Hydrodynamic model of fish orientation in a channel flow
Figures
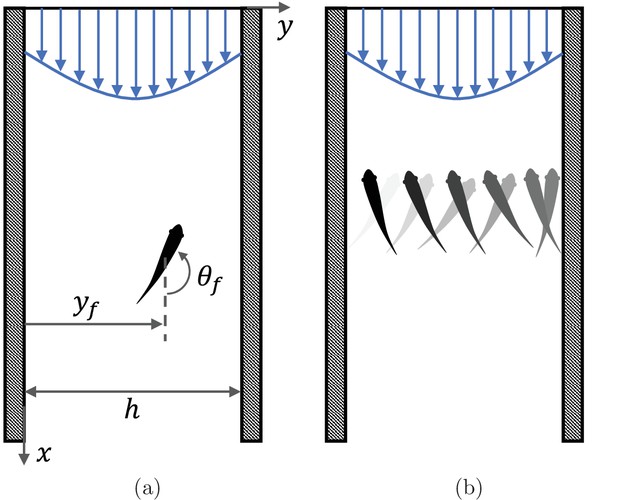
Fish rheotaxis.
(a) Illustration of the problem with notation, showing a fish swimming in a background flow described by Equation 4. (b) Schematic of the cross-stream sweeping movement of some fish species swimming without visual cues; snapshots of fish at earlier time instants are illustrated by lighter shading.
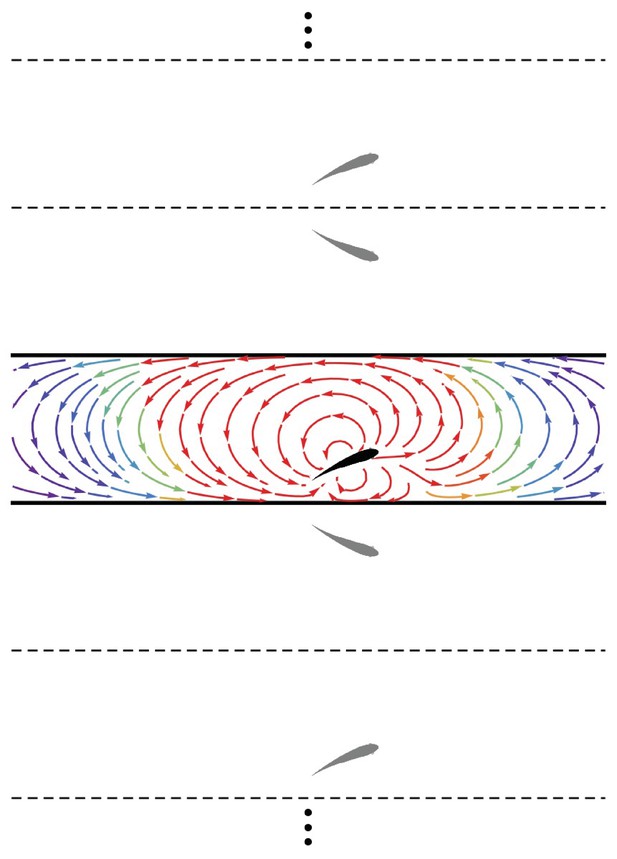
Method of images.
Schematic of the fish (black) in the channel (thick lines) and the set of images (gray) needed to generate the channel. The streamlines generated by the fish in an otherwise quiescent fluid are shown in the channel colored by local velocity magnitude (red: high; blue: low). Dashed and solid lines are mirroring planes for the method of images, the pattern for which continues ad infinitum.
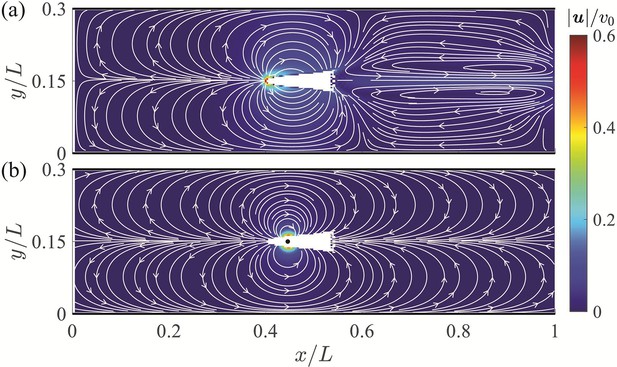
Velocity field around a swimming fish from computational fluid dynamics.
(a) Mean velocity field around the steady swimming giant danio relative to the background flow. (b) Velocity field predicted by a dipole with located at from the fish head along its centerline relative to the background flow. The selection of the dipole location and strength is detailed in Appendix 2.
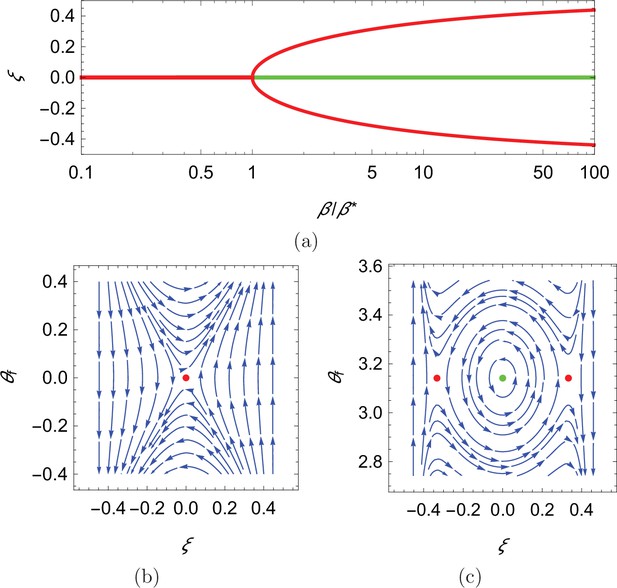
Qualitative dynamics of equation set Equation 10a.
(a) Cross-stream equilibria for upstream swimming as a function of β. (b,c) Phase plot for downstream and upstream swimming in the case , , and , so that . In all panels, red refers to unstable equilibria and green to stable equilibria.
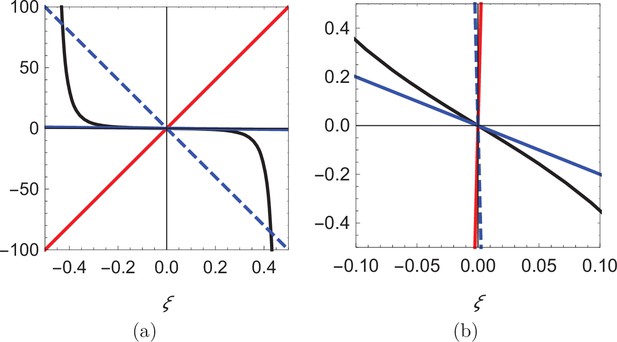
Visual illustration of the process of determining the roots of Equation 14.
(a) Plot of the function (black), superimposed with three lines of different slope: 200 (red), -200 (dashed blue), and -2 (solid blue). (b) Zoomed-in view of the curves in (a) showing that the blue line can only intersect the black curve at the origin.
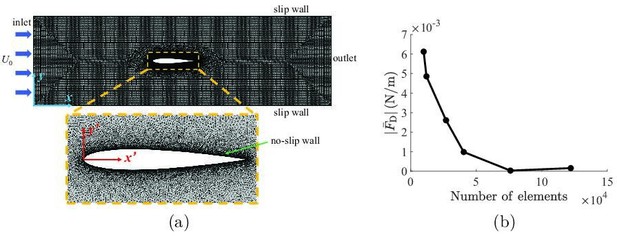
Details about the implementation of the computational fluid dynamics simulations.
(a) Mesh implemented in the simulations, with definitions of coordinate systems and a zoomed-in view of the refined mesh around the fish. (b) Mesh convergence analysis, showing the mean drag as a function of number of elements in the simulation.
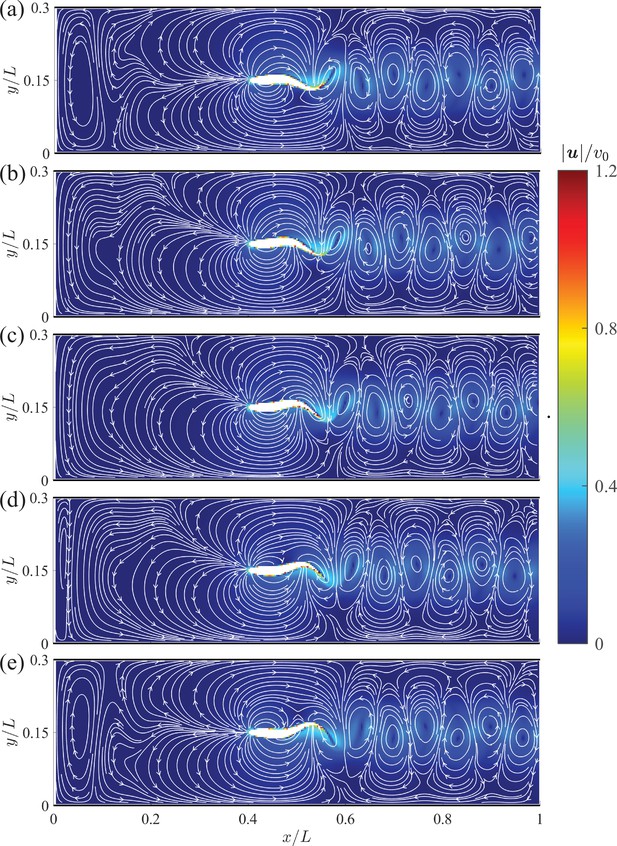
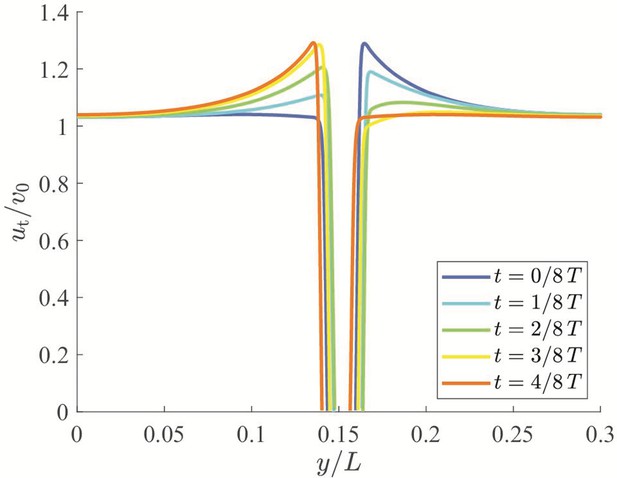
Instantaneous velocity fields around a swimming fish relative to the background flow from computational fluid dynamics.
(a) – (e) correspond to , , , , and , respectively, where is the period of a tail beat. White curves are streamlines with arrows indicating flow directions.
Tables
Estimation of model parameters from data in the literature.
-
LL+ cavefish swimming speed in zero background flow in Bak-Coleman and Coombs, 2014 is used to estimate α.
Parameters employed in (27) to describe the locomotory pattern of a giant danio.
| Parameters | |||||
|---|---|---|---|---|---|
| Values | 0.004 |
Relevant publications on fish rheotaxis in the absence of visual cues, identified through literature review.
| Reference | Fish | Swimming domain | Flow properties | *Sensory cues | Rheotaxis threshold speed | ||
|---|---|---|---|---|---|---|---|
| Species | Length | Flow speed | Flow gradient | ||||
| Bak-Coleman et al., 2013 | Giant danio (Devario aequipinnatus) | 6.0 –7.3 cm | Flow tank of () | 0, 3, and | at LL+ threshold speed; flow gradient expected to be small near center of tank | LL+/LL- | |
| Bak-Coleman and Coombs, 2014 | blind cavefish (Astyanax mexicanus) | 4.2 –5.0 cm | Flow tank of () | 0, 1, 2, 3, 4, 7 and | at LL+ threshold speed; flow gradient expected to be small near center of tank | LL+/LL-; fish made transient contacts with substrate | LL+: ; LL-: |
| †Baker and Montgomery, 1999 | blind cavefish (Astyanax fasciatus) | 4 –7 cm | Flow tank of () | 0, 2, 3, 5, 9 and | at LL+ threshold speed; flow gradient expected to be small near center of tank | LL+/LL-; tactile senses | LL+: 2–; LL-: 9– |
| Elder and Coombs, 2015 | Mexican tetras (Astyanax mexicanus) | Flow tank of () | 0, 1, 2, 4, 7, and | at threshold speed; flow gradient expected to be small near center of tank | LL+/LL- | for LL+ and LL- | |
| Kulpa et al., 2015 | blind cavefish (Astyanax mexicanus) | 4.4 –5.3 cm | Flow tank of () | Maximum speed of | Jet flow across center of tank; flow gradient expected to be large | LL+/LL- | |
| ‡Lyon, 1904 | blind Fundulus | unspecified | Trough with unspecified dimensions; tideway leading to pond | “not too strong current” in trough and current with “more or less eddy and irregularity” in tideway | Flow gradient expected to be small | LL+; some fish gained tactile senses | Not measured; rheotaxis elicited only by tactile cues |
| ‡Lyon, 1904 | blind Fundulus | unspecified | Trough with unspecified dimensions | flow “gushing rather violently” | Jet flow; flow gradient expected to be large | LL+ | Not measured; rheotaxis elicited by flow |
| †Montgomery et al., 1997 | blind cavefish (Astyanax fasciatus) | 4 –7 cm | §Flow tank of () | 0, 2, 3, 5, 9, and | at LL+ threshold speed; flow gradient expected to be small near center of tank | LL+/LL-; tactile senses | LL+: 2–3 cm/s; LL-: 9–16 cm/s |
| Oteiza et al., 2017 | zebrafish (Danio rerio) larva 5–7 days post fertilization (dpf) | unspecified | 13 cm-long circular tube with diameter 1.27– | 0.2–0.8 cm/s | Low to high flow gradients identified through particle image velocimetry | LL+/LL- | LL+: rheotaxis observed as low as |
| Peimani et al., 2017 | zebrafish (Danio rerio) larva 5–7 dpf | estimated | Flow channel of () | 0.95–3.8 cm/s | at threshold speed; flow gradient expected to be large | LL+ | |
| Suli et al., 2012 | zebrafish (Danio rerio) larva 5 dpf | Flume of () | 0.075, 0.15, | ; flow gradient expected to be large | LL+/LL- | Not quantified | |
| Van Trump and McHenry, 2013 | blind Mexican cavefish (Astyanax fasciatus) | 3 –7 cm | Cylindrical channel of () | 0, 1, 2, 4, 6, 8, 10, 13, | at threshold speed; flow gradient expected to be small near center of tank | LL+/LL- | 2–4 cm/s |
-
*
LL+: lateral line enabled; LL−: lateral line disabled
-
†
Data are extracted from the same set of experiments
-
‡
Two experiments are considered from the same paper
-
§
Data are from Baker and Montgomery, 1999.
Results of the bibliographical research on fish rheotaxis in the absence of visual cues, used to validate the proposed model.
| Reference | Fish species | *Evidence | Comparison with model | |
|---|---|---|---|---|
| Supportive | Inconclusive | |||
| Within studies | ||||
| Effect of lateral line | ||||
| Bak-Coleman et al., 2013 | Giant danio (Devario aequipinnatus) | No significant difference in fish heading angle against current was detected between LL+ and LL- | × | |
| Bak-Coleman and Coombs, 2014 | blind cavefish (Astyanax mexicanus) | Rheotaxis threshold speed was slightly (but not significantly) lower in LL- condition | × | |
| Baker and Montgomery, 1999 and Montgomery et al., 1997 | blind cavefish (Astyanax fasciatus) | Rheotaxis threshold speed was significantly higher in LL- condition; fish received intermittent tactile senses | × | |
| Elder and Coombs, 2015 | Mexican tetras (Astyanax mexicanus) | No significant influence of LL condition was detected on rheotactic performance | × | |
| Kulpa et al., 2015 | blind cavefish (Astyanax mexicanus) | Significantly higher rheotaxis index in LL+ fish than LL- fish in jet stream | × | |
| Oteiza et al., 2017 | zebrafish (Danio rerio) larva 5–7 dpf | Posterior lateral line ablation or chemical neuromast ablation severely reduced rheotaxis | × | |
| Suli et al., 2012 | zebrafish (Danio rerio) larva 5 dpf | LL hair cell damage led to a significant decrease in rheotaxis; regeneration of LL hair cells restored rheotaxis | × | |
| Van Trump and McHenry, 2013 | blind Mexican cavefish (Astyanax fasciatus) | In LL+ and LL-, fish exhibited statistically indistinguishable rheotaxis behavior | × | |
| Effect of flow gradient | ||||
| Lyon, 1904 | blind Fundulus | In a flow with small gradient, rheotaxis was elicited only when fish received tactile cues; in jet flow with large gradient, rheotaxis was elicited by flow without tactile cues. Lack of data on statistical significance | × | |
| Oteiza et al., 2017 | zebrafish (Danio rerio) larva 5–7 dpf | Rheotaxis of fish improved with increasing gradient magnitudes | × | |
| Across studies | ||||
| Effect of channel width | ||||
| Bak-Coleman and Coombs, 2014; Baker and Montgomery, 1999 | blind cavefish (Astyanax mexicanus); blind cavefish (Astyanax fasciatus) | Significantly different threshold speed for LL+ fish: (mean ±s.e.m.) in wide tunnel; between and in wide tunnel. Tactile cues available to fish in Bak-Coleman and Coombs, 2014 | × | |
| Bak-Coleman and Coombs, 2014; Van Trump and McHenry, 2013 | blind cavefish (Astyanax mexicanus); blind cavefish (Astyanax fasciatus) | Significantly different threshold speed for LL+ fish: (mean ±s.e.m.) in wide tunnel; between and in diameter tunnel. Tactile cues available to fish in Bak-Coleman and Coombs, 2014 | × | |
| Oteiza et al., 2017; Peimani et al., 2017 | zebrafish (Danio rerio) larva 5–7 dpf | Onset of rheotaxis in LL+ fish observed at flow speed in wide tunnel; rheotaxis observed in LL+ fish at flow speed in diameter tunnel | × | |
| Effect of body length | ||||
| Bak-Coleman and Coombs, 2014; Elder and Coombs, 2015 | blind cavefish (Astyanax mexicanus); Mexican tetras (Astyanax mexicanus) | Significantly different threshold speed for LL+ fish: (mean ±s.e.m.) for 4.2– long fish; (mean ±s.e.m.) for long fish. Tactile cues available to fish in Bak-Coleman and Coombs, 2014 | × | |
| Total | 5 | 9 | ||
-
*
LL+: lateral line enabled; LL−: lateral line disabled