Hippocampal-hypothalamic circuit controls context-dependent innate defensive responses
Figures
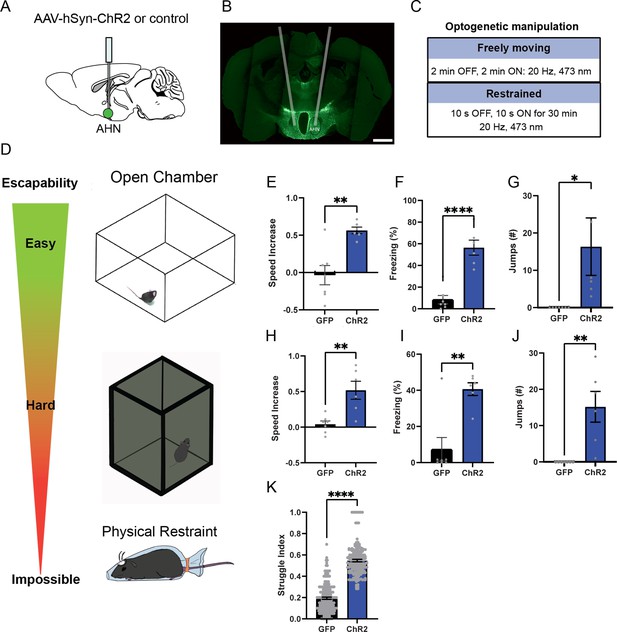
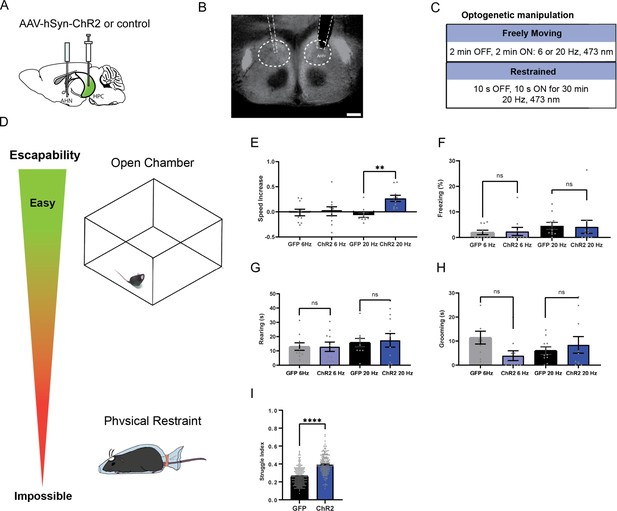
AHN stimulation induces escape-associated behaviors.
(a) Schematic illustration of optogenetic activation in the AHN (green circle depicts the AAV infusion). (b) An example of histological confirmation showing the expression of ChR2 and placement of optic fiber in the AHN. (c) Schematic describing optogenetic stimulation paradigm. (d) Three different escape conditions where the effects of AHN stimulation was examined. Top: open field arena with short transparent walls (condition 1, easy). Middle: tall opaque walls (condition 2, hard). Bottom: physical restraint tube (condition 3, impossible). (e) Condition 1: speed increase from the light OFF epoch to ON epoch (GFP N=7, ChR2 N=6 unpaired t-test, two-tailed, t=4.119, df=11, **p=0.0017). (f) Condition 1: freezing time during the light ON epoch (GFP N=7, ChR2 N=6, unpaired t-test, two-tailed, t=6.695, df=11, ****p<0.0001). (g) Condition 1: number of jumps during the light ON epoch (unpaired t-test, two-tailed, t=2.308, df=11, *p=0.0414). (h) Condition 2: speed increase from the light OFF epoch to ON epoch (GFP N=7, ChR2 N=6, unpaired t-test, two-tailed, t=3.778, df=11, **p=0.0031). (i) Condition 2: freezing time during the light ON epoch (GFP N=7, ChR2 N=6, unpaired t-test, two-tailed, t=4.259, df=11, **p=0.0013). (j) Condition 2: number of jumps during the light ON epoch (GFP N=7, ChR2 N=6, unpaired t-test, two-tailed, t=3.796, df=11, **p=0.003). (k) Condition 3: struggle movement during the 30 min of physical restraint (GFP N=4, ChR2 N=6, unpaired t-test, two-tailed, t=26.05, df=366, ****p<0.0001). All results reported are mean ± s.e.m. *p < 0.05, **p < 0.01, ***p < 0.001, ****p<0.0001. Scale bar=1 mm.
-
Figure 1—source data 1
Numerical data shown in Figure 1.
AHN stimulation induces escape-associated behaviors.
- https://cdn.elifesciences.org/articles/74736/elife-74736-fig1-data1-v2.xlsx
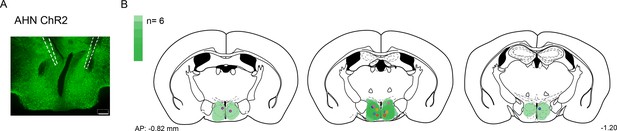
Compilation of viral expression and optic fiber implantation sites for optogenetic manipulation experiments.
(a,b) AHN soma activation. (N=6, each pair of colored circles shows the optic fiber tip placements in one animal). Viral spread in all injection areas (AHN, HPC) are depicted by the green blots across coronal plates of mouse brain atlas. Color bar, the number of mice with viral expression in the area (DAPI, blue; ChR2 and ArchT, green). Scale bar=200 µm.
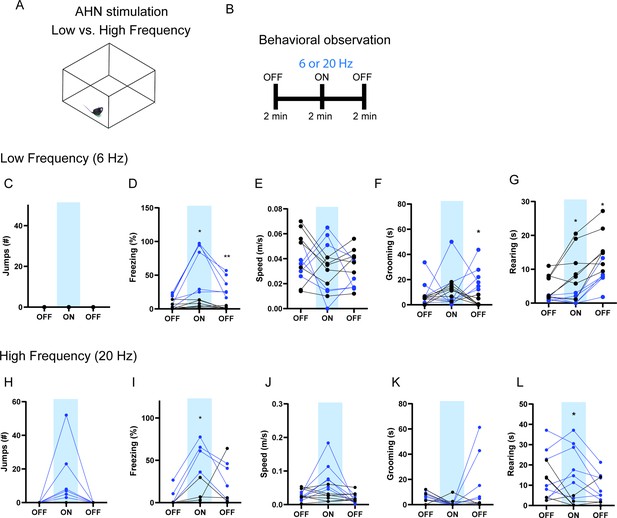
The effects of low vs high-frequency AHN stimulation.
(ChR2 N=6, GFP N=7). (a) Schematic illustration of open field box where the low- and high-frequency stimulation occurred. (b) Testing paradigm for with low- vs. high-frequency AHN stimulation. (c–g) Behavioral changes induced by low-frequency (6 Hz) AHN stimulation. (c) No jumping observed upon light stimulation. (d) Freezing was increased upon AHN stimulation (two-way RM ANOVA, light x genotype, F(2,22)=13.13, ***p=0.0002, light effect, F (1.610, 17.71) = 16.95, ***p=0.0002, genotype effect, F (1, 11) = 35.33, ****p<0.0001, Sidak’s multiple comparison test, ON *p=0.0167, Off 2 **p=0.0098). (e) Speed did not change (two-way RM ANOVA, light x genotype, F(2,22)=0.9463, p=0.4034, NS,), light effect, F (1.868, 20.54) = 1.426, p=0.2622, NS, genotype effect, F (1, 11) = 0.2379, p=0.6352, NS. (f) Grooming was increased after AHN stimulation during the second light OFF period. (two-way RM ANOVA, light x genotype, F(2,22)=2.430, p=0.1113, NS, Sidak’s multiple comparison test, Off 2, *p=0.0164). (g) Rearing was decreased (two-way RM ANOVA, F(2,22)=2.407, p=0.1135, NS, F (1.691, 18.60) = 21.68, ****p<0.0001, F (1, 11) = 13.98, **p=0.0033, Sidak’s multiple comparison test, ON, *p=0.0317, the second light OFF period, *p=0.0297). (h–l) Behavioral changes induced by high frequency (20 Hz) AHN stimulation. (h) Jumping was increased upon AHN stimulation (two-way RM ANOVA, light x genotype, F (2, 22) = 5.328, *p=0.0130, light effect, F (1.000, 11.00) = 5.328, *p=0.0414, genotype effect, F (1, 11) = 5.328, *p=0.0414). (i) Freezing was increased upon AHN stimulation. (two-way RM ANOVA, light x genotype, F (2, 22) = 5.432, *p=0.0121, light effect, F (1.772, 19.50) = 8.363, **p=0.0031, genotype effect, F (1, 11) = 9.633, *p=0.01, Sidak’s multiple comparison test, ON, *p=0.0286). (j) Speed was increased upon AHN stimulation, light x genotype, F (2, 22) = 15.55, ****p<0.0001, light effect, F (1.119, 12.30) = 15.12, **p=0.0017, genotype effect, F (1, 11) = 2.073, p=0.1777, NS. (k) Grooming was increased during the second light OFF period. (two-way RM ANOVA, light x genotype, F (2, 22) = 4.473, *p=0.0234, light effect, F (1.153, 12.69) = 3.175, p=0.0949, genotype effect, F (1, 11) = 6.429, *p=0.0277) (l) Rearing is reduced upon AHN stimulation (two-way RM ANOVA, light x genotype, F (2, 22) = 5.564, *p=0.0111, light effect, F (1.406, 15.47) = 4.790, *p=0.0339, genotype effect, F (1, 11) = 6.128, *p=0.0308, Sidak’s multiple comparison test, *p=0.0146). All results reported are mean ± s.e.m. Sidak’s multiple comparison test: *p < 0.05, **p < 0.01, ***p < 0.001, ****p<0.0001.
-
Figure 1—figure supplement 2—source data 1
Numerical data shown in Figure 1—figure supplement 2.
The effects of low- vs. high-frequency AHN stimulation.
- https://cdn.elifesciences.org/articles/74736/elife-74736-fig1-figsupp2-data1-v2.xlsx
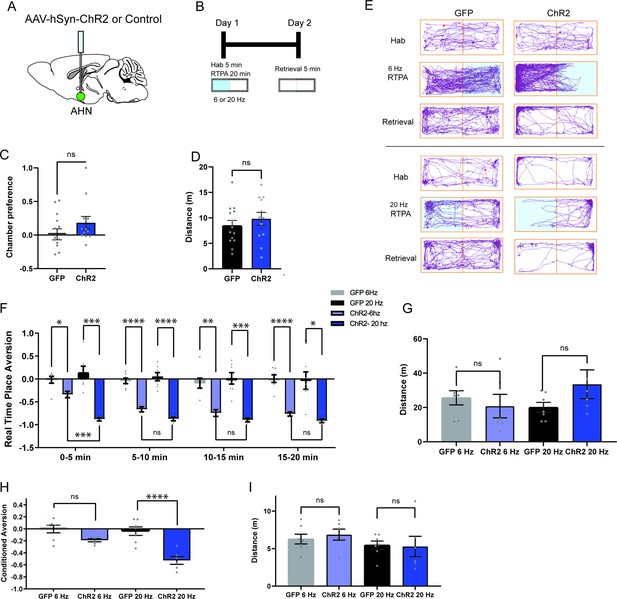
AHN stimulation is aversive and induces conditioned place aversion.
(a) Schematic illustration of optogenetic activation in the AHN (green circle depicts the AAV infusion). (b) Schematic describing the RTPA and CPA test paradigm: day 1 consisting of habituation and real-time place preference (20 min) and day 2 for testing conditioned place preference (5 min). (c) Chamber preference during habituation (GFP N=14, ChR2 N=12, unpaired t-test, t=1.390, df=24, p=0.1772, NS). (d) Distance travelled during habituation (GFP N=14, ChR2 N=12, unpaired t-test, t=0.8396, df=24, p=0.41, NS). (e) Representative locomotion trajectory for a GFP control animal (left column) and a ChR2-expressing animal (right column) during habituation (hab), 6 Hz or 20 Hz real-time stimulation (6 Hz RTPA, 20 Hz RTPA), and conditioned place aversion test (Retrieval). Light-coupled chambers are shown in blue. (f) Realtime place aversion monitored across 20-min test (GFP N=7, ChR2 N=6). GFP 6 Hz vs. ChR2 6 Hz (two-way RM ANOVA, time x treatment, F(3,33)=3.965, *p=0.016, time effect, F(2.252, 24.77)=4.739, p=0.152, NS, treatment effect, F(1, 11)=77.41, ****p<0.0001, Sidak’s multiple comparisons test, 0-5 min, *p=0.0359, 5-10 min, ****p<0.0001, 10-15 min, **p=0.0022, 15-20 min, ****p<0.0001). GFP 20 Hz vs. ChR2 20 Hz (two-way RM ANOVA, time x treatment, F(3,33)=0.6059, p=0.6158, NS, time effect, F(1.938, 21.32)=1.305, p=0.2911, NS, treatment effect, F(1,11)=43.38, ****p<0.0001, 24 multiple comparisons test, 0-5 min, ***p=0.0008, 5-10 min, ****p<0.0001, 10-15 min, ***p=0.0007, 15-20 min, *p=0.0127). GFP 6 Hz vs. GFP 20 Hz (two-way RM ANOVA, time x frequency, F(2.071, 12.42)=1.076, p=0.3730, NS, time effect, F(1.964, 11.78)=0.5391, p=0.5939, NS, frequency effect, F(1, 6)=0.2474, p=0.6366, NS, Sidak’s multiple comparisons test, 0-5 min, p=0.8256, NS, 5-10 min, p=0.8824, NS, 10-15 min, p=0.9794, NS, 15-20 min, p=0.9995, NS). ChR2 6 Hz vs. ChR2 20 Hz (2-WAY RM ANOVA, time x frequency, F(1.455, 7.274)=7.391, *p=0.0223, time effect, F(1.514, 7.571)=11.05, **p=0.0075, frequency effect, F(1, 5)=20.99, **p=0.0059, Sidak’s multiple comparisons test, 0-5 min, ***p=0.0008, 5-10 min, p=0.2586, NS, 10–15 min, p=0.5763, NS, 15–20 min, p=0.3504, NS). (g) Distance travelled during 6 Hz and 20 Hz real-time stimulation (2-WAY ANOVA, frequency x genotype, F(1,22)=2.581, p=0.1224, NS, frequency effect, F(1, 22) = 0.3967, p=0.5353, NS, genotype effect, F(1, 22)=0.5732, p=0.457, NS, Sidak’s multiple comparisons test, 6 Hz GFP vs. ChR2, p=0.8013, NS, 20 Hz GFP vs. ChR2, p=0.2058, NS). (h) Conditioned aversion memory tested 24-hr after real time place aversion tests (two-way ANOVA, frequency x genotype, F(1,22)=6.208, *p=0.0207, frequency effect, F(1, 22) = 9.411, **p=0.0056, genotype effect, F(1, 22)=31.19, ****p<0.0001, Sidak’s multiple comparisons test, 6 Hz GFP vs. ChR2, p=0.0778, NS, 20 Hz GFP vs. ChR2, ****p<0.0001). (i) Distance travelled during the conditioned place aversion test (two-way ANOVA, frequency x genotype, F(1,22)=0.2058, p=0.6545, NS, frequency effect, F(1, 22) = 1.998, p=0.1715, NS, genotype effect, F(1, 22)=0.06095, p=0.8073, NS, Sidak’s multiple comparisons test, 6 Hz GFP vs. ChR2, p=0.8596, NS, 20 Hz GFP vs. ChR2, p=0.9868, NS). All results reported are mean ± s.e.m. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
-
Figure 2—source data 1
Numerical data shown in Figure 2.
AHN stimulation is aversive and induces conditioned place aversion.
- https://cdn.elifesciences.org/articles/74736/elife-74736-fig2-data1-v2.xlsx
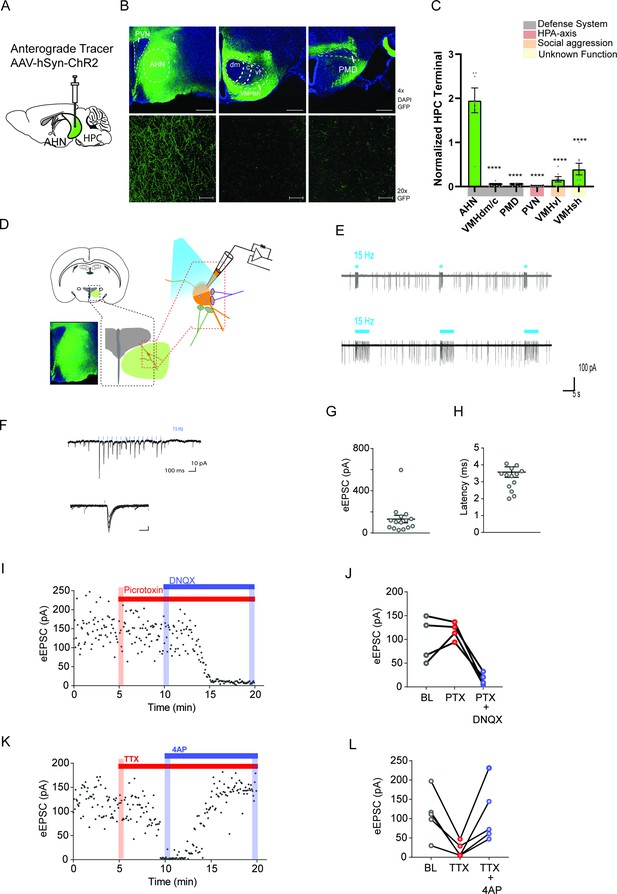
Hippocampus sends monosynaptic excitatory inputs to the anterior hypothalamic nucleus.
(a) Schematic illustration of anterograde tracing experiment. (b) HPC terminals (green) in the hypothalamus, including anterior hypothalamic nucleus (AHN), dorsomedial and central regions of ventromedial hypothalamus (VMHdm/c), premammillary dorsal nucleus (PMD), paraventricular nucleus (PVN), ventrolateral region of ventromedial hypothalamus (VMHvl), shell of ventromedial hypothalamus (VMHsh). DAPI staining (blue). (c) Quantification of HPC terminal intensity (N=2 animals, ~7 sections per ROI, One-Way ANOVA, F(5,35)=33.24, ****p<0.0001, Dunnett’s multiple comparisons test, AHN vs. VMHdm/c, ****p<0.0001, AHN vs. PMD, ****p<0.0001, AHN vs. PVN, ****p<0.0001, AHN vs. VMHvl, ****p<0.0001, AHN vs. VMHsh, ****p<0.0001). (d) Schematic illustration for patch clamp recordings of AHN neurons in coronal brain slices that express ChR2 in HPC terminals. (e) Examples of cell attach recordings. Illumination of blue light (480 nm, 5ms pulse at 15 Hz) triggered firing of AHN neurons. (f) Examples of whole-cell voltage-clamp recordings of AHN neurons. Blue light illumination (5 ms) evoked inward current. (g-h) Summary of light-evoked EPSCs (g) amplitude and latency (h). (i) Light-evoked EPSCs persisted in the presence of GABA A receptor antagonist picrotoxin (PTX, 100 µM) and eliminated by AMPA/kainite receptor antagonist DNQX (20 µM). (j) Summary of eEPSC change after PTX and DNQX application. (k) Light-evoked EPSCs were eliminated by TTX (0.5 µM) and then recovered by a low dose 4-AP (100 µM). (l) Summary of eEPSC changes after TTX and 4-AP application. All results reported are mean ± s.e.m. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p<0.0001. Scale bar=100 µm.
-
Figure 3—source data 1
Numerical data shown in Figure 3.
Hippocampus sends monosynaptic excitatory inputs to the anterior hypothalamic nucleus.
- https://cdn.elifesciences.org/articles/74736/elife-74736-fig3-data1-v2.xlsx
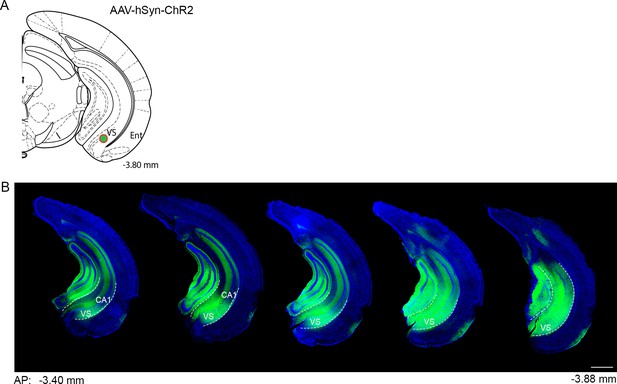
Viral expression of the anterograde tracer ChR2-eYFP.
(a) Schematic showing the viral injection target (green dot) in the ventral subiculum. (b) Representative coronal sections of the hippocampus showing the spread of AAV-hSyn-ChR2-eYFP along the anterior-posterior axis of the hippocampus. DAPI, blue; ChR2, green; VS, ventral subiculum; CA1, cornu ammonis 1 of hippocampus. Scale bar = 500 µm.
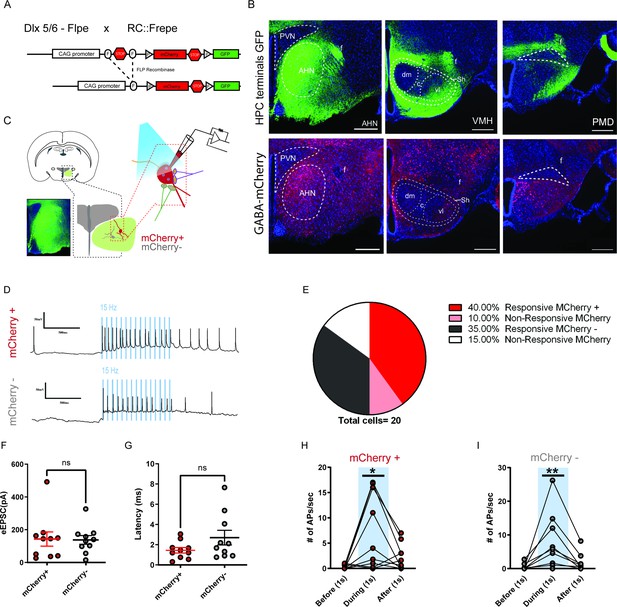
HPC inputs innervate both GABA and non-GABA cells in the AHN.
(a) Schematic showing the reporter allele, RC::FrePe, containing FRT-flanked and loxP-flanked transcriptional stop cassettes. Dlx 5/6 FLPe-mediated stop cassette removal results in mCherry expression in forebrain GABA cells. The RC::FrePe allele is knocked in to the Gt(ROSA)26Sor(R26) locus with CAG (chicken beta-actin and CMV enhancer) promoter elements. (b) Top: HPC terminals (green) in the hypothalamus, including anterior hypothalamic nucleus (AHN), dorsomedial and central regions of ventromedial hypothalamus (VMHdm/c), premammillary dorsal nucleus (PMD), paraventricular nucleus (PVN), ventrolateral region of ventromedial hypothalamus (VMHvl), shell of ventromedial hypothalamus (VMHsh). DAPI staining (blue). Bottom: mCherry expression in GABA cells in the respective three regions from the same brain. (c) Schematic illustration for patch clamp recordings of AHN neurons in coronal brain slices that express ChR2 in HPC terminals. Red: mCherry-positive GABA cells, Gray: mCherry-negative non-GABAergic cells. (d) Examples of cell attached recordings. Illumination of blue light (480 nm, 5 ms pulse at 15 Hz) triggered action potential firing of AHN mCherry+ and mCherry- neurons. (e) A pie chart depicting the percentage of AHN GABA or non-GABA cells that evoked light-evoked action potentials (mCherry-positive GABA cells, N=10; mCherry-negative non-GABAergic cells, N=10). (f, g) Summary of light-evoked EPSC amplitude (f) and latency (g). (h,i) Number of action potentials evoked 1 s before, 1 s during and 1 s after light onset from mCherry-positive GABA cells (h) of the AHN (one-wa RM ANOVA, F (1.119, 10.07) = 8.271, *p = 0.0146) and mCherry-negative non-GABAergic cells (i) of the AHN (one-way RM ANOVA, F (1.088, 9.791) = 7.116, *p = 0.0223). All results reported are mean ± s.e.m. *p < 0.05. Scale bar = 100 µm.
-
Figure 3—figure supplement 2—source data 1
Numerical data shown in Figure 3—figure supplement 2.
Viral expression of the anterograde tracer ChR2-eYFP.
- https://cdn.elifesciences.org/articles/74736/elife-74736-fig3-figsupp2-data1-v2.xlsx
HPC→AHN pathway activation induces escape-associated locomotion.
(a) Schematic illustration of optogenetic activation of hippocampal terminals in the AHN (GFP N=10, ChR2 N=10). (b) An example of histological confirmation showing the expression of HPC terminals and placement of optic fibers in the AHN. (c) Schematic describing optogenetic stimulation paradigm. (d) Two different escape conditions where the effects of HPC terminal stimulation was examined. Top: open field arena with short transparent walls (condition 1, easy). Bottom: physical restraint tube (condition 2, impossible). (e) Condition 1: speed increase from the light OFF epoch to ON epoch (two-way ANOVA, frequency x genotype, F(1,36)=5.298,*p=0.0272, frequency effect, F(1, 36)=2.337, p=0.135, NS, genotype, F(1, 36)=7.164, *p=0.0111, Sidak’s multiple comparisons test, 6 Hz GFP vs. ChR2, p=0.957, NS, 20 Hz GFP vs. ChR2, **p=0.0024). (f) Condition 1: freezing time during the light ON epoch (two-way ANOVA, frequency x genotype, F(1,36)=0.04839, p=0.8273, NS, frequency effect, F(1, 36)=1.637, p=0.2089, NS, genotype effect, F(1, 36) = 2.385e-005, p=0.9961, NS, Sidak’s multiple comparisons test, 6 Hz GFP vs. ChR2, p=0.9856, NS, 20 Hz GFP vs. ChR2, p=0.9843, NS). (g) Condition 1: rearing time during the light ON epoch (two-way ANOVA, frequency x genotype, F(1,36)=0.06028, p=0.8075, NS, frequency effect, F(1, 36)=1.08, p=0.3057, NS, genotype effect, F(1, 36)=0.04343, p=0.8361, NS, Sidak’s multiple comparisons test, 6 Hz GFP vs. ChR2, p=0.9996, NS, 20 Hz GFP vs. ChR2, p=0.9375, NS) (h) Condition 1: grooming time during the light ON epoch (two-way ANOVA, frequency x genotype, F(1,36)=3.858, p=0.0573, NS, frequency effect, F(1,36) = 0.03451, p=0.8537, NS, genotype effect, F(1,36)=1.024, p=0.3184, NS, Sidak’s multiple comparisons test, 6 Hz GFP vs. ChR2, p=0.083, NS, 20 Hz GFP vs. ChR2, p=0.7549, NS). (i) Condition 2: struggle movement during the 30 minutes of physical restraint (GFP N=7, ChR2 N=9, unpaired t-test, two-tailed, t=12.22, df=356 ****p<0.0001). All results reported are mean ± s.e.m. *p < 0.05, **p < 0.01, ***p < 0.001, ****p<0.0001. Scale bar=200µm.
-
Figure 4—source data 1
Numerical data shown in Figure 4.
HPC→AHN pathway activation induces escape-associated locomotion.
- https://cdn.elifesciences.org/articles/74736/elife-74736-fig4-data1-v2.xlsx
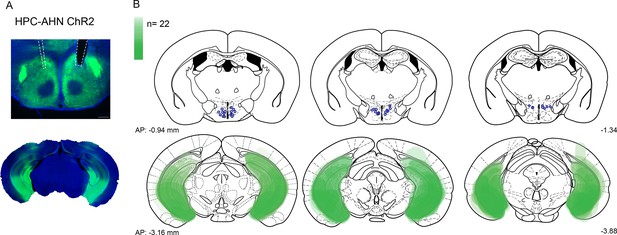
viral expression and optic fiber implantation sites for HPC→AHN pathway optogenetic activation a,b, HPC terminal activation in the AHN (N=22).
Viral spread in all injection areas (AHN, HPC) are depicted by the green blots across coronal plates of mouse brain atlas. Color bar, the number of mice with viral expression in the area (DAPI, blue; ChR2, green). Scale bar 200 µm.
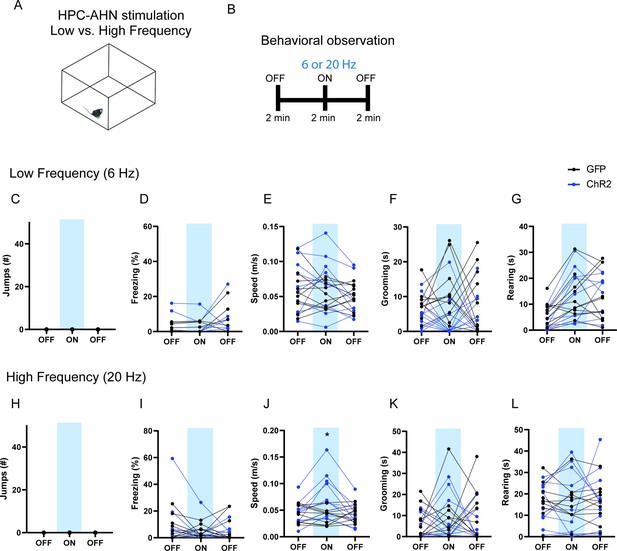
The effects of low- vs high-frequency HPC→AHN pathway activation.
(a) Schematic illustration of open field box where the low- and high-frequency HPC→AHN pathway stimulation occurred. (b) Testing paradigm for with low- vs. high-frequency HPC→AHN pathway stimulation. (c–g) Behavioral changes induced by low-frequency (6 Hz) stimulation. (c) No jumping observed upon stimulation. (d) Freezing did not change (two-way RM ANOVA, light x genotype, F(2, 36) = 0.0762, p=0.9268, NS, light effect, F(1.040, 18.73) = 1.947, p=0.1793, NS, genotype effect, F(1, 18) = 0.1065, p=0.7479, NS). (e) Speed did not change (two-way RM ANOVA, light x genotype, F (2, 36) = 1.656, p=0.2051, NS, light effect, F(1.860, 33.48) = 2.281, p=0.1211, NS, genotype effect, F (1, 18) = 0.5189, p=0.4806, NS). (f) Grooming was decreased upon stimulation (two-way RM ANOVA, light x genotype, F(2, 36) = 0.6101, p=0.5488, NS, light effect, F(1.640, 29.53) = 0.08480, p=0.8846, NS, genotype, F(1, 18) = 10.22, **p=0.005). (g) Rearing did not change (two-way RM ANOVA, light x genotype, F (2, 36) = 0.5721, p=0.5694, NS, light effect, F (1.927, 34.69) = 13.27, ****p<0.0001, Sidak’s multiple comparison test, NS, genotype effect, F (1, 18) = 0.07171, p=0.7919, NS). (h–l) Behavioral changes induced by highfrequency (20 Hz) stimulation. (h) No jumping observed upon stimulation. (i) Freezing did not change (two-way RM ANOVA, light x genotype, F (2, 36) = 0.5866, p=0.5614, NS, light effect, F(1.693, 30.47) = 1.614, p=0.2173, NS, genotype effect, F (1, 18) = 0.0006919, p=0.9793, NS). (j) Speed was increased upon stimulation (two-way RM ANOVA, light x genotype, F (2, 36) = 15.26, ****p<0.0001, light effect, F (1.779, 32.02) = 8.293, **p=0.0018, genotype effect, F (1, 18) = 1.916, p=0.1832, NS, Sidak’s multiple comparison test, ON, *p=0.0402). (k) Grooming did not change (two-way RM ANOVA, light x genotype, F(2, 36) = 0.3965, p=0.6756, NS, light effect, F (1.839, 33.09) = 0.5543, p=0.5653, NS, genotype effect, F(1, 18) = 2.738, p=0.1153.) (l) Rearing did not change (two-way RM ANOVA, light x genotype, F (2, 36) = 0.2581, p=0.7739, NS, light effect, F (1.715, 30.86) = 0.03206, p=0.9524, NS, genotype effect, F (1, 18) = 0.03084, p=0.8626, NS). Sidak’s multiple comparison test: *p < 0.05, **p < 0.01, ***p < 0.001, ****p<0.0001.
-
Figure 4—figure supplement 2—source data 1
Numerical data shown in Figure 4—figure supplement 2.
The effects of low- vs. high-frequency HPC→AHN pathway activation.
- https://cdn.elifesciences.org/articles/74736/elife-74736-fig4-figsupp2-data1-v2.xlsx
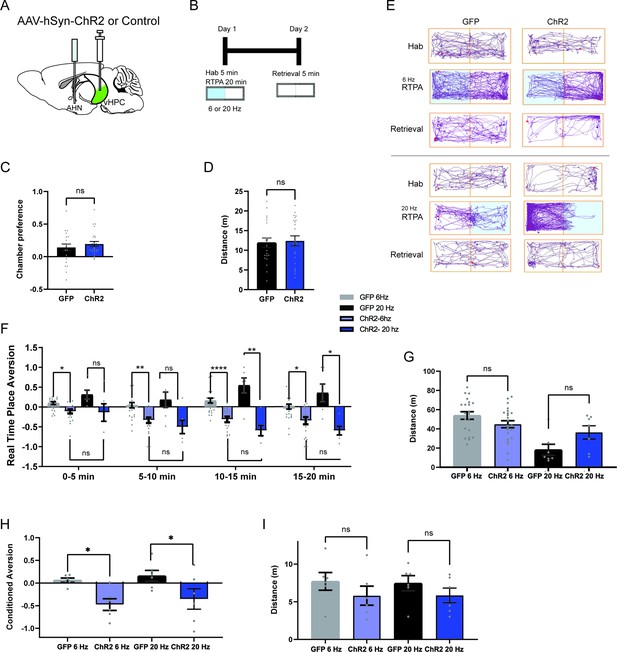
HPC→AHN pathway activation is aversive and instructs learning of a conditioned place aversion.
(a) Schematic illustration of optogenetic activation of hippocampal terminals in the AHN (GFP N=21, ChR2 N=22). (b) Schematic describing the RTPA and CPA test paradigm: day 1 consisting of habituation and real-time place preference (20 min) and day 2 for testing conditioned place preference (5 min). (c) Chamber preference during habituation (unpaired t-test, two-tailed, t=0.8339, df=41, p=0.4089, NS). (d) Distance travelled during habituation (unpaired t-test, two-tailed, t=0.2674, df=41, p=0.7905, NS). (e) Representative locomotion trajectory for a GFP control animal (left column) and a ChR2-expressing animal (right column) during habituation (hab), 6 Hz or 20 Hz real-time stimulation (6 Hz RTPA, 20 Hz RTPA), and conditioned place aversion test (Retrieval). Light-coupled chambers are shown in blue. (f) Real time place aversion monitored across 20 minute test. GFP 6 Hz vs. ChR2 6 Hz (two-way RM ANOVA, time x treatment F(3,120)=3.539, *p=0.0168, time effect, F(2.633, 105.3)=4.648, **p=0.0062, treatment effect, F(1,40)=21.57, ****p<0.0001, Sidak’s multiple comparisons test, 0–5 min, *p=0.0206, 5–10 min, **p=0.0017, 10-15 min, ****p<0.0001, 15-20 min, *p=0.0124), GFP 20 Hz vs. ChR2 20 Hz (two-way RM ANOVA, time x treatment, F(3,30)=4.132, *p=0.0145, time effect, F(2.228, 22.28)=2.056, p=0.1476, NS, treatment effect, F(1,10)=13.59, **p=0.0042, Sidak’s multiple comparisons test, 0-5 min, p=0.5916, NS, 5-10 min, p=0.1451, NS, 10-15 min, **p=0.0031,15-20 min, *p=0.0229). GFP 6 Hz vs. GFP 20 Hz (two-way RM ANOVA, time x treatment, F (3, 75) = 1.249, p=0.2980, NS, time effect, F(2.711, 67.77)=3.977, *p=0.0139, treatment effect, F(1,25)=4.669, *p=0.0405, Sidak’s multiple comparisons test, 0-5 min, p=0.3668, NS, 5-10 min, p=0.9733, 10-15 min, p=0.396, NS, 15-20 min, p=0.5472, NS) ChR2 6 Hz vs. ChR2 20 Hz (two-way RM ANOVA, time x treatment, F (3, 75) = 1.828, p=0.1492, NS, time effect, F(1.944, 48.61)=10.74, ***p=0.0002, treatment effect, F(1,25) = 1.279, p=0.2687, NS, Sidak’s multiple comparisons test, 0-5 min, P=0.9998, NS, 5-10 min, p=0.959, NS, 10-15 min, p=0.3079, NS, 15-20 min, p=0.3884, NS) (g) Distance travelled during 6 Hz and 20 Hz real-time stimulation (two-way ANOVA, frequency x treatment, F(1, 51) = 4.679, *p=0.0352, frequency effect, F(1,51)=13.87, ***p=0.0005, treatment effect, F(1,51)=0.2719, p=0.6043, NS, Sidak’s multiple comparisons test, 6 Hz GFP vs. ChR2 P=0.1626, NS, 20 Hz GFP vs. ChR2 p=0.252, NS). (h) Conditioned aversion memory tested 24-hr after real-time place aversion tests (two-way ANOVA, frequency x treatment, F(1,20)=0.009471, p=0.9234, NS, frequency effect, F(1,20)=0.5755, p=0.4569, NS, treatment effect, F(1,20)=13.05, **p=0.0017, Sidak’s multiple comparisons test, 6 Hz GFP vs. ChR2, *p=0.0323, 20 Hz GFP vs. ChR2, *p=0.0433). (i) Distance travelled during the conditioned place aversion test (two-way ANOVA, frequency x treatment, F(1, 20)=0.01613, p=0.902, NS, frequency effect, F(1,20)=0.009512, p=0.9233, treatment effect, F(1,20)=2.486, p=0.1305, Sidak’s multiple comparisons test, 6 Hz GFP vs. ChR2, p=0.4260, NS, 20 Hz GFP vs. ChR2, p=0.5342, NS). All results reported are mean ± s.e.m. *p < 0.05, **p < 0.01, ***p < 0.001, ****p<0.0001.
-
Figure 5—source data 1
Numerical data shown in Figure 5.
HPC→AHN pathway activation is aversive and instructs learning of a conditioned place aversion.
- https://cdn.elifesciences.org/articles/74736/elife-74736-fig5-data1-v2.xlsx
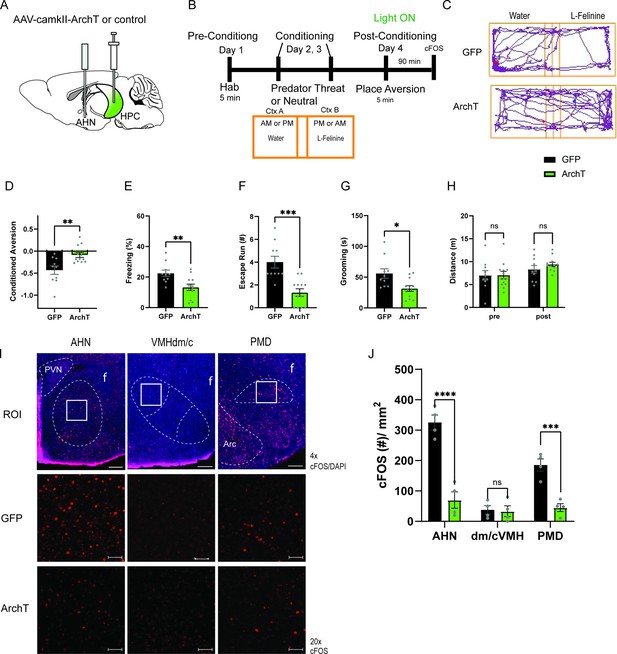
HPC input to the AHN is necessary for remembering the context-associated with predatory threat.
(a) Schematic illustration of optogenetic HPC terminal inhibition in the AHN (GFP N=10, ArchT N=12). (b) Schematic describing the behavioral paradigm for the contextual fear conditioning with the predator odor (L-Felinine-); day 1 for habituation (5 min), days 2–3 for two daily conditioning sessions where mice were enclosed either L-Felinine- or water-paired chamber for 20 minutes in AM and PM in a counterbalanced manner, and day 4 for testing conditioned place preference (5 min) and the immunochemical detection of c-Fos. (c) Representative locomotion trajectory for a GFP control animal (top) and a ChR2-expressing animal (down) during the conditioned place aversion test with optogenetic HPC terminal inhibition in the AHN (left: water-coupled chamber, right: L-Felinine-coupled chamber). (d) Conditioned aversion memory tested 24 hr after conditioning. GFP vs. ArchT (unpaired t-test, t=3.223, df=20. **p=0.0043). (e) Freezing time during the conditioned place aversion test. GFP vs. ArchT (unpaired t-test, t=3.056, df=20, **p=0.0062). (f) Number of escape runs from the L-Felinine-paired chamber to the water-paired chamber. GFP vs. ArchT (unpaired t-test, t=4.479, df=20, ***p=0.0002). (g) Time spent grooming during the conditioned place aversion test. GFP vs. ArchT (unpaired t-test, t=2.816, df=20, *p=0.0107). (h) Distance travelled during habituation (pre) and conditioned place aversion test (post) (two-way ANOVA, training x treatment, F(1, 20)=0.9938, p=0.3307, NS, training effect, F(1,20) = 12.29, **p=0.0022, treatment effect, F(1,20)=0.3235, p=0.5759, NS). (i) c-Fos immunochemical detection across the medial hypothalamic defense system (AHN, VMHdm/c, PMD). First row, representative 4 x epi-fluorescence microscope images of the medial hypothalamic defense system in GFP control mice. The regions of interest (ROI, white squares) within AHN, VMHdm/c, and PMD were imaged by confocal microscopy for cell counting. Second and third row: representative 20 x confocal images of c-Fos signals in AHN, VMHdm/c, and PMD activated by the conditioned place aversion test in GFP and ArchT mice, respectively. (j) Density of c-Fos signals in AHN, VMHdm/c, and PMD in GFP control (black) and ArchT mice (green) (N=4 mice for each group; two-way ANOVA, ROI x treatment, F(2, 18)=19.65, ****p<0.0001, ROI effect F(2,18)=32.79, ****p<0.0001, treatment effect F(1, 18)=66.19, ****p<0.0001, Sidak’s multiple comparison test, GFP AHN vs ArchT AHN, ****p<0.0001, GFP VMHdm/c vs. ArchT dm/cVMH p=0.9984, NS, GFP PMD vs ArchT PMD, ***p=0.0003). All results reported are mean ± s.e.m. *p < 0.05, **p < 0.01, ***p < 0.001, ****p<0.0001. Scale bar = 100 μm for 4 x epi-fluorescence microscope images and 10 μm for 20 x confocal images. (PVN, paraventricular nucleus. f, fornix. Arc, arcuate nucleus).
-
Figure 6—source data 1
Numerical data shown in Figure 6.
HPC input to the AHN is necessary for remembering the context-associated with predatory threat.
- https://cdn.elifesciences.org/articles/74736/elife-74736-fig6-data1-v2.xlsx
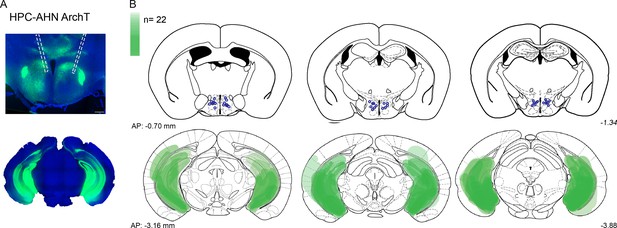
Viral expression and optic fiber implantation sites for HPC→AHN pathway optogenetic inhibition.
(a,b) HPC terminal inhibition in the AHN (N=22). Viral spread in all injection areas (AHN, HPC) are depicted by the green blots across coronal plates of mouse brain atlas. Color bar, the number of mice with viral expression in the area (DAPI, blue; ArchT, green). Scale bar=200 µm.
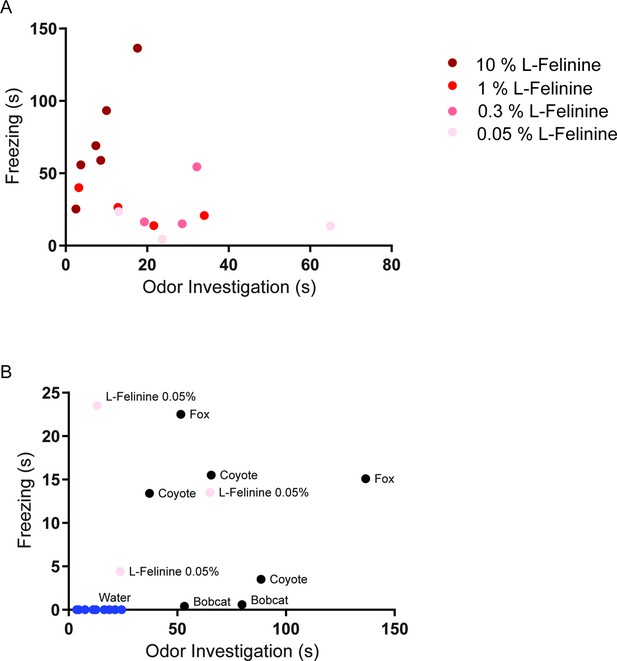
L-Felinine increases freezing in a dose-dependent manner.
Each dot represents behavioral responses to different concentrations of L-Felinine that were measured in home cage during a 5 min trial. (a) Freezing and odor investigation evoked by different concentrations of L-Felinine. Higher concentrations of L-Felinine induced greater freezing and shorter odor investigation. (b) Freezing and odor investigation evoked by water, natural predator urine, and L-Felinine 0.05% (concentration found in cat urine).
-
Figure 6—figure supplement 2—source data 1
Numerical data shown in Figure 6—figure supplement 2.
L-Felinine increases freezing in a dose-dependent manner.
- https://cdn.elifesciences.org/articles/74736/elife-74736-fig6-figsupp2-data1-v2.xlsx
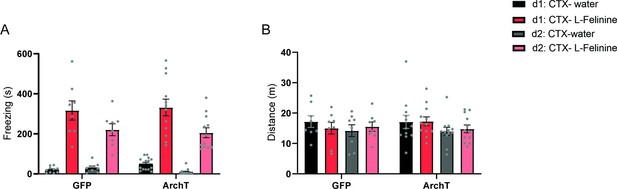
Behavioral responses to L-Felinine during conditioning sessions.
(a) Freezing induced by water and L-Felinine during two days of conditioning (d1 and d2) in GFP and ArchT mice (GFP N=8, ArchT N=12). Black: Day 1 water, Red: Day 1 L-Felinine, Grey: Day 2 water, Pink: Day 2 L-Felinine. (Mixed-effects analysis, genotype x odorant x day, F(1,76)=0.0837, p=0.7731, NS, genotype x odorant, F(1,76)=0.01045, genotype x day, F(1,76)=1.242, odorant x day, F(1,76)=6.931, *p=0.0103, genotype effect, F(1,76)=0.009447, p=0.9228, NS, odorant effect, F(1,76)=175, ****p<0.0001, day effect, (1,76)=12.06, ***p=0.0009, Sidak’s multiple comparisons test, GFP:d1: CTX- water vs. ArchT:d1: CTX- water, p=0.9974, NS, GFP:d2: CTX-water vs. ArchT:d2: CTX-water, p>0.9999, NS, GFP:d1: CTX- L-Felinine vs. ArchT:d1: CTX- L-Felinine, p>0.9999, NS, GFP:d2: CTX- L-Felinine vs. ArchT:d2: CTX- L-Felinine, p>0.9999, NS, GFP:d1: CTX- water vs. GFP:d2: CTX-water, p>0.9999, NS, GFP:d1: CTX- L-Felinine vs. GFP:d2: CTX- L-Felinine, p=0.2251, ArchT:d1: CTX- water vs. ArchT:d2: CTX-water, p=0.9381, NS, ArchT:d1: CTX- L-Felinine vs. ArchT:d2: CTX- L-Felinine, **p=0.0036, GFP:d1: CTX- water vs. GFP:d1: CTX- L-Felinine, ****p<0.0001, GFP:d2: CTX-water vs. GFP:d2: CTX- L-Felinine, ***p=0.0002, ArchT:d1: CTX- water vs. ArchT:d1: CTX- L-Felinine, ****p<0.0001, ArchT:d2: CTX-water vs. ArchT:d2: CTX- L-Felinine, ****p<0.0001.) (b) Distance travelled during water and L-Felinine exposure during two days of conditioning (Mixed-effects analysis, genotype x day x odorant, F(1,6)=0.4017, p=0.5496, NS, day x odorant, F(1,22)=0.7140, p=0.4072, NS, genotype x odorant, F(1,22)=0.4008, p=0.5332, NS, genotype x day, F(1,6)=0.1722, p=0.6926, NS, genotype effect, F(1,22)=0.1401, p=0.7118, NS, day effect, F(1,22)=0.0002211, p=0.9883, odorant effect, F(1,22)=2.322, p=0.1418, NS, Sidak’s multiple comparisons test, GFP:d1: CTX- water vs. ArchT:d1: CTX- water, p>0.9999, NS, GFP:d2: CTX-water vs. ArchT:d2: CTX-water, p>0.9999, NS, GFP:d1: CTX- L-Felinine vs. ArchT:d1: CTX- L-Felinine, p=0.9937, NS, GFP:d2: CTX- L-Felinine vs. ArchT:d2: CTX- L-Felinine, p>0.9999, NS, GFP:d1: CTX- water vs. GFP:d2: CTX-water, p=0.9882, NS, GFP:d1: CTX- L-Felinine vs. GFP:d2: CTX- L-Felinine. p>0.9999, NS, ArchT:d1: CTX- water vs. ArchT:d2: CTX-water, p=0.9379. NS, ArchT:d1: CTX- L-Felinine vs. ArchT:d2: CTX- L-Felinine, p=0.9872, NS, GFP:d1: CTX- water vs. GFP:d1: CTX- L-Felinine, p=0.9987, NS, GFP:d2: CTX-water vs. GFP:d2: CTX- L-Felinine, p>0.9999, NS, ArchT:d1: CTX- water vs. ArchT:d1: CTX- L-Felinine, p>0.9999, NS, ArchT:d2: CTX-water vs. ArchT:d2: CTX- L-Felinine, p>0.9999, NS). All results reported are mean ± s.e.m.
-
Figure 6—figure supplement 3—source data 1
Numerical data shown in Figure 6—figure supplement 3.
Behavioral responses to L-Felinine during conditioning sessions.
- https://cdn.elifesciences.org/articles/74736/elife-74736-fig6-figsupp3-data1-v2.xlsx
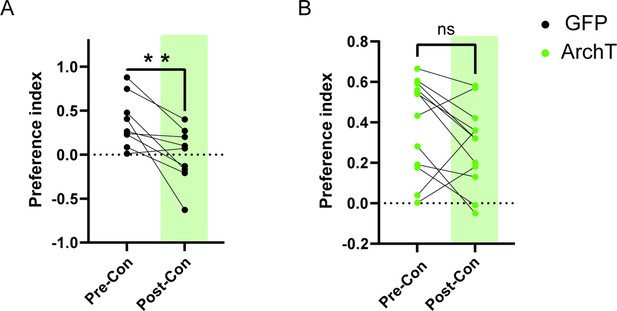
HPC→AHN pathway inhibition impairs the retrieval of place aversion memory.
Preference index of GFP (a) and ArchT mice (b) (GFP = 8, ArchT N = 12) for the L-Felinine-coupled chamber during habituation (pre-con: pre-conditioning) and after conditioning session (post-con: post-conditioning). GFP pre-conditioning vs. GFP post-conditioning (paired t-test, t=3.381, df=7, **p=0.0096). ArchT pre-conditioning vs. ArchT post-conditioning (paired t-test, t=1.704, df=11, p=0.1164).
-
Figure 6—figure supplement 4—source data 1
Numerical data shown in Figure 6—figure supplement 4.
HPC→AHN pathway inhibition impairs the retrieval of place aversion memory.
- https://cdn.elifesciences.org/articles/74736/elife-74736-fig6-figsupp4-data1-v2.xlsx
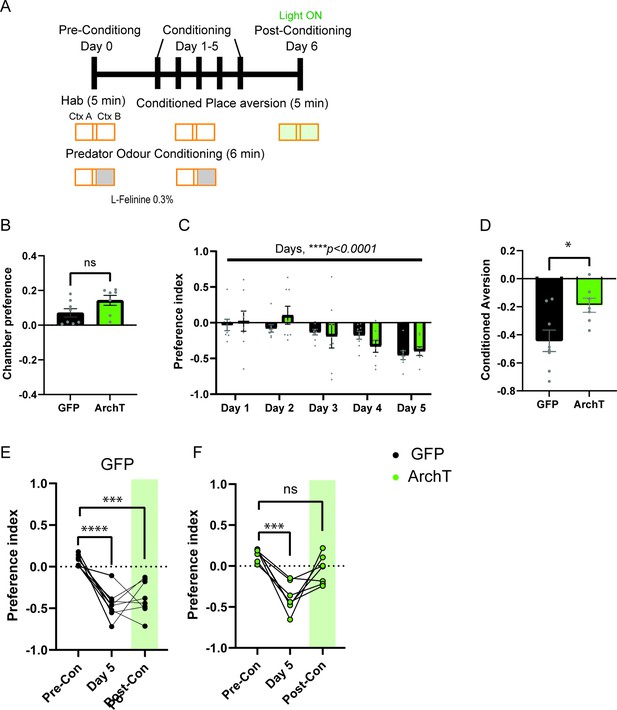
Development of predator associated context avoidance and impairment of retrieval of place aversion memory upon HPC-AHN pathway inhibition.
(a) Schematic illustration of the testing paradigm which consisted of multiple context-odor pairing sessions. It incorporated free chamber exploration (5 min) phase prior to the predator odor conditioning (6 min) with L-Felinine (0.3%). On Day 0, the free exploration or the Habituation (Hab), preferred chamber is selected as the predator odor chamber. (b) Chamber preference during habituation (GFP N=8, ArchT N=7, unpaired t-test, t=1.936, df=13, p=0.0749, NS). (c) Predator-context aversion developed across conditioning days. The day number equals the number of odor pairing throughout the training course. (two- way RM ANOVA, day x genotype, F(4, 52)=1.319, p=0.2753, NS, day effect, F(4, 52) = 8.656, ****p<0.0001, genotype effect, F(1, 13) = 0.1516, p=0.7033, NS). (d) Conditioned aversion memory. GFP N=8, ArchT N=7, GFP vs. ArchT (unpaired t-test, t=2.680, df=13. *p=0.0189). (e) GFP animals developed conditioned aversion and light did not impair predator context retrieval (1-WAY RM ANOVA, Treatment effect, F(2,14)=20.83, ****p<0.0001, Dunnett’s multiple comparison test for Pre-con vs. Day 5, ****p<0.0001, Pre-con vs. Post-Con, ***p=0.0004). (f) ArchT animals developed conditioned aversion and light impaired predator context retrieval (1-WAY RM ANOVA, Treatment effect, F(2,12)=19.33, ***p=0.0002, followed by Dunnett’s multiple comparison test for Pre-Con vs. Day 5, ***p=0.0001, Pre-Con vs. Post-Con, p=0.0885, NS).
-
Figure 6—figure supplement 5—source data 1
Numerical data shown in Figure 6—figure supplement 5.
Development of predator associated context avoidance and impaired retrieval of place aversion memory upon HPC-AHN pathway inhibition.
- https://cdn.elifesciences.org/articles/74736/elife-74736-fig6-figsupp5-data1-v2.xlsx
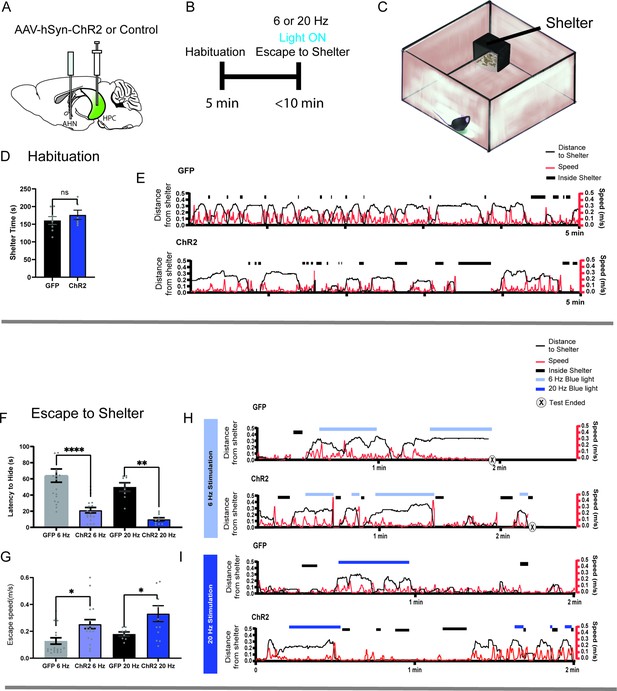
HPC→AHN pathway activation induces goal-directed escape.
(a) Schematic illustration of optogenetic HPC terminal activation in the AHN. (b) Schematic describing a test paradigm consisting of habituation (5 min) and a 6 or 20 Hz stimulation stage to induce shelter-directed escapes. (c), A cartoon drawing of the open field arena with a shelter box. (d) Time spent in the shelter during habituation (GFP N=8, ChR2 N=6, unpaired t-test, two-tailed, t=0.9241, df=12, p=0.3736, NS). (e) Representative line graphs for GFP (top) and ChR2 (bottom) mice, showing distance from shelter (black lines), speed (red lines), and moments when mice were inside the shelter (black boxes) over the 5 min habituation period. (f) Latency to escape to the shelter after optogenetic HPC terminal activation. (two-way ANOVA, frequency x genotype, F(1, 52) = 0.04138, p=0.8396, NS, frequency effect, F(1, 52)=3.268, p=0.0764, NS, genotype effect, F(1, 52)=34.71, ****p<0.0001, Sidak’s multiple comparisons test, 6 Hz GFP vs. ChR2, ****p<0.0001, 20 Hz GFP vs. ChR2, **p=0.0023). (g) Speed of escape running. 2-WAY ANOVA, frequency x genotype, F(1, 55) = 0.1134, p=0.7375, NS, frequency effect, F(1, 55)=2.78, p=0.1011, NS, genotype effect, F(1, 55)=12.65, ***p=0.008, Sidak’s multiple comparisons test, 6 Hz GFP vs. ChR2, *p=0.0139, 20 Hz GFP vs. ChR2, *p=0.0413. (h, i) Representative line graphs for GFP and ChR2 mice, showing distance from shelter (black lines), speed (red lines), and moments when mice were inside the shelter (black boxes) during the 6 Hz (h) and 20 Hz (i) HPC terminal stimulation stage. Light and dark blue highlights indicate the duration of 6 Hz and 20 Hz light stimulation, respectively, and (x) denotes test termination time. All results reported are mean ± s.e.m. *p < 0.05, **p < 0.01, ***p < 0.001, ****p<0.0001.
-
Figure 7—source data 1
Numerical data shown in Figure 7.
HPC→AHN pathway activation induces goal-directed escape.
- https://cdn.elifesciences.org/articles/74736/elife-74736-fig7-data1-v2.xlsx
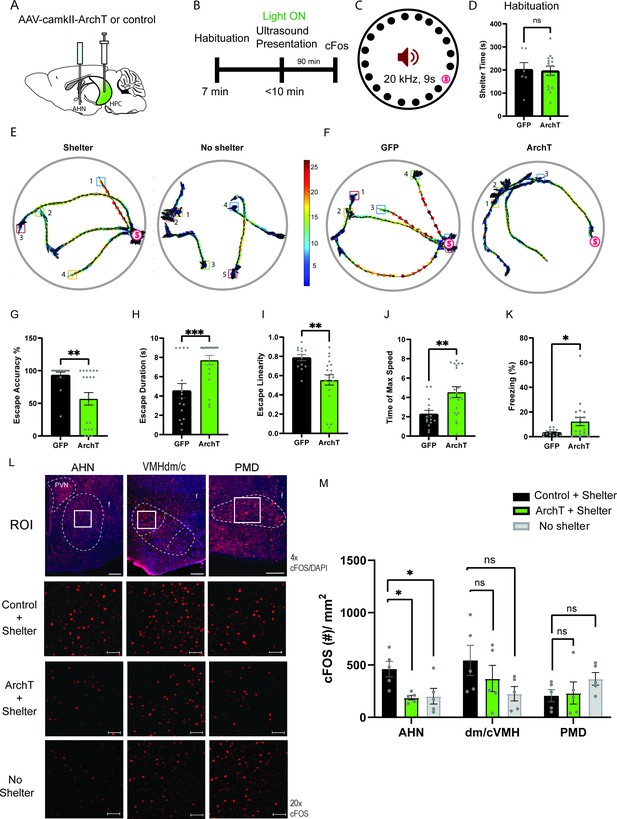
HPC→AHN pathway is necessary for goal-directed escape.
(a) Schematic illustration of optogenetic HPC terminal inhibition in the AHN (GFP N=7 and ArchT N=15). (b) Schematic describing a test paradigm consisting of habituation (7 min) and a threat delivery stage during which ultrasound (20 kHz, 9s) is turned on after mice voluntarily come out of the shelter to induce a shelter-directed escape. (c), Top view of testing apparatus, a modified Barnes maze. ‘s’ denotes the position where a shelter was placed. The red speaker sign denotes the auditory threat played from a speaker above the apparatus centre. (d) Time spent in the shelter during habituation stage (unpaired t-test, t=0.1698, df=20, p=0.8668, NS). (e) Representative ultrasound-evoked escape trajectories for a Wild type (WT) when a shelter is available (left) vs. WT when no shelter is available (right). (f) Representative ultrasound-evoked escape trajectories for a GFP control (left) and ArchT (right). (e,f) Individual threat presentation as a trial is numbered next to a square box, which denotes the animals’ starting position at the beginning of 9 s of 20 kHz sound. Dot color along the trajectory lines reflects animals’ speed. The arrows track animals’ head direction. The heatmap colorbar displays the scale of speed (pix/s). (g) Accuracy of reaching the shelter during escape. (unpaired t-test, t=3.149, df=33, **p=0.0034). (h), The linearity of escape trajectories expressed as the percentage ratio between the length of escape trajectory and a linear distance from escape onset position (i.e. ultrasound onset) to the shelter (unpaired t-test, t=3.266, df=31, **p=0.0027). (i) Time elapsed from the ultrasound onset to the shelter arrival (unpaired t-test, t=3.666, df=33, ***p=0.0008). (j) Time elapsed to reach the maximum speed during escape running to the shelter (unpaired t-test, t=3.134, df=31, **p=0.0036). (k) Time spent in freezing between the ultrasound onset and the shelter arrival (unpaired t-test, t=2.261, df=33, *p=0.0305). (l) c-Fos immunochemical detection across the medial hypothalamic defense system (AHN, VMHdm/c, PMD). First row, representative 4x epi-fluorescence microscope images of the medial hypothalamic defense system in GFP control mice. The regions of interest (ROI, white squares) within AHN, VMHdm/c, and PMD were imaged by confocal microscopy for cell counting. Second and third row: representative 20x confocal images of c-Fos signals in AHN, VMHdm/c, and PMD activated by ultrasound-evoked escapes with a shelter available in GFP (second row) and ArchT mice (third row). Fourth row: representative 20x confocal images of c-Fos signals activated by ultrasound-evoked escapes without shelter in controls. (m) Density of c-Fos signals activated by ultrasound-evoked escapes in AHN, VMHdm/c, and PMD in GFP controls with shelter (black), ArchT mice with shelter (green), and controls without shelter (grey) (N=5 mice for each group). AHN (1-WAY ANOVA, F(2,12)=6.171, *p=0.0144, Sidak’s multiple comparison test, Control Shelter vs. ArchT Shelter, *p=0.0177, Control Shelter vs. Control No Shelter, *p=0.0237), VMHdm/c (one-way ANOVA, F(2,12)=1.824, p=0.2035, NS, Sidak’s multiple comparison test, Control Shelter vs. ArchT Shelter, p=0.5391, NS, Control Shelter vs. Control No Shelter, p=0.1548), PMD (one-way ANOVA, F(2,12)=1.25, p=0.3212, NS, Sidak’s multiple comparison test, Control Shelter vs. ArchT Shelter, p=0.9665, NS, Control Shelter vs. Control No Shelter, p=0.3058, NS). Two-way ANOVA (ROI x treatment, F(4,36)=2.347, p=0.0729, NS, ROI effect, F(2,36) = 1.391, p=0.262, NS, treatment effect, F(2,36)=2.44, p=0.1015, NS, Sidak’s multiple comparisons test). AHN (Control Shelter vs. ArchT Shelter, p=0.1064, NS, Control Shelter vs. No Shelter, p=0.1344, ArchT Shelter vs. No Shelter, p=0.9993, NS), VMHdmd/c (Control Shelter vs. ArchT Shelter, p=0.4470, NS, Control Shelter vs. No Shelter, *p=0.0476, ArchT Shelter vs. No Shelter, p=0.5876, NS), PMD (Control Shelter vs. ArchT Shelter, p=0.9957, NS, Control Shelter vs. No Shelter, p=0.4999, NS, ArchT Shelter vs. No Shelter, p=0.6378, NS). Scale bar = 100 μm for 4x epi-fluorescence microscope images and 10 μm for 20 x confocal images. (PVN, paraventricular nucleus. f, fornix).
Mice display shelter-directed escape or freezing depending on the shelter availability.
(a) During ultrasound (US)-evoked escape responses, C57BL6 wild type mice (WT) run toward the shelter and reach the maximum speed in the middle of the escape trajectory to the shelter (WT-Best fit, quadratic, y=19.57–3.757x+ 0.5372–75.48 x2, maximum speed at x=0.5372). (b) Mice turn their head toward the shelter quickly after they initiate escape flights Best fit, sigmoidal, y = 7.433 + (1412478–7.433)/(1+10Log0.4760-x). (c) Rastor plots of speed profile for WT animals’ escape flights to the shelter. (d) Representative escape trajectories of wildtype mice when shelter is not available. Right, Rastor plots of speed profile for WT animals’ escape flights when shelter is not available. Pink line denotes the US onset which lasted 9 s. Animals’ arrivals at the shelter are denoted by ‘(s)’. Dot colors along the trajectory denote speed according to the color map on the right. (e) Changes in speed (pix/s) 1 second before and 9 s after the US onset (WT-NS N = 13, WT-S N = 8, two-way RM ANOVA, time x shelter availability, F(100,1900) = 2.156, ****p < 0.0001, time effect, F(7.912, 150.3) = 4.619, ****p < 0.0001, shelter availability, F(1,19)=10.15, **p = 0.0049). (f) Maximum speed during escape running (unpaired t-test, t = 4.761, df = 22, ****p < 0.0001), (g) Time elapsed to reach the maximum speed during escape running (unpaired t-test, t = 1.869, df = 22, p = 0.075, NS). (h) Time spent in freezing during the US presentation (unpaired t-test, t = 4.266, df = 19, ***p = 0.0004). WT-NS: Wild-type mice with no shelter. WT-S: Wild-type mice with shelter. All results reported are mean ± s.e.m. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
-
Figure 8—figure supplement 1—source data 1
Numerical data shown in Figure 8—figure supplement 1.
Mice display shelter-directed escape or freezing depending on the shelter availability.
- https://cdn.elifesciences.org/articles/74736/elife-74736-fig8-figsupp1-data1-v2.xlsx
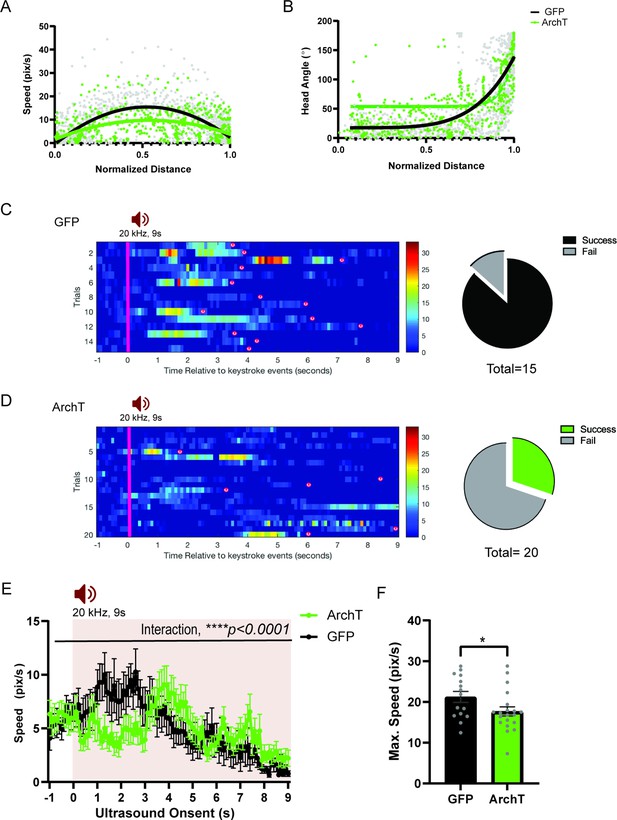
HPC→AHN pathway inhibition impairs ultrasound (US)-evoked escape responses.
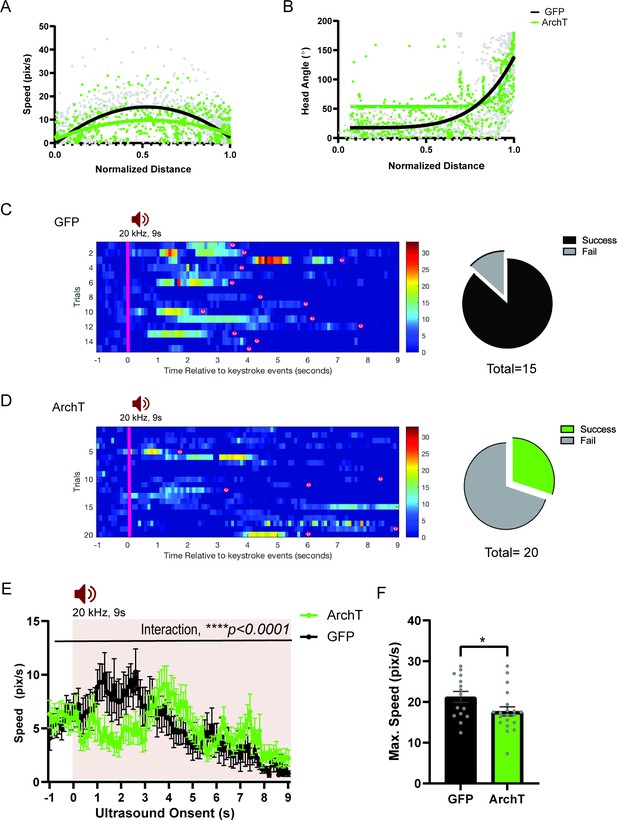
(a) Comparison between GFP (N=16) and ArchT (N=20) mice for speed (pixel/s) plotted in relation to the normalized distance to the shelter (GFP best fit line, y=15.34–5.226 x-58.01x2,, maximum speed at x=0.5706; ArchT best fit line, y=9.768–0.2301x-28.23x2, maximum speed at x=0.5444). (b) Head angle plotted in relation to the normalized distance to the shelter (GFP best fit line, y = 17.50 + (126075–4.765)/(1+10Log0.6336-x); ArchT best fit line, y=53.72 + (35041837–14.89)/(1+10Log0.3752-x)). (c) Left, Rastor plots of speed profile for GFP. Right, Pie chart comparing the number of successful or failing escape responses upon ultrasound presentation (GFP: total 15 trials, 2 fails, 13 successes), (d) Left Rastor plots of speed profile for ArchT. Right, Pie chart comparing the number of successful or failing escape responses upon ultrasound presentation (ArchT: total 20 trials, 14 fails, 6 successes). Pink line denotes the US onset which lasted 9s. Animals’ arrivals at the shelter are denoted by ‘(s)’. (e) Changes in speed (pix/s) 1 s before and 9 s after the US onset (two-way RM ANOVA, time x genotype, F (100, 3400) = 1.638, ****p < 0.0001, time effect, F(7.109, 241.7) = 3.668, ***p = 0.0008, genotype effect, F(1,34)=2.974e-005, p=0.9957, NS). (f) Maximum speed during escape running (unpaired t-test, t=2.047, df=33, *p = 0.0487). All results reported are mean ± s.e.m. *p < 0.05, **p < 0.01, ***p < 0.001, ****p<0.0001.
-
Figure 8—figure supplement 2—source data 1
Numerical data shown in Figure 8—figure supplement 2.
HPC→AHN pathway inhibition impairs ultrasound (US)-evoked escape responses.
- https://cdn.elifesciences.org/articles/74736/elife-74736-fig8-figsupp2-data1-v2.xlsx
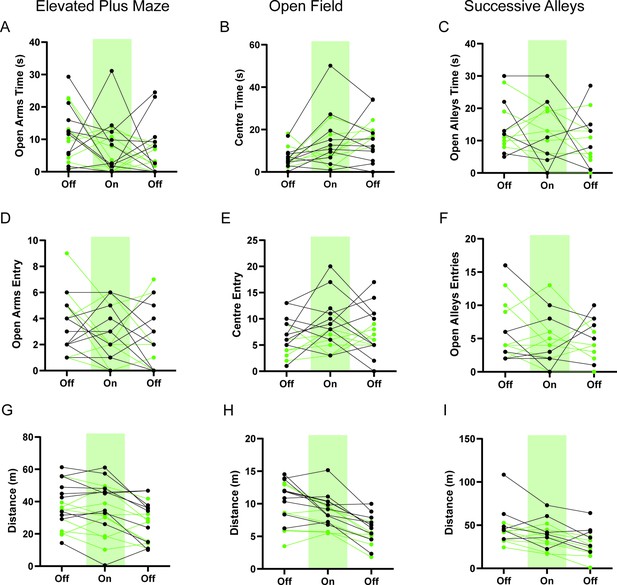
HPC→AHN pathway inhibition does not change anxiety-related behaviors.
(a) Time spent in the open arms in the elevated plus maze (EPM) (GFP N=10, ArchT N=9, two-way RM ANOVA, time x genotype, F(2,34)=0.1412, NS, time effect, F(1.902, 32.33)=2.278, p=0.1209, NS genotype effect, F(1,17)=1.244, p=0.2802, NS). (b) Time spent in the center of the open field (OF) (GFP N=10, ArchT N=7, 2-WAY RM ANOVA, time x genotype, F(2,30)=1.142, p=0.3326, NS, time effect, F(1.896, 28.44)=6.736, **p=0.0046, genotype effect, F(1,15)=0.001859, p=0.9662, NS). (c) Time spent in the open alleys of successive alleys (SA) (GFP N=6 ArchT N=8, two-way RM ANOVA, time x genotype, F(2,24)=0.07654, p=0.9265, NS, time effect, F(1.758, 21.1)=1.147, p=0.3303, NS, genotype effect, F(1,12)=0.04910, p=0.8284, NS). (d) Number of entries into the open arms of EPM (two-way RM ANOVA, time x genotype, F(2,34)=1.578, p=0.2211, NS, time effect, F(1.967, 33.45)=3.07, p=0.0604, NS, genotype effect, F(1, 17)=0.0005564, p=0.9815, NS). (e) Number of entries into the centre of the OF (two-way RM ANOVA, time x genotype effect, F(2,30)=0.8540, p=0.4358, NS, time effect, F(1.966, 29.49)=0.8676, p=0.4288, NS, genotype effect, F (1, 15) = 2.676, p=0.1227, NS). (f) Number of entries into the open alleys of SA (two-way RM ANOVA, time x genotype, F(2,24)=0.7369, p=0.4891, NS, time effect, F(1.976, 23.72)=1.154, p=0.332, NS, genotype effect, F(1,12)=0.01358, p=0.9092, NS). (g) Distance travelled in the EPM (2-WAY RM ANOVA, time x genotype, F(2,34)=0.4192, p=0.6609, NS, time effect, F (1.541, 26.20) = 14.80, ****p=0.0001, genotype effect, F (1, 17) = 1.855, p=0.1910, NS). (h) Distance travelled in the OF (two-way RM ANOVA, time x genotype, F(2,30)=0.5938, p=0.5586, NS, time effect, F(1.711, 25.67)=28.53, ***p<0.0001, genotype effect, F (1, 15) = 5.255, *p=0.0368). (i) Distance travelled in the SA (two-way RM ANOVA, time x genotype, F(2,24)=0.5972, NS, time effect, F(1.902, 22.82) = 13.14, ***p = 0.0002, genotype effect, F(1, 12) = 4.473, p = 0.0560, NS). All results reported are mean ± s.e.m.
-
Figure 8—figure supplement 3—source data 1
Numerical data shown in Figure 8—figure supplement 3.
HPC→AHN pathway inhibition does not change anxiety-related behaviors.
- https://cdn.elifesciences.org/articles/74736/elife-74736-fig8-figsupp3-data1-v2.xlsx
Videos
High-frequency (20 Hz) stimulation of AHN induced running, freezing and jumping in easy escape conditions.
During Light OFF, AHN-ChR2 animals are walking, grooming and rearing. Upon 20 Hz light photostimulation, AHN-ChR2 animals display running, freezing and jumping responses in the escapable chamber. In contrast, AHN-GFP animals display no change in behaviors between light OFF and light ON epoch.
High-frequency (20 Hz) stimulation of AHN induced running, freezing and jumping in difficult escape conditions.
During Light OFF, AHN-ChR2 animals are walking, grooming and rearing. Upon 20 Hz light photostimulation, AHN-ChR2 animals display running, freezing and jumping responses in the inescapable chamber. In contrast, AHN-GFP animals display no change in behaviors between light OFF and light ON epoch.
Low-frequency (6 Hz) stimulation of AHN carries negative valence and induces conditioned avoidance.
AHN-ChR2 animals run away from the light-paired chamber when photostimulation is delivered real time. Twenty-four hr later, the same animals remember the negative valence of the light-paired chamber and avoid and escape from the same chamber and remain in the light-off chamber. AHN-GFP animals display no aversion to light-paired chamber real time and 24 hr later.
Low-frequency (6 Hz) stimulation of HPC-AHN carries negative valence and induces conditioned place avoidance.
HPC-AHN ChR2 animals run away from the 6 Hz light-paired chamber when photostimulation is delivered real time. Twenty-four hr later, the same animals remember the negative valence of the light-paired chamber and avoid and escape from the same chamber and remain in the light-off chamber. HPC-AHN GFP animals display no aversion to light-paired chamber real time and 24 hr later.
High-frequency (20 Hz) stimulation of HPC-AHN carries negative valence and induces conditioned place avoidance.
HPC-AHN ChR2 animals run away from the 20 Hz light-paired chamber when photostimulation is delivered real time. Twenty-four hr later, the same animals remember the negative valence of the light-paired chamber and avoid and escape from the same chamber and remain in the light-off chamber. HPC-AHN GFP animals display no aversion to light-paired chamber real time and 24 hr later.
HPC-AHN pathway inhibition impairs the retrieval of contextual memory of predator cue.
HPC-AHN ArchT animals do not avoid the L-felinine paired chamber during the green light illumination. HPC-AHN GFP animals display avoidance of the L-felinine chamber and display predator cue associated chamber.
Low frequency (6 Hz) stimulation of HPC-AHN pathway induces escape to shelter.
HPC-AHN ChR2 animals display escape to shelter when 6 Hz photostimulation is delivered. HPC-AHN GFP animals do not escape to shelter upon 6 Hz photostimulation.
High frequency (20 Hz) stimulation of HPC-AHN pathway induces escape to shelter.
HPC-AHN ChR2 animals display escape to shelter when 20 Hz photostimulation is delivered. HPC-AHN GFP animals do not escape to shelter upon 20 Hz photostimulation.
HPC-AHN pathway inhibition impairs goal-directed escape to shelter.
HPC-AHN ArchT animals display fragmented and impaired escape to shelter compared to the GFP controls upon hearing the 20 kHz ultrasound during the green light illumination.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Mus musculus, male) | AHN-ChR2/GFP; HPC-AHN ChR2/GFP; HPC-AHN ArchT/GFP | Charles River | C57BL/6 | |
| Strain, strain background (Mus musculus) | GABA-mCherry or Dlx5/6-FLPe;RC::FrePe | PMID:22151329 | JAX#029486 x JAX#010815 | Obtained by crossing homozygous RC::FrePe6 x Dlx5/6-FLPe mice |
| Recombinant DNA reagent | AAV2/9-hSyn-ChR2-eYFP (ChR2) | Addgene Vrial Vector Core | 26,973 | |
| Recombinant DNA reagent | AAV2/9 or AAV2/5-CB7-CI-eGFP (Control) | Addgene Vrial Vector Core | 105,542 | |
| Recombinant DNA reagent | AAV2/5-camkIIa-ArchT-GFP (ArchT) | Addgene Vrial Vector Core | 99,039 | |
| Antibody | anti-GFP (chicken polyclonal) | Abcam | ab13970 | (1:1000) |
| Antibody | anti-cFOS (rabbit polyclonal) | Santa Cruz Biotechnology | SC-52 | (1:1000) |
| Antibody | Alexa Fluor 594-conjugated anti-rabbit secondary antibody (donkey polyclonal) | Jackson ImmunoResearch Laboratories | AB_2340621 | (1:500) |
| Antibody | Alexa Fluor 488-conjugated anti-chicken secondary antibody (donkey polyclonal) | Jackson ImmunoResearch Laboratories | AB_2340375 | (1:1000) |
| Chemical compound, drug | DAPI | Cell Signaling Technology | 4,083 S | |
| Chemical compound, drug | L-Felinine | Toronto Research Chemicals | F231250 | |
| Software, algorithm | DeepLabCut | PMID:31227823 | ||
| Software, algorithm | ANY-MAZE | StoeltingCo | ||
| Software, algorithm | MATLAB | Mathworks | ||
| Software, algorithm | Prism | GraphPad | ||
| Software, algorithm | Code for MATLAB | Custom written code | 10.5281/zenodo.5899428 | Custom written code for MATLAB used for ultrasound evoked escape |





