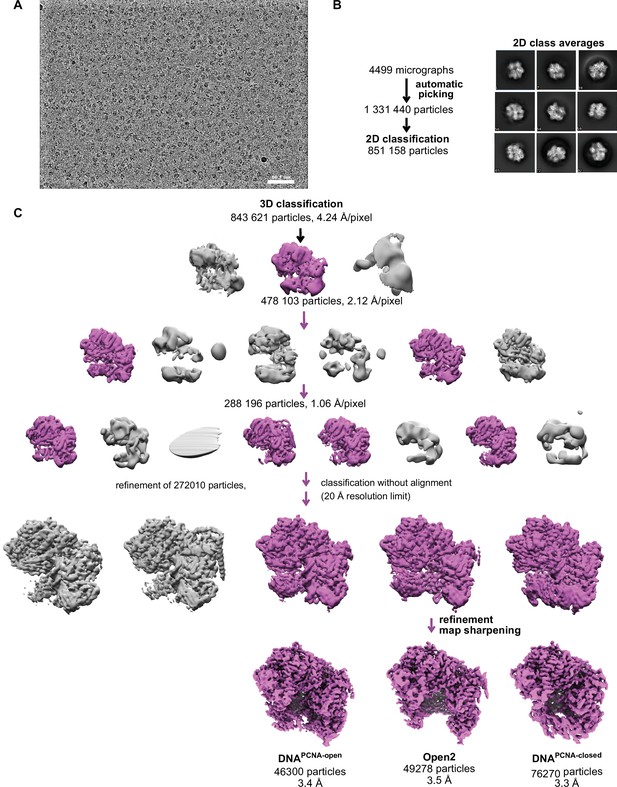
Schematic of yRFC:PCNA:DNA cryo-EM processing.
(A) A downfiltered micrograph taken on a Thermo Fisher Scientific Titan Krios with a Gatan K3 detector is displayed. (B) 2D class averages show different side views. (C) Class Open1 of the dataset without DNA was downfiltered to 60 Å and used as reference for 3D classification. The first round of classification was performed with the 4× binned particle stack and the second round of classification with the 2× binned stack. Further classification with the unbinned stack with resolution limit and without alignment helped to separate different conformational states and improved the resolution.
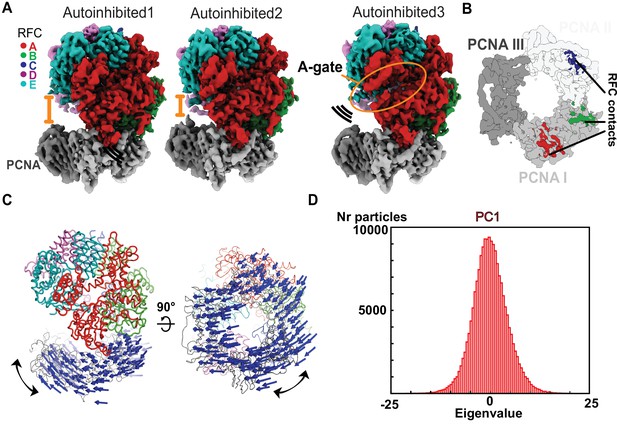
The Autoinhibited state is dynamic.
(A) Cryo-EM maps of the three Autoinhibited conformations of the RFC:PCNA complex. PCNA tilts closer relative to RFC in Autoinhibited2. The subunit arrangement of the AAA+ module of Autoinhibited3 is changed slightly, which leads to a crack in the A-gate. (B) Top view on the contact sites of PCNA with RFC in the autoinhibited conformation. (C) Principal component analysis of all Autoinhibited particles reveals a rocking motion of PCNA relative to RFC. The Cα displacement of principal component 1 (PC1) is indicated by arrows, scaled down by a factor of 2. (D) Principal component analysis reveals a range of motions within the initial RFC:PCNA complex. Amplitude histogram of the first principal component (PC1) reveals a unimodal distribution of particles, suggesting that this state consists of related particles in continuous motion.
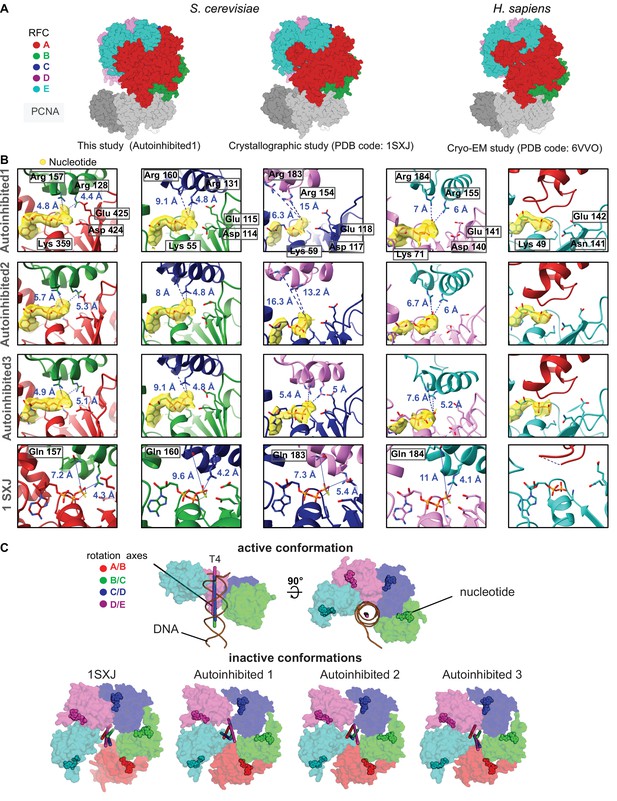
RFC:PCNA complexes in autoinhibited conformations.
(A) Side views of the atomic models of Autoinhibited1, the RFC:PCNA crystal structure (Bowman et al., 2004), and of the human RFC:PCNA complex show similarity. (B) Closeup of the nucleotide-binding sites. The cryo-EM map is shown in yellow overlaying the atomic model. The catalytically important Walker A lysine, Walker B glutamates, and trans-acting arginine fingers are shown. The arginine fingers are distant within the active sites of RFC-B,C,D in Autoinhibited1 and 2 and in RFC-B,D of Autoinhibited3, rendering these active sites inactive. For comparison, the nucleotide-binding sites in the atomic model of the RFC:PCNA crystal structure (PDB 1SXJ) are shown. Here, the SRC motif arginine fingers were mutated to glutamines. RFC-E is not catalytically competent and has ADP bound. The A′ domain does not donate trans-acting arginine fingers. (C) Top views on the AAA+ spiral of the T4 clamp loader, RFC:PCNA crystal structure and Autoinihibited1–3. The T4 clamp loader, which has DNA bound, is in an active conformation. Here, the rotation axes that relate the subunits are coincident with each other and the central axis of DNA. In contrast, the symmetry of the AAA+ spiral of RFC in the autoinhibited conformation is distorted, and the axes are skewed in all these structures.
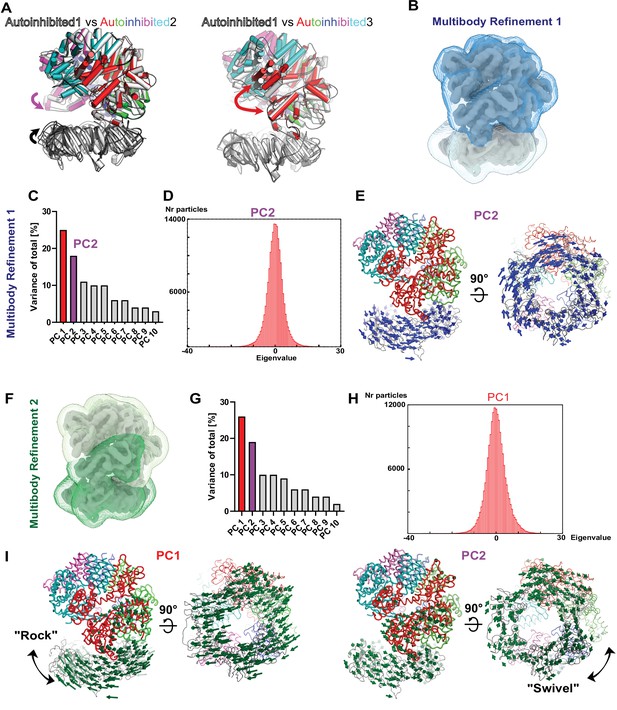
Multibody analyses with all 183,571 particles combined from the three Autoinhibited states to investigate the dynamic initial complex of RFC with PCNA.
(A) Superpositions of Autoinhibited 1 and 2 and 1 and 3 highlight differences in the orientation of PCNA toward RFC as well as the opening of the A-gate. (B) The two masks used for the first multibody refinement define PCNA and RFC as separate rigid bodies. (C) Principal component (PC) analysis revealed the two most dominant motions with RFC and PCNA. (D) Amplitude histogram of PC2 is unimodal. (E) PC2 reveals a swiveling motion. The Cα displacement is indicated by modevector generated arrows, scaled down by a factor of 2. (F) The two masks used for the second multibody refinement were chosen to match domain boundaries determined with the ENM DynOmics server (Li et al., 2017) and to capture motion between the A′ and the AAA+ module of the A-gate. (G) PC analysis revealed two dominant motions. (H) Amplitude histogram of PC1 is unimodal. (I) Multibody refinement two also revealed rocking and swiveling as dominant motions.
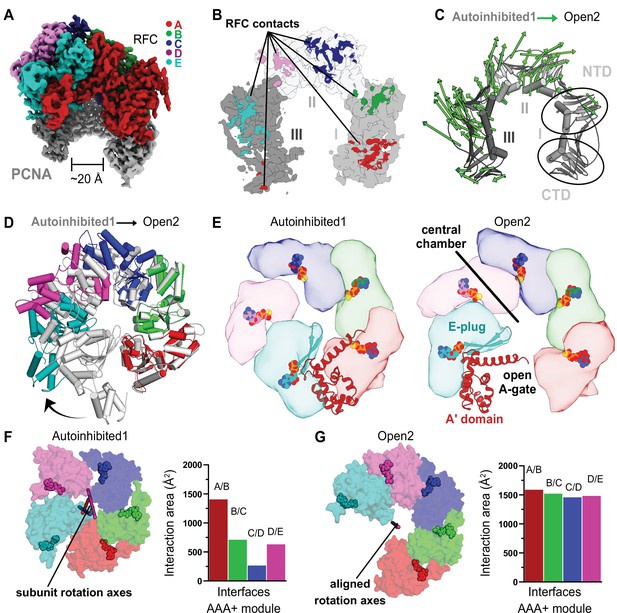
RFC undergoes a large conformational change to open PCNA.
(A) Cryo-EM map of RFC bound to an open PCNA ring. (B) PCNA is held open by contacts with all five subunits of RFC. (C) The Cα displacement from closed to open PCNA is indicated by arrows, scaled up by a factor of 4. (D) The AAA+ modules widen from the Autoinhibited state (gray) to an open spiral conformation. (E) Top view of the AAA+ spiral shows that the E-plug and A-gate block access to RFC’s central DNA-binding chamber in the Autoinhibited conformation but retract in the open conformation. RFC opens wide enough for DNA to directly enter the central chamber. (F) Top view of the Rossmann fold arrangement in the Autoinhibited conformation. The rotation axes that relate neighboring subunits are shown in different colors and are skewed, indicating asymmetric rotations which lead to gaps between the subunits. (G) The rotation axes overlay in the Open2 state of RFC, indicating a symmetric arrangement of the AAA+ spiral. Symmetrization closes the gaps, and results in an increased interaction area between neighboring subunits.
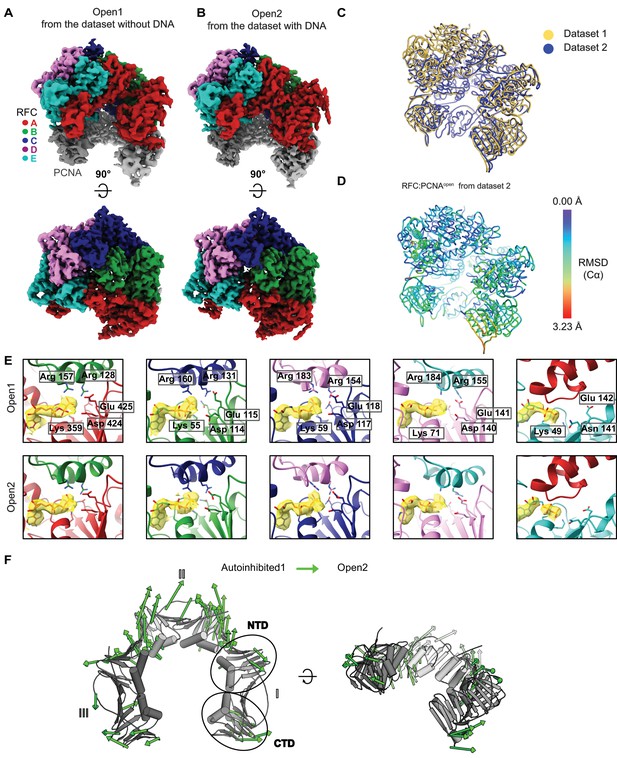
Comparison of RFC bound to open PCNA from different datasets.
(A) Top and side views of the cryo-EM map of RFC bound to open PCNA, which was obtained from the dataset without DNA. (B) Top and side views of the cryo-EM map of RFC bound to open PCNA obtained from the dataset with DNA. (C) Overlay of the two models for Open1 and Open2 shows that the two models strongly resemble each other. (D) Open1 superposed to Open2. Open2 is colored by RMSD. (E) Closeup of the nucleotide-binding sites in Open1 and in Open2. The cryo-EM map is shown in yellow overlaying the atomic model. Critical catalytic residues are shown as sticks. All active sites are occupied with ATPγS. (F) PCNA intrasubunit distortions that occur for opening. The Cα displacement is indicated by modevector-generated arrows, scaled up by a factor of 4.
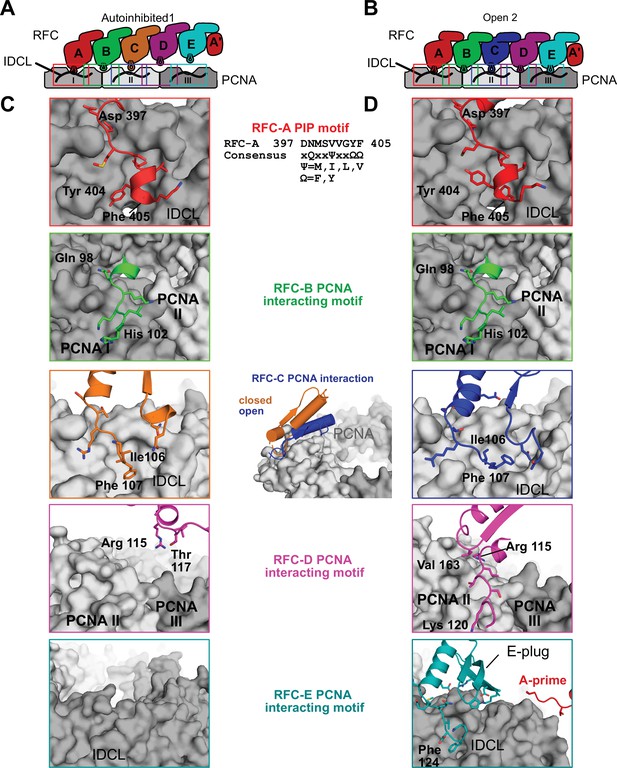
RFC interacts with all five subunits to hold PCNA open.
(A) Overview of interaction sites of RFC with PCNA in the autoinhibited conformation. The three PCNA subunits and RFC’s AAA + module are shown in a cartoon flattened onto the page. RFC-D and RFC-E are not in contact with PCNA. (B) Cartoon overview of interaction sites of RFC with PCNA in the open conformation. RFC-D and RFC-E now contact PCNA. (C) Closeup views of the RFC–PCNA interaction sites in Autoinhibited1 are shown, the rest is omitted for clarity. The contact between PCNA and RFC-A is mediated by a short helix and adjacent hydrophobic and aromatic residues that insert into PCNA’s hydrophobic pockets. This conformation is commonly seen in binding partners which contain a PCNA-interacting protein (PIP) motif or derived motifs. (D) Contacts of RFC-A and RFC-B with PCNA do not change significantly upon PCNA opening. The interaction of PCNA with RFC-C becomes more extensive, and RFC-D and RFC-E establish new contacts to PCNA. RFC-C and RFC-E insert into PCNA’s hydrophobic pocket but do not employ a PIP motif. The E-plug and A′ domain reinforce the interaction with PCNA.
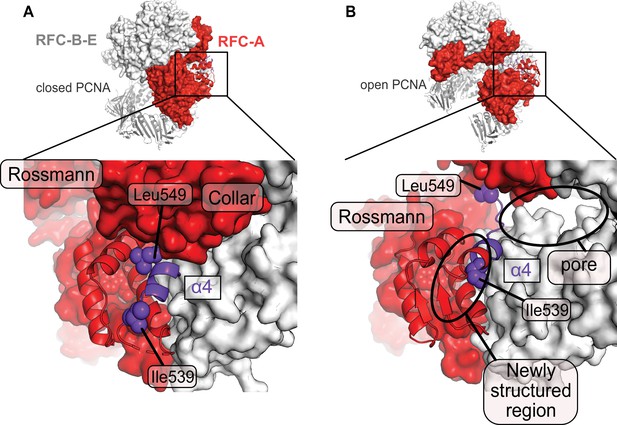
A fold-switch mechanism for opening a pore in the Open state of RFC:PCNA.
(A) Helix 4 of the RFC-A subunit in Autoinhibited1 is shown in purple. (B) In the open conformation, Lid Helix four is displaced and partially unravels, whereby the packing arrangement of the hydrophobic core of the lid domain in RFC-A changes. Ile536 and Leu549 move ~13 and ~22 Å from their original position and a pore is formed between the RFC-A and RFC-B subunits.
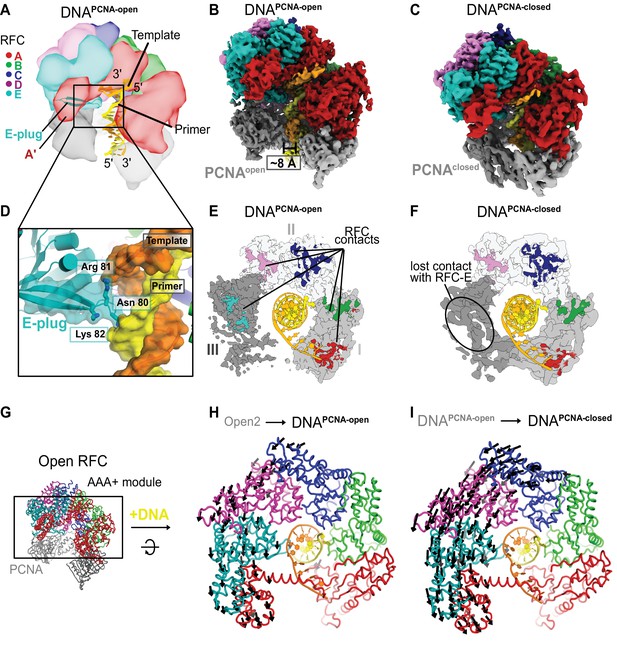
Structures of RFC:PCNA bound to DNA.
(A) Schematic representation of the structure of RFC:PCNA bound to primer–template (p/t) DNA. (B) Cryo-EM map of RFC:PCNA bound to p/t-DNA and open PCNA (termed DNAPCNA-open). (C) Cryo-EM map of RFC:PCNA bound to p/t-DNA with closed PCNA (termed DNAPCNA-closed). (D) The E-plug inserts into the major groove and interacts with both strands of the p/t-DNA. (E) Top view of contact sites of RFC with PCNA. PCNA is held open by contacts with all five subunits in DNAPCNA-open. (F) In DNAPCNA-closed, the interaction between RFC-E and PCNA-III is lost.(G) Overview of structure of Open2. (H) Top view of the AAA+ spiral of DNAPCNA-open. Displacement vectors between Open2 and DNAPCNA-open are indicated by arrows, scaled up by a factor of 2. The AAA+ spiral constricts around DNA. (I) The AAA+ spiral of DNAPCNA-closed. Displacement vectors between DNAPCNA-open and DNAPCNA-closed indicate that the AAA+ spiral constricts further around DNA, leading to changes in ATPase sites.
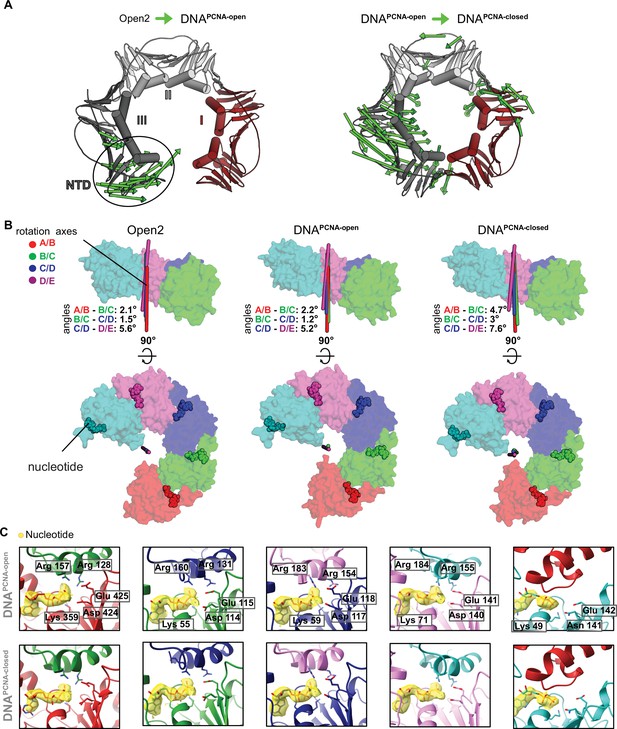
Conformational changes upon DNA binding and ring closure.
(A) PCNA constriction in DNAPCNA-open and DNAPCNA-closed. Displacement vectors between Open2 and DNAPCNA-open are shown as green arrows, scaled by a factor of 4 (left). Displacement vectors between DNAPCNA-open and DNAPCNA-closed are shown as green arrows, scaled by a factor of 4 (right). Upon DNA binding, the PCNA lock-washer constricts in DNAPCNA-open, due to a motion at the NTD of PCNA-III. PCNA is closed in a puckered conformation in DNAPCNA-closed through constricting motions of PCNA-I and PCNA-III. (B) The overlay of the rotation axes in the open conformation of RFC is indicative for spiral symmetry. The tilt angles show that in the DNA-bound structures (DNAPCNA-open and DNAPCNA-closed), the rotation axes become more tilted upon PCNA closure, indicating that DNA binding and PCNA closure slightly disrupt the symmetric arrangement of the AAA+ spiral. (C) Closeup of the nucleotide-binding sites in DNAPCNA-open and DNAPCNA-closed. The cryo-EM map is shown in yellow overlaying the atomic model. Critical catalytic residues are shown as sticks. All active sites are occupied with ATPγS.
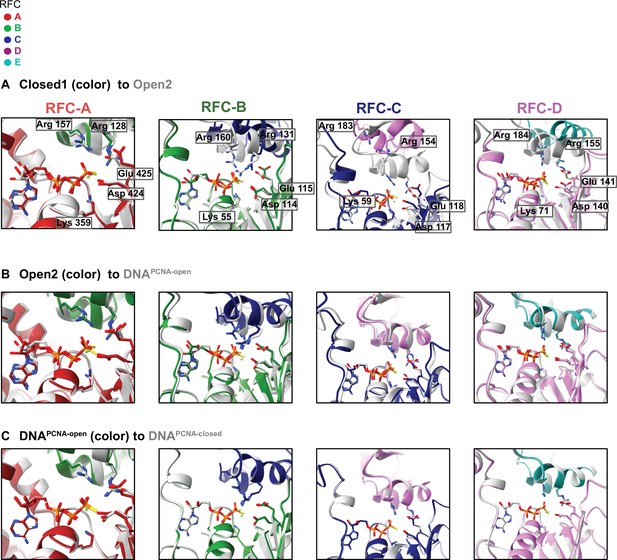
Superposition of the ATPase active sites across conformations.
In all cases, the active sites were aligned about their Walker A and Walker B motifs. (A) Comparing the active sites of Closed1 to Open2. (B) Comparing the active sites of Open2 to DNAPCNA-open. (C) Comparing the active sites of DNAPCNA-open to DNAPCNA-closed. The most substantial change occurs upon clamp opening (A), which brings the arginine fingers in the RFC B–D active sites close to the γ-phosphate. This change does not occur in RFC-A, whose hydrolysis is dispensable for clamp loading. After opening, DNA binding (B) and then clamp closure (C) do not change the active sites much, indicating that DNA binding does not stimulate hydrolysis by reorganizing the ATPase-binding interface.
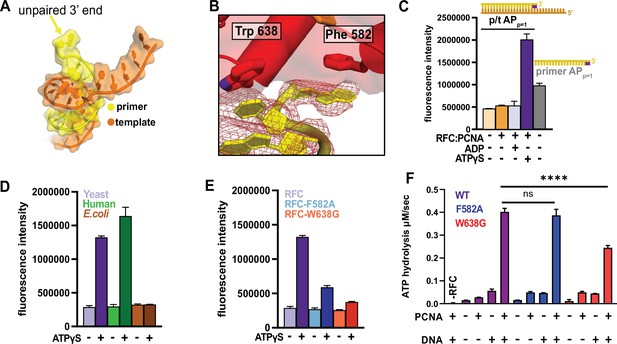
RFC separates the 3′ end of the primer strand.
(A) The last nucleotide in the primer strand is separated from the duplex. (B) The collar of RFC-A contains a ‘separation pin’ with two critical residues (Trp638 and Phe582) that stabilize the flipping of the 3′ primer nucleotide into the pore between RFC-A and RFC-B. The cryo-EM map is shown in red mesh. (C) The primer strand of p/t-DNA contains 3′ nucleotide with a 2-aminopurine (2AP) base, an adenine analog that reports on base-pairing and base-stacking. 2AP fluorescence increases in the presence of ATPγS and RFC:PCNA to a higher level than in the unpaired 2AP-labeled primer strand. (D) The human RFC:PCNA complex also induces an increase in 2AP fluorescence emission, whereas the E. coli clamp loader, which does not flip the 3’ end of the primer (Simonetta et al., 2009), does not increase 2AP fluorescence. (E) Mutation of Phe582 and Trp638 reduces 2-AP fluorescence in the presence of ATPγS. (F) ATPase activity of the ‘separation pin’ mutants.The ATP hydrolysis rate of the RFC-W638G variant is significantly reduced compared to wild type in the presence of PCNA and DNA (p value from one-way ANOVA test: ****p ≤ 0.0001).
-
Figure 6—source data 1
2-Aminopurine fluorescence and ATPase data.
- https://cdn.elifesciences.org/articles/74175/elife-74175-fig6-data1-v2.zip
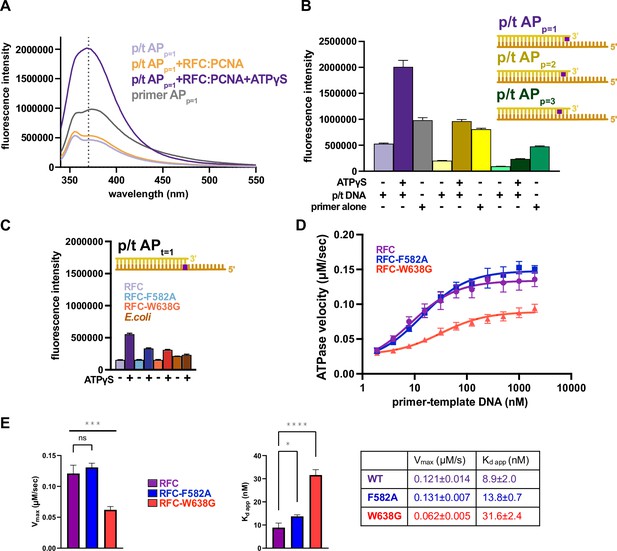
The separation wedge has two critical residues.
(A) Fluorescence intensity traces for 375 nM of p/t APp=1 in the presence of RFC:PCNA with and without nucleotide or primer APp=1. (B) Placement of 2-aminopurine (2AP) at different positions. The fluorescence with p/t APp=1 in the presence of ATPγS and RFC:PCNA increases ~fourfold in relation to the sample without ATPγS, whereas placement of the oligo further away from the 3′OH end reduces the fluorescence increase to ~twofold. (C) Results from (Figure 6B) could be recapitulated using p/t-DNA with 2-AP in the template strand (t = 1). The E. coli clamp loader, which does not have a separation pin, does not change fluorescence in the presence of ATPγS. (D) ATPase activity of the ‘separation pin’ mutants. DNA-binding affinity and maximum ATP hydrolysis rate are reduced in RFCW638G. (E) The steady-state ATP hydrolysis rate of the RFC-W638G variant is significantly reduced compared to wild type (p value = 0.0005). The RFC-F582A variant binds DNA with similar affinity than wild type (p value = 0.0288). DNA-binding affinity for RFC-W638G is slightly reduced (p value <0.0001). Error bars reflect the standard deviation from three replicates. The asterisks correspond to p values from one-way ANOVA tests comparing variants to wild type, ‘ns’ = not significant p > 0.05, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001 .
-
Figure 6—figure supplement 1—source data 1
2-Aminopurine fluorescence and ATPase data.
- https://cdn.elifesciences.org/articles/74175/elife-74175-fig6-figsupp1-data1-v2.zip
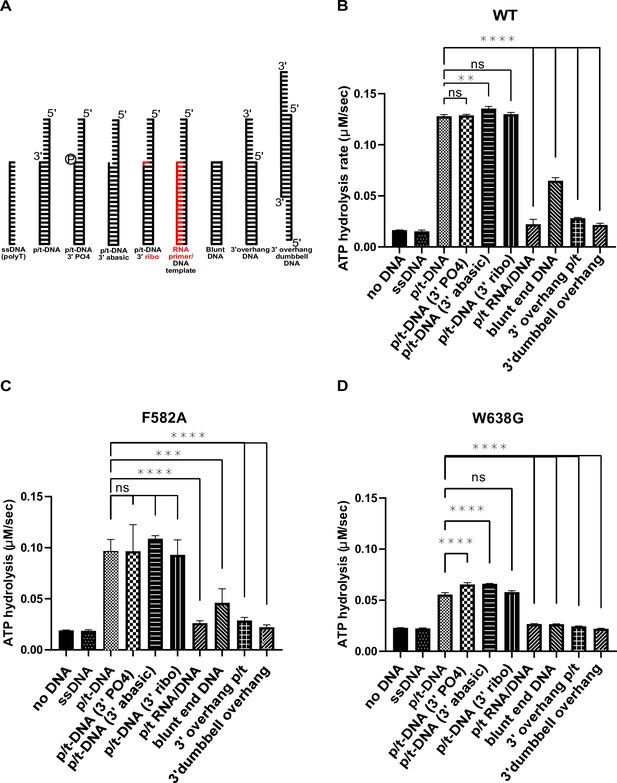
Discrimination of different primer–template junctions by RFC.
(A) Cartoon depiction of different DNA substrates that were used to probe if the separation pin acts to discriminate different moieties at the primer–template junction. (B–D) Similar ATPase activity profiles of the wild type and the two separation pin variants indicate that the separation pin is not critical to discriminate between these DNA substrates. Error bars on these bar graphs reflect the standard deviation from three replicates. The asterisks on these graphs correspond to p values from one-way ANOVA tests comparing different DNA substrates with an unmodified primer–template junction (p/t-DNA): ‘ns’ = not significant p > 0.05, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001.
-
Figure 6—figure supplement 2—source data 1
ATPase data.
- https://cdn.elifesciences.org/articles/74175/elife-74175-fig6-figsupp2-data1-v2.zip
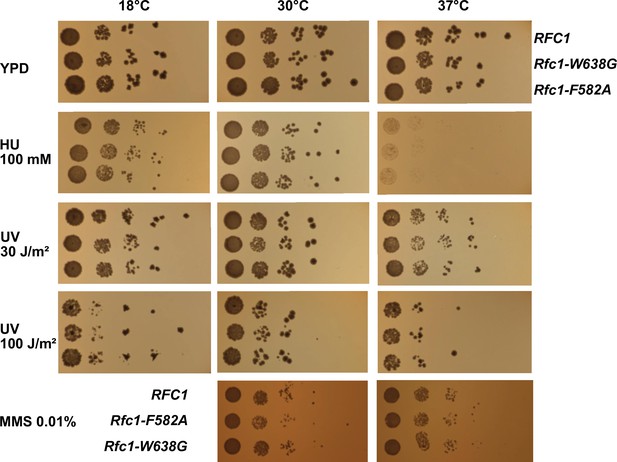
Functional characterization of separation pin mutants in S. cerevisiae.
To directly assess the physiological role of the separation pin using yeast, we performed spot assays with strains that express either wild type RFC1 or the F582A or the W638G variant. The two variants and the wild type display similar colony sizes across a variety of conditions, including varying temperatures, UV radiation, and treatment with hydroxyurea (HU) or methyl methanesulfonate (MMS), indicating that there is no striking phenotype with the tested conditions.
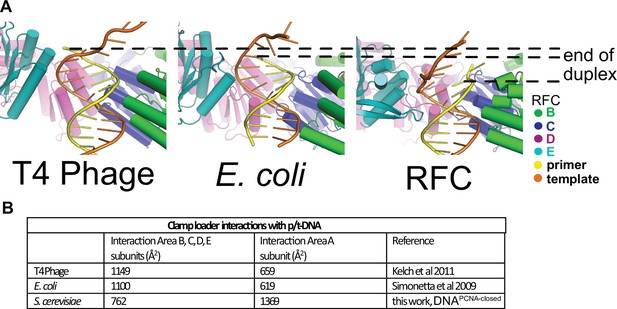
Differences in how duplex p/t-DNA is held in the central chamber of clamp loaders.
(A) In the T4 and E. coli clamp loaders, the duplex p/t-DNA extends farther into the central chamber, enabling more substantial contacts with the B–E subunits compared to RFC. (B) Contribution of RFC subunits to DNA binding. RFC-A dominates contact with p/t-DNA when compared to other clamp loaders.
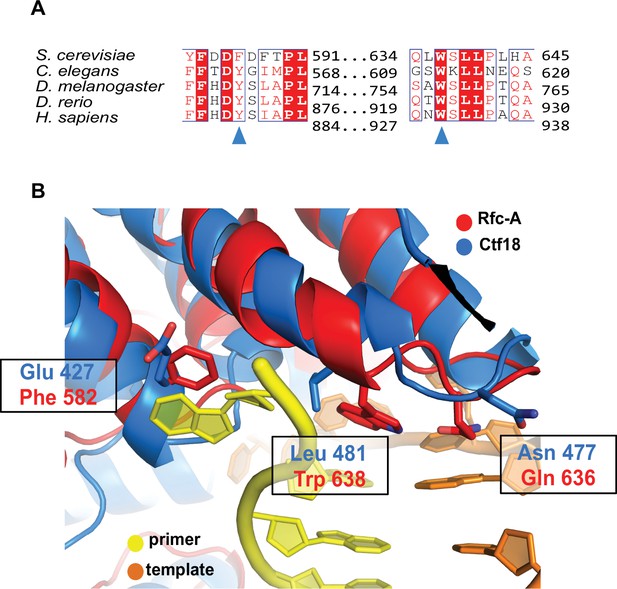
Conservation of the separation pin.
(A) The separation pin is conserved in RFC1 across eukaryotes. Sequence alignment shows the conservation of the ‘separation pin’ among five eukaryotic species. The conserved sequences are marked by blue boxes. The fully conserved residues are in white with a red background, the highly conserved residues are in red, and the less conserved ones are in black. F582 and W638 are pointed out by the blue arrow. (B) A potential separation pin in the Ctf18 clamp loader. The structure of yeast Ctf18 (blue) predicted by AlphaFold (Jumper et al., 2021; Varadi et al., 2022) is superposed onto collar domain of the DNAPCNA-open structure of yeast RFC (RFC1 in red, primer strand in yellow, and template strand in orange). Key residues of the RFC1 separation pin are highlighted (Phe 582, Gln 636, and Trp 638), as well as their predicted counterparts in Ctf18 (Glu427, Asn477, and Leu481). While the separation pin helical hairpin is likely conserved, the key residues that mediate base-flipping in RFC1 are not conserved.
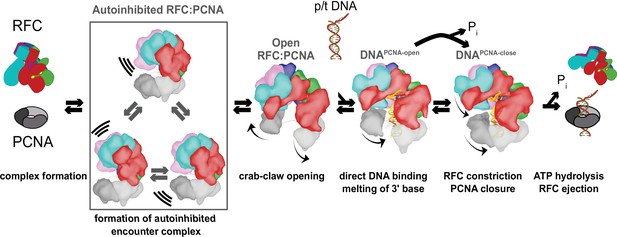
Clamp loading by RFC.
Initial binding of RFC to PCNA places the complex in an Autoinhibited state, whereby closed PCNA and the E-plug preclude DNA binding, and an overtightened AAA+ helix inhibits ATPase activity. The Autoinhibited state is dynamic, rocking PCNA relative to RFC as captured by multibody refinement. Upon complete binding to PCNA, RFC uses the crab-claw mechanism to simultaneously open both PCNA and the A-gate, providing an entryway for p/t-DNA. p/t-DNA then binds directly through the A-gate and open PCNA, which are wide enough to accommodate dsDNA entry. The 3′ end of the primer is flipped into the pore that is formed between RFC-A and RFC-B. PCNA closes to form additional contacts with DNA, partially detaching from RFC at the E subunit. Finally, ATPase activity and inorganic phosphate release eject RFC, leaving PCNA bound to p/t-DNA in the correct orientation.
Videos
RFC:PCNA motion along Eigenvalue 1 with masks on PCNA and RFC.
RFC:PCNA motion along Eigenvalue 2 with masks on PCNA and RFC.
Morph closed to open.
PCNA loading by RFC.
Tables
Clamp loader structures previously obtained for the various states in the clamp loading cycle.
| Clamp loader prior to clamp binding | |||
|---|---|---|---|
| Species | Composition | Reference | PDB accession number |
| E. coli | Clamp loader alone | Jeruzalmi et al., 2001a | 1JR3 |
| E. coli | Clamp loader, ADP | Kazmirski et al., 2004 | 1XXI |
| E. coli | Clamp loader, ATP analog | Kazmirski et al., 2004 | 1XXH |
| E. coli | Clamp loader, ATP analog, primer/template DNA | Simonetta et al., 2009 | 3GLF |
| Encounter complex of clamp loader bound to the closed clamp | |||
| H. sapiens | Clamp loader bound to the clamp, ATP analog | Gaubitz et al., 2020 | 6VVO |
| S. cerevisiae | Clamp loader bound to the closed clamp, ATP analog | Bowman et al., 2004 | 1SXJ |
| Clamp loader bound to the clamp and primer/template DNA | |||
| T4 phage | Clamp loader, open clamp, ATP analog, DNA | Kelch et al., 2011 | 3U60 |
| T4 phage | Clamp loader, closed clamp, ATP analog, DNA | Kelch et al., 2011 | 3U5Z |
| T4 phage | Clamp loader, closed clamp, ATP analog, ADP, DNA | Kelch et al., 2011 | 3U61 |
List of BS3 crosslinks.
| XlinkX score | Type | # Crosslink spectral matches | Sequence A | Position A | Sequence B | Position B | Protein A | Protein B |
|---|---|---|---|---|---|---|---|---|
| 58,66 | Inter | 1 | [K]LHLPPGK | 100 | [K]LAATR | 274 | RFC4 | RFC1 |
| 58,64 | Inter | 3 | [K]LELNVVSSPYHLEITPSDMGNNDR | 82 | S[K]TLLNAGVK | 385 | RFC5 | RFC1 |
| 56,99 | Inter | 3 | [K]YVNTFMK | 285 | DIL[K]R | 220 | RFC2 | RFC5 |
| 56,47 | Inter | 1 | NQI[K]DFASTR | 98 | [K]LAATR | 274 | RFC3 | RFC1 |
| 52,59 | Inter | 2 | E[K]VKNFAR | 109 | TME[K]YSK | 160 | RFC2 | RFC5 |
| 50,97 | Inter | 1 | NQI[K]DFASTR | 98 | RPDANSI[K]SR | 484 | RFC3 | RFC1 |
| 48,17 | Inter | 1 | GASEALA[K]R | 182 | [K]IVKER | 269 | RFC1 | RFC5 |
| 45,16 | Inter | 1 | YT[K]NTR | 139 | [K]EEER | 267 | RFC3 | RFC1 |
| 41,65 | Inter | 1 | [K]LEEQHNIATK | 249 | YT[K]NTR | 139 | RFC1 | RFC3 |
| 91,6 | Intra | 3 | [K]LEEQHNIATK | 249 | RPDANSI[K]SR | 484 | RFC1 | RFC1 |
| 72,73 | Intra | 4 | EAELLV[K]KEEER | 266 | [K]LAATR | 274 | RFC1 | RFC1 |
| 71,87 | Intra | 12 | QLIAGMPAEGGDGEAAE[K]AR | 245 | R[K]LEEQHNIATK | 249 | RFC1 | RFC1 |
| 71,27 | Intra | 2 | E[K]FKLDPNVIDR | 495 | [K]LAATR | 274 | RFC1 | RFC1 |
| 71,03 | Intra | 1 | F[K]LDPNVIDR | 497 | [K]LAATR | 274 | RFC1 | RFC1 |
| 71,03 | Intra | 9 | [K]TSTPLILICNER | 446 | S[K]TLLNAGVK | 385 | RFC1 | RFC1 |
| 64 | Intra | 1 | EAELLV[K]KEEER | 266 | S[K]KLAATR | 273 | RFC1 | RFC1 |
| 62,71 | Intra | 1 | RPDANSI[K]SR | 484 | SA[K]YYR | 678 | RFC1 | RFC1 |
| 62,2 | Intra | 2 | YAPTNLQQVCGN[K]GSVMK | 314 | L[K]NWLANWENSKK | 321 | RFC1 | RFC1 |
| 61,3 | Intra | 4 | EAELLVK[K]EEERSK | 267 | [K]LAATR | 274 | RFC1 | RFC1 |
| 60,15 | Intra | 1 | FAFACNQSN[K]IIEPLQSR | 149 | VT[K]NLAQVK | 275 | RFC4 | RFC4 |
| 60,15 | Intra | 3 | YS[K]LSDEDVLKR | 165 | VT[K]NLAQVK | 275 | RFC4 | RFC4 |
| 58,98 | Intra | 1 | IPATV[K]SGFTR | 767 | HAG[K]DGSGVFR | 340 | RFC1 | RFC1 |
| 58,55 | Intra | 4 | GASEALA[K]R | 182 | VT[K]SISSK | 190 | RFC1 | RFC1 |
| 57,1 | Intra | 3 | RPDANSI[K]SR | 484 | [K]EEER | 267 | RFC1 | RFC1 |
| 56,99 | Intra | 1 | KLEEQHNIAT[K]EAELLVK | 259 | [K]EEER | 267 | RFC1 | RFC1 |
| 56,99 | Intra | 1 | DNVVREED[K]LWTVK | 296 | [K]EEER | 267 | RFC1 | RFC1 |
| 56,41 | Intra | 1 | [K]YNSMTHPVAIYR | 773 | LGTSTD[K]IGLR | 698 | RFC1 | RFC1 |
| 53,33 | Intra | 1 | Y[K]CVIINEANSLTK | 136 | L[K]IDVR | 69 | RFC5 | RFC5 |
| 52,59 | Intra | 2 | [K]ASSPTVKPASSK | 77 | [K]TKPSSK | 90 | RFC1 | RFC1 |
| 52,59 | Intra | 2 | HAG[K]DGSGVFR | 340 | GSVM[K]LK | 319 | RFC1 | RFC1 |
| 52,59 | Intra | 2 | ASSPTV[K]PASSK | 84 | [K]TKPSSK | 90 | RFC1 | RFC1 |
| 51,79 | Intra | 2 | [K]LEEQHNIATK | 249 | [K]LAATR | 274 | RFC1 | RFC1 |
| 50,97 | Intra | 1 | [K]TATSKPGGSK | 845 | S[K]TLLNAGVK | 385 | RFC1 | RFC1 |
| 50,34 | Intra | 1 | KMPVSNVIDVSETPEGE[K]K | 68 | LPLPA[K]R | 75 | RFC1 | RFC1 |
| 49,59 | Intra | 4 | EKF[K]LDPNVIDR | 497 | RPDANSI[K]SR | 484 | RFC1 | RFC1 |
| 47,92 | Intra | 1 | LGTSTD[K]IGLR | 698 | [K]LAATR | 274 | RFC1 | RFC1 |
| 47,92 | Intra | 1 | S[K]TLLNAGVK | 385 | [K]LAATR | 274 | RFC1 | RFC1 |
| 47,92 | Intra | 1 | GASEALA[K]R | 182 | [K]LAATR | 274 | RFC1 | RFC1 |
| 47,85 | Intra | 2 | SISS[K]TSVVVLGDEAGPK | 195 | [K]LEEQHNIATK | 249 | RFC1 | RFC1 |
| 47,85 | Intra | 1 | [K]YNSMTHPVAIYR | 773 | [K]TATSKPGGSK | 845 | RFC1 | RFC1 |
| 46,57 | Intra | 4 | R[K]LEEQHNIATK | 249 | GASEALA[K]R | 182 | RFC1 | RFC1 |
| 46,35 | Intra | 1 | [K]ASSPTVKPASSK | 77 | VT[K]SISSK | 190 | RFC1 | RFC1 |
| 45,16 | Intra | 1 | YAPTNLQQVCGN[K]GSVMK | 314 | [K]EEER | 267 | RFC1 | RFC1 |
| 45,16 | Intra | 1 | E[K]FKLDPNVIDR | 495 | [K]EEER | 267 | RFC1 | RFC1 |
| 45,16 | Intra | 2 | [K]LEEQHNIATK | 249 | [K]EEER | 267 | RFC1 | RFC1 |
| 44,72 | Intra | 1 | E[K]FKLDPNVIDR | 495 | RPDANSI[K]SR | 484 | RFC1 | RFC1 |
| 44,45 | Intra | 1 | YAPTNLQQVCGN[K]GSVMK | 314 | [K]LEEQHNIATK | 249 | RFC1 | RFC1 |
| 44,14 | Intra | 2 | NLP[K]MRPFDR | 462 | S[K]TLLNAGVK | 385 | RFC1 | RFC1 |
| 44,14 | Intra | 1 | RPDANSI[K]SR | 484 | GASEALA[K]R | 182 | RFC1 | RFC1 |
| 44,12 | Intra | 1 | [K]LEEQHNIATK | 249 | [K]TKPSSK | 90 | RFC1 | RFC1 |
| 43,7 | Intra | 1 | NLP[K]MRPFDR | 462 | LGTSTD[K]IGLR | 698 | RFC1 | RFC1 |
| 43,7 | Intra | 1 | [K]YNSMTHPVAIYR | 773 | TATS[K]PGGSK | 850 | RFC1 | RFC1 |
| 41,98 | Intra | 2 | LGTSTD[K]IGLR | 698 | RPDANSI[K]SR | 484 | RFC1 | RFC1 |
| 41,98 | Intra | 1 | [K]LEEQHNIATK | 249 | F[K]LDPNVIDR | 497 | RFC1 | RFC1 |
| 41,98 | Intra | 1 | HAG[K]DGSGVFR | 340 | VT[K]SISSK | 190 | RFC1 | RFC1 |
| 41,94 | Intra | 1 | NQI[K]DFASTR | 98 | YT[K]NTR | 139 | RFC3 | RFC3 |
| 40,95 | Intra | 1 | NLAQV[K]ESVR | 281 | IHKLNN[K]A | 322 | RFC4 | RFC4 |
| 40,92 | Intra | 1 | KLPLPA[K]R | 75 | [K]EEER | 267 | RFC1 | RFC1 |
Cryo-EM data collection, processing, and model statistics.
| Dataset | No DNA | DNA | |||||
|---|---|---|---|---|---|---|---|
| Magnification | 130,000 | 81,000 | |||||
| Voltage (keV) | 300 | 300 | |||||
| Cumulative exposure(e−/Å 2) | 49–51 | 40 | |||||
| Detector | K2 Summit | K3 | |||||
| Pixel size (Å) | 1.059 | 1.06 | |||||
| Defocus range (μm) | −1.1 to −2.4 | −1.2 to −2.3 | |||||
| Micrographs used (no.) | 6109 | 4499 | |||||
| Initial particle images (no.) | 954,291 | 1,331,440 | |||||
| Symmetry | C | ||||||
| Class name | Autoinhibited1 | Autoinhibited2 | Autoinhibited3 | Open1 | Open2 | DNA-open | DNA-closed |
| Final refined particles (no.) | 55,308 | 68,227 | 60,036 | 46,069 | 63,752 | 46,300 | 76,270 |
| Applied B factor (Å2) | −100 | −159.352 | −163.938 | −100 | −106.457 | −105.857 | −105.313 |
| Map resolution(Å, FSC 0.143) | 3.8 | 3.9 | 4.0 | 4.0 | 3.5 | 3.4 | 3.3 |
| Model-Map CC_mask | 0.78 | 0.77 | 0.77 | 0.76 | 0.78 | 0.79 | 0.77 |
| Bond lengths (Å), angles (°) | 0.002,0.585 | 0.002,0.561 | 0.002,0.558 | 0.002,0.574 | 0.002,0.542 | 0.002,0.518 | 0.002,0.523 |
| Ramachandran outliers, allowed, favored | 0.00,3.16, 96.84 | 0.00,3.11, 96.89 | 0.00,2.89, 97.11 | 0.00,3.08, 96.92 | 0.00,3.38, 96.62 | 0.00,2.23, 97.77 | 0.00,2.16, 97.84 |
| Poor rotamers (%),MolProbity score, Clashscore (all atoms) | 0.00,1.68,9.05 | 0.00,1.68,9.42 | 0.00,1.68,9.95 | 0.00,1.67,9.26 | 2.01,1.91,8.67 | 1.09,1.54,8.44 | 1.09,1.55,9.18 |
| Accession number,EMDB, PDB | 25568,7THJ | 25569,7TIC | 25614,7THV | 25615,7TKU | 25753,7TI8 | 25616,7TIB | 25617, 7TID |
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Escherichia coli) | BL21(DE3) | Novagen | 69,450 | Chemically competent cells |
| Recombinant DNA reagent | pET(11a)-RFC[2 + 3 + 4] (plasmid) | Finkelstein et al., 2003 | Expression plasmid | |
| Recombinant DNA reagent | pLANT-2/RIL[1 + 5] (plasmid) | Finkelstein et al., 2003 | Expression plasmid | |
| Recombinant DNA reagent | pRS413-RFC1(plasmid) | This study | Plasmid for yeast expression of Rfc1 from endogenous promotor | |
| Strain, strain background (S. cerevisiae) | BY4743his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 LYS2/lys2Δ0 met15Δ0/MET15 ura3Δ0/ura3Δ0 ∆rfc1::KanMX4/RFC1 (YOR217W) | Dharmacon | YSC1055 (22473) | Yeast Heterozygous Collection |
| Software, algorithm | RELION | doi:10.7554/eLife.42166 | Relion 3.0.2 | |
| Software, algorithm | cisTEM | doi:10.7554/eLife.35383 | cisTEM-1.0.0-beta | https://cistem.org/software |
| Software, algorithm | Ctffind | doi:10.1016/j.jsb.2015.08.008 | Ctffind 4.1 | |
| Software, algorithm | UCSF Chimera | UCSF, doi:10.1002/jcc.20084 | http://plato.cgl.ucsf.edu/chimera/ | |
| Software, algorithm | ChimeraX | UCSF, doi:10.1002/pro.3943 | ChimeraX-1.2 | https://www.cgl.ucsf.edu/chimerax/ |
| Software, algorithm | COOT | doi:10.1107/S0907444910007493 | Coot-0.9.4 | http://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/ |
| Software, algorithm | Phenix | doi:10.1107/S0907444909052925 | Phenix-dev-3699 | https://phenix-online.org |
| Software, algorithm | PyMOL | PyMOL Molecular Graphics System, Schrodinger LLC | https://www.pymol.org/ | |
| Software, algorithm | GraphPad PRISM | GraphPad | GraphPad PRISM 9.2.1 | http://www.graphpad.com/ |
| Other | Pyruvate kinase | Calzyme | 107A0250 | |
| Other | Lactate Dehydrogenase | Worthington Biochemical Cooperation | LS002755 | |
| Other | Phosphoenol-pyruvic acid monopotassium salt | Alfa Aesar | B20358 |
DNA sequences.
| Template name | Sequence | Primer name | Sequence | Name used in assay |
|---|---|---|---|---|
| Template30-20-A | TTTTTTTTTTAATGTACTCGTAGTGTCTGC | Primer20-3’abasic | GCAGACACTACGAGTACAT/3dSp/ | p/t-DNA 3'-abasic |
| Primer20-3’-T-phosphate | GCAGACACTACGAGTACATT/3Phos/ | p/t-DNA 3’ PO4 | ||
| Primer20-3’-T-RNA | rGrCrArGrArCrArCrUrArCrGrArGrUrArCrArUrU | RNA primer/DNA template | ||
| Primer20-3’-riboT | GCAGACACTACGAGTACATrU | p/t-DNA 3’ ribo | ||
| Primer20-3’-T | GCAGACACTACGAGTACATT | p/t-DNA | ||
| Primer20-2AP-0 | GCAGACACTACGAGTACAT/32AmPu/ | p/t-AP, P = 1 | ||
| Primer20-2AP-2 | GCAGACACTACGAGTAC/i2AmPr/TA | p/t-AP, P = 3 | ||
| Template30-T-1 | TTTTTTTTTTTTTGTACTCGTAGTGTCTGC-3’ | Primer20-2AP-1 | GCAGACACTACGAGTACA/i2AmPr/A | p/t-AP, P = 2 |
| Template30-20-2AP | TTTTTTTTTT/i2AmPr/ATGTACTCGTAGTGTCTGC-3’ | Primer20-3’-T | GCAGACACTACGAGTACATT | p/t-AP, t = 1 |
| Template20-5’-A | AATGTACTCGTAGTGTCTGC | Primer20-3’-T | GCAGACACTACGAGTACATT | Blunt DNA |
| Primer 20–3'-T-10ext | GCAGACACTACGAGTACATTTTTTTTTTTT | 3' overhang DNA | ||
| Template30-20-A-3’T | AATGTACTCGTAGTGTCTGCTTTTTTTTTT | Primer 20–3'-T-10ext | GCAGACACTACGAGTACATTTTTTTTTTTT | 3' overhang dumbbell DNA |
| polyT 20 | TTTTTTTTTTTTTTTTTTTT | ssDNA (poly T) |





