A novel monocyte differentiation pattern in pristane-induced lupus with diffuse alveolar hemorrhage
Figures
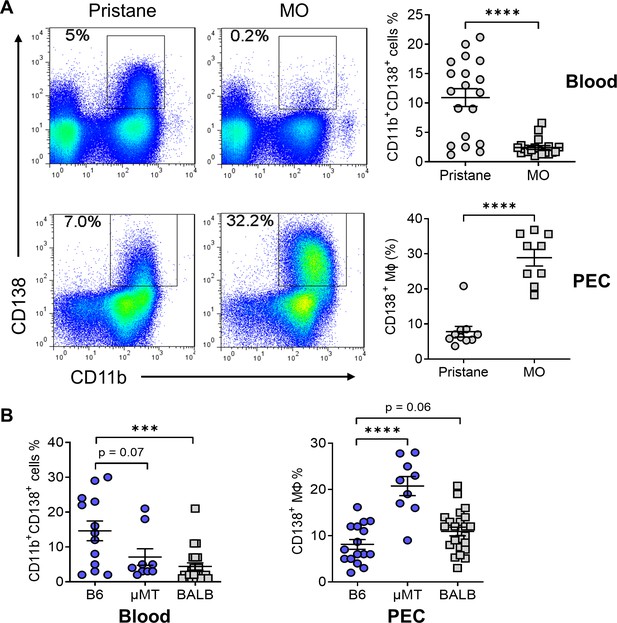
CD138+CD11b+ cells in the circulation and peritoneum.
(A) B6 mice were injected with either pristane or mineral oil (MO). CD138 and CD11b surface expression on peripheral blood leukocytes and peritoneal exudate cells (PECs) was analyzed by flow cytometry at day 14. (B) Comparison of the percentages of CD138+ cells in blood and PECs from B6, B6 μMT (B cell deficient), and BALB/c mice. ***p < 0.001; ****p < 0.0001 (Student’s t-test).
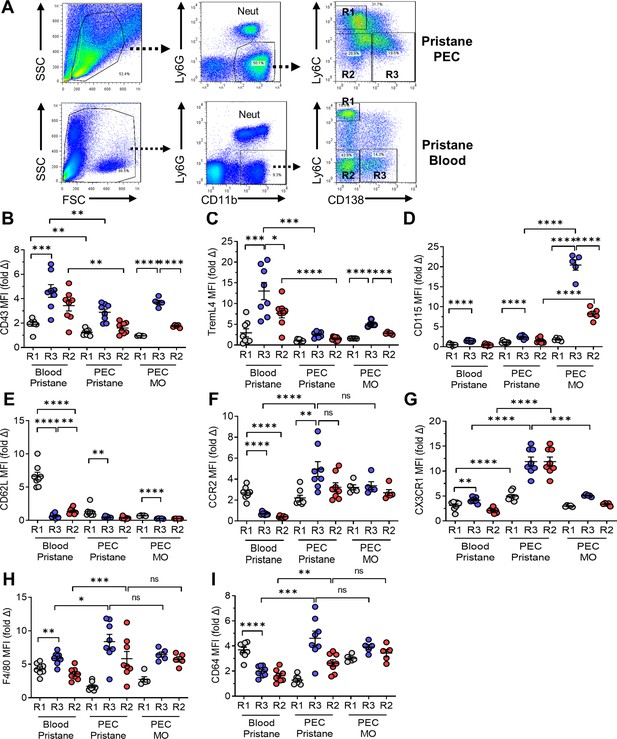
Flow cytometry of monocytes surface markers.
Peripheral blood mononuclear cells and peritoneal exudate cells (PECs) from pristane- or mineral oil (MO)-treated mice were stained with fluorescently labeled anti-CD11b, Ly6G, Ly6C, and CD138 antibodies. Mean fluorescence intensity (MFI) of CD43, TREML4, CD115, and CD62L was determined on cell subsets. (A) Gating strategy for analysis of CD11b+Ly6G− PEC and circulating mononuclear cells (blood). R1, CD11b+Ly6G−Ly6ChiCD138− cells; R2, CD11b+Ly6G−Ly6C−CD138− cells; R3, CD11b+Ly6G−Ly6C−CD138+ cells. Staining intensity of CD43 (B), TREML4 (C), CD115 (D), CD62L (E), CCR2 (F), CX3CR1 (G), F4/80 (H), and CD64 (I) in the R1, R2, and R3 subsets from peripheral blood or PEC from pristane- or MO-treated mice. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 (Student’s t-test); ns, not significant.
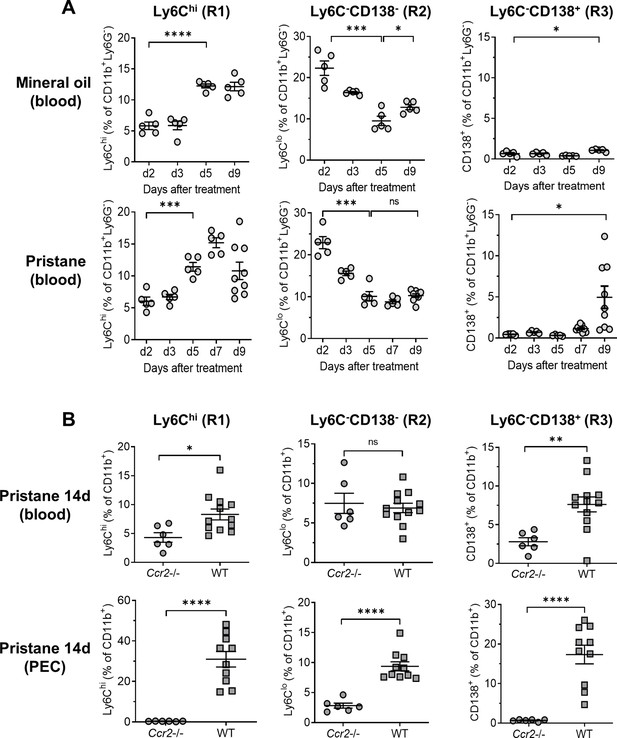
Ccr2 dependence of monocyte egress from the bone marrow (BM).
B6 mice were treated with either mineral oil or pristane and CD11b+Ly6G− myeloid subsets in the blood and peritoneal exudate cell (PEC) were gated as in Figure 2A. (A) Circulating Ly6Chi (R1), Ly6C−CD138− (R2) and Ly6C−CD138+ (R3) monocytes from mineral oil- and pristane-treated mice were assessed at 0–9 days after treatment by flow cytometry. Data are expressed as a percentage of CD11b+Ly6G− cells. (B) B6 wild-type (WT) and Ccr2−/− mice were treated with pristane. R1, R2, and R3 cells as a percentage of CD11b+Ly6G− cells were measured in the blood and PEC by flow cytometry 14 days later. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 (Student’s t-test). ns, not significant.
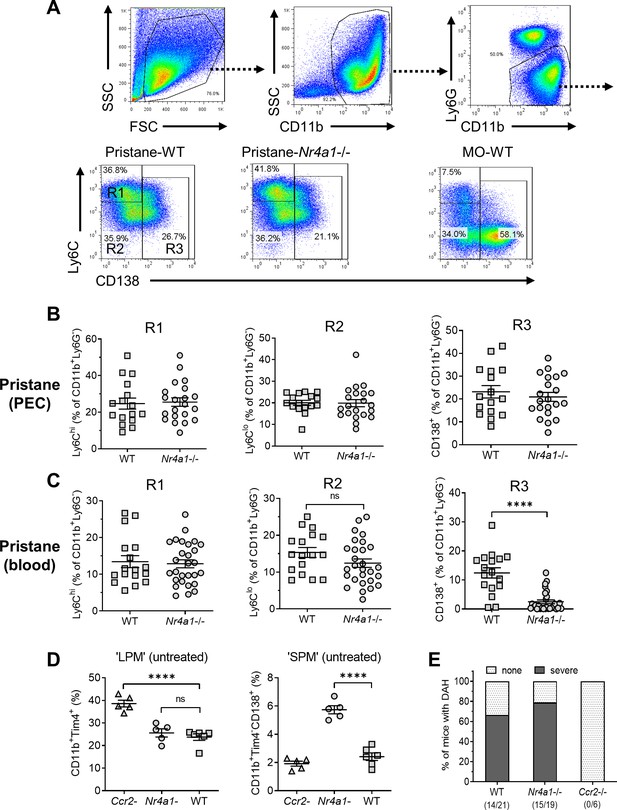
Monocyte/Mϕ subsets in Nr4a1−/− mice.
(A) Gating strategy. Peritoneal exudate cells (PECs) from pristane- or mineral oil (MO)-treated wild-type and Nr4a1−/− mice were stained with anti-CD11b, Ly6G, Ly6C, and CD138 and analyzed by flow cytometry. PECs were gated on the CD11b+Ly6G− population. R1, Ly6ChiCD138− PECs; R2, Ly6CloCD138− PECs; R3, Ly6CloCD138+ PECs. Gating for circulating (blood) cells was similar. SSC, side scatter; FSC, forward scatter. (B) R1, R2, and R3 subsets in the peritoneum (percentage of CD11b+Ly6G− cells). (C) R1, R2, and R3 subsets in the peripheral blood (percentage of CD11b+Ly6G− cells). (D) Flow cytometry of peritoneal cells in untreated Ccr2−/−, Nr4a1−/−, and wild-type (WT) mice. Left, CD11b+Tim4+ large peritoneal macrophages (LPMs) as a % of total peritoneal cells. Right, CD11b+Tim4−CD138+ small peritoneal macrophages (SPMs) as a % of total peritoneal cells. (E) Frequency of diffuse alveolar hemorrhage (day 14) in pristane-treated WT, Nr4a1−/−, and Ccr2−/− mice. ****p < 0.0001 (Student’s t-test). ns, not significant.
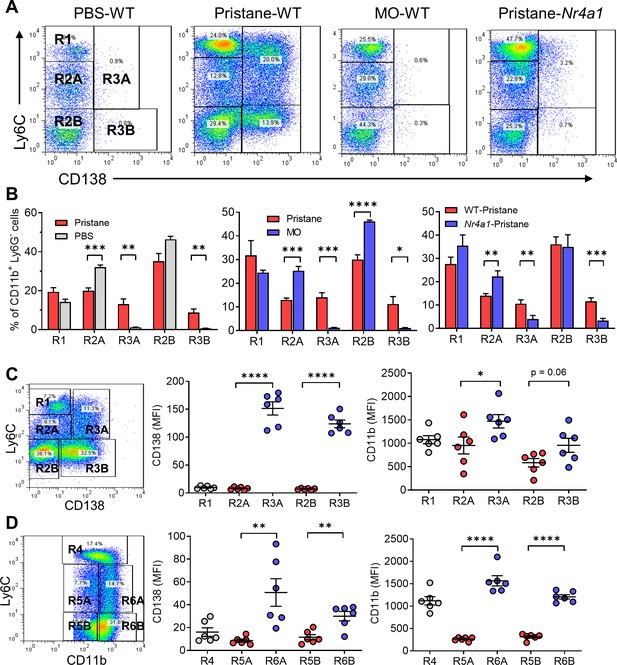
Circulating monocyte subsets.
(A) Gating strategy. Blood cells from pristane- or mineral oil (MO)-treated B6 mice, Nr4a1−/− mice, and PBS-treated controls were stained with anti-CD11b, Ly6G, Ly6C, and CD138 and analyzed by flow cytometry, gating on the CD11b+Ly6G− population. R1, Ly6ChiCD138−; R2A, Ly6CloCD138−; R2B, Ly6C−CD138−; R3A, Ly6CloCD138+; Ly6C−CD138+. SSC, side scatter; FSC, forward scatter. (B) Comparison of circulating CD11b+Ly6G− cells in pristane-treated mice vs. PBS-treated (left) and MO-treated (middle) mice. Right panel compares wild-type (WT) vs. Nr4a1−/− mice treated with pristane. (C) Mean fluorescence intensity (MFI) of CD138 and CD11b staining in the R1, R2A, R2B, R3A, and R3B subsets from pristane-treated mice. (D) Alternative gating strategy for circulating CD11b+Ly6G− cells from pristane-treated mice: R4, CD11b+Ly6Chi; R5A, Ly6CloCD11b+; R5B, Ly6C−CD11b+; R6A, Ly6CloCD11bhi; R6B, Ly6C−CD11bhi. Right, CD138 and CD11b staining (MFI) of the R4, R5A/B, and R6A/B subsets. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 (Student’s t-test).
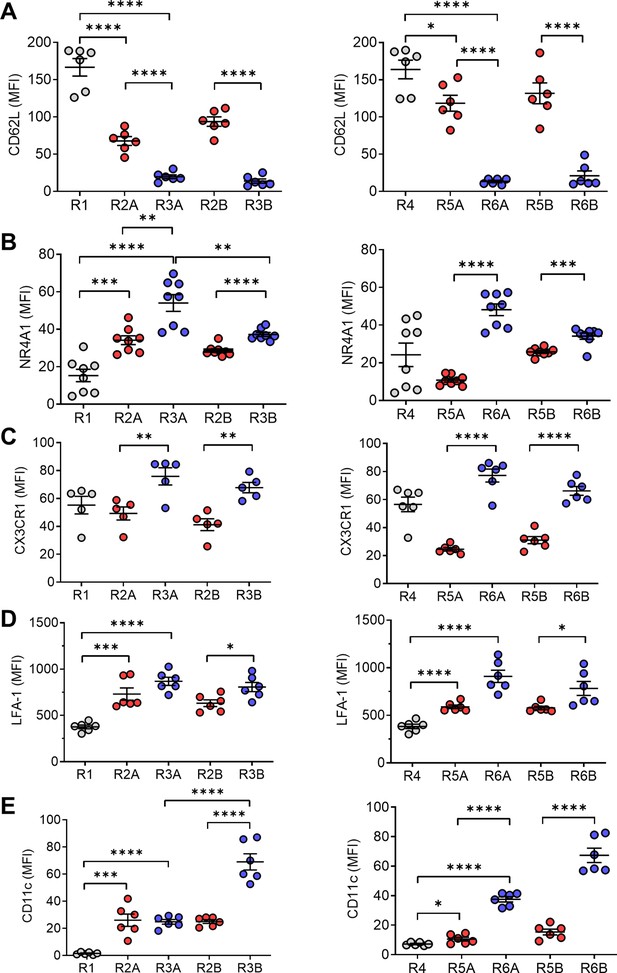
Circulating monocyte phenotypes.
Circulating monocytes are gated as in Figure 5 and the staining intensity (MFI) of various markers was ascertained by flow cytometry. (A) CD62L, (B) Nr4a1, (C) Cx3Cr1, (D) LFA-1, and (E) CD11c. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 (Student’s t-test).
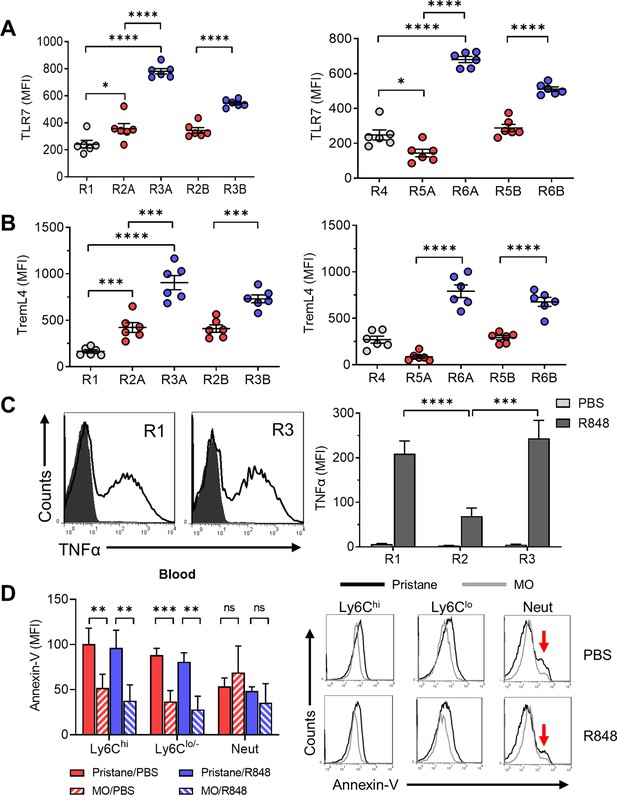
Function of CD138+ monocytes.
Circulating monocytes from pristane-treated B6 mice were gated as in Figure 5 and the staining intensity (MFI) of TLR7 (A) and TremL4 (B) was ascertained by flow cytometry. (C) R848-stimulated TNFα production in R1, R2, and R3 monocytes from pristane-treated mice (14 days). Circulating leukocytes were incubated 5 hr with R848 or PBS and then surface stained with anti-CD11b, Ly6G, Ly6C, and CD138 antibodies and intracellularly stained with anti-TNFα. Left, histograms of intracellular TNFα staining (R1 and R3 subsets). Right, TNFα staining of blood cells cultured 5 hr with R848 or PBS. (D) Annexin-V staining (left, mean fluorescence intensity; right, representative histograms) of circulating CD11b+ monocyte subsets (Ly6ChiLy6G− and Ly6Clo/−Ly6G−) and CD11b+Ly6G+ neutrophils (Neut) from pristane- or mineral oil (MO)-treated mice cultured for 20 hr with R848 or PBS. Red arrows, annexin-Vhi apoptotic neutrophils. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 (Student’s t-test); ns, not significant.
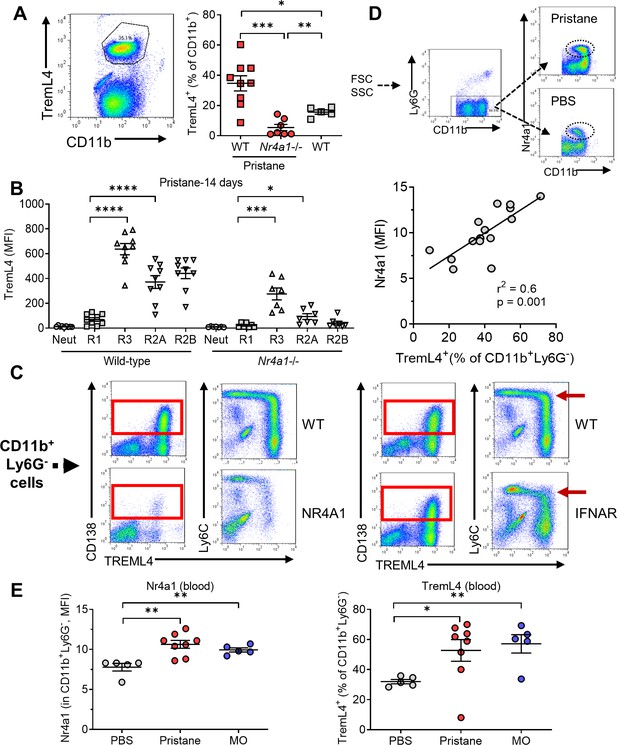
Low TremL4 expression in Nr4a1−/− mice.
(A) Blood cells were stained with anti-CD11b and anti-Treml4 monoclonal antibodies. CD11b+Treml4+ cells as a percentage of total CD11b+ cells were determined by flow cytometry in pristane-treated wild-type (WT) and Nr4a1−/− mice and in untreated WT mice. (B) Wild-type and Nr4a1−/− mice were treated with pristane and CD11b+Ly6G− blood cells were gated as in Figure 2A. CD11b+Ly6G+ neutrophils (% of total CD11b+ cells) and Ly6Chi (R1), Ly6C−/loCD138− (R2), and Ly6C−/loCD138+ (R3) monocytes (% of CD11b+Ly6G− cells) were determined. (C) Left, TremL4, CD138, and Ly6C staining of circulating CD11b+Ly6G− cells from pristane-treated WT vs. Nr4a1−/− mice. Right, CD138, and Ly6C staining of circulating CD11b+Ly6G− cells from wild -type (WT) vs. Ifnar1−/− mice. Nearly all CD138+ cells were TremL4+ (red boxes). Red arrow, Ly6Chi cells. (D) Top, gating strategy for identifying Nr4a1+ cells. Bottom, linear regression analysis of intracellular Nr4a1 and TremL4 staining in total CD11b+Ly6G− cells from PBS- plus pristane-treated B6 mice. (E) Flow cytometry of Nr4a1 (intracellular staining) and TremL4 (surface staining) in circulating CD11b+Ly6G− cells from wild-type mice treated with PBS, pristane, or mineral oil (MO). *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 (Student’s t-test).
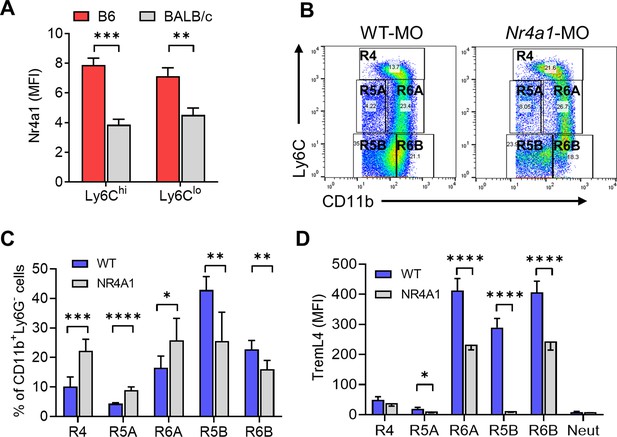
Ly6CloCD138+ monocytes are increased in mice that develop diffuse alveolar hemorrhage (DAH).
(A) Intracellular Nr4a1 staining in the CD11b+Ly6Chi and CD11b+Ly6C−/lo monocytes from untreated B6 and BALB/c mice. (B) CD11b and Ly6C staining of circulating Ly6G- cells in mineral oil (MO)-treated mice. Gated as in Figure 5A. (C) Percentages of R4, R5A, R5B, R6A, and R6B monocytes in wild-type B6 (WT) vs. B6 Nr4a1−/− mice treated with MO. (D) TremL4 staining of circulating R4, R5A, R5B, R6A, and R6B monocytes and neutrophils (Neut) from MO-treated mice. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 (Student’s t-test).
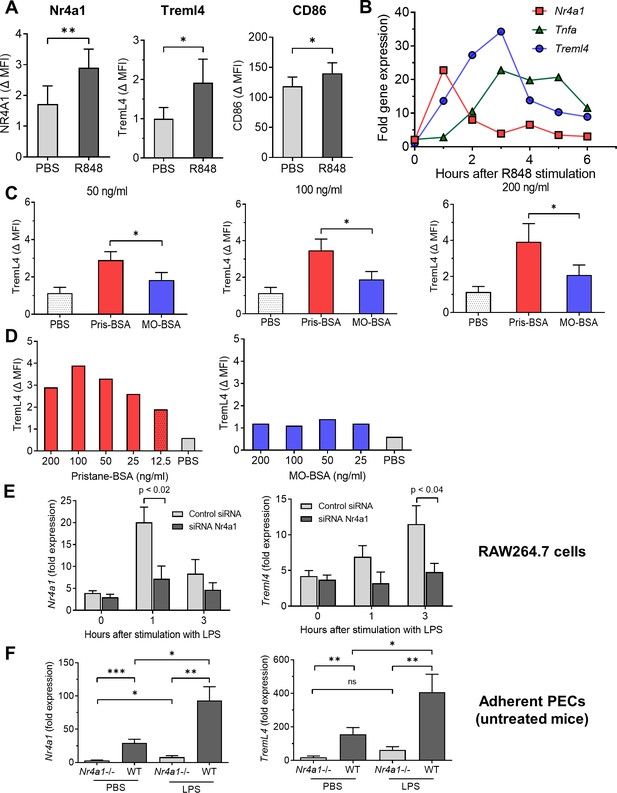
Nr4a1 regulates TremL4.
(A) R848 activated expression (24 hr) of Nr4a1, TremL4, and CD86 proteins in RAW264.7 cells (flow cytometry; MFI, mean fluorescence intensity). (B) R848-stimulated mRNA expression of Nr4a1, Tnfa, and TremL4 (qPCR). (C, D) TremL4 expression (MFI) in RAW264.7 cells by flow cytometry after culture with pristane or mineral oil (MO) 12.5–200 ng/ml emulsified in bovine serum albumin (BSA) or with PBS for 24 hr. (E) Nr4a1 and Treml4 expression (qPCR) in LPS-stimulated RAW264.7 cells cultured with Nr4a1 or control siRNA. (F) Nr4a1 and Treml4 expression (qPCR) in adherent peritoneal exudate cells (PECs) from untreated wild-type (WT) or Nr4a1−/− mice treated for 3 hr with LPS or PBS. *p < 0.05; **p < 0.01; ***p < 0.001 (Student’s t-test).
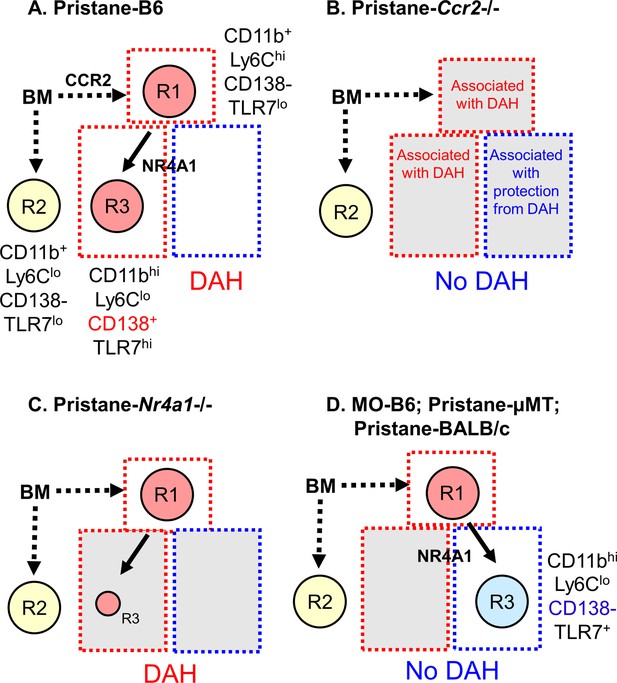
Monocyte subsets in pristane- and mineral oil (MO)-treated mice.
(A) Pristane-treated B6 mice; (B) pristane-treated B6 Ccr2−/− mice; (C) pristane-treated B6 Nr4a1−/− mice; (D) MO-treated B6 mice, pristane-treated B6 μMT mice, and pristane-treated BALB/c mice. Phenotypes of bone marrow (BM)-derived monocyte subsets R1, R2, and R3 and their dependence on CCR2 and Nr4a1 are indicated. Subsets that are associated with susceptibility to diffuse alveolar hemorrhage (DAH) are highlighted in red. Subsets associated with resistance to DAH are highlighted blue. Subsets that have no apparent relationship with DAH are highlighted yellow. R3 monocytes are Nr4a1 dependent, but have somewhat different phenotypes in pristane- (A) vs. MO-treated (B) mice. In pristane-treated mice, they appear to be permissive for induction of DAH, whereas in MO-treated mice, they may inhibit the development of DAH.
Tables
Phenotypes of monocyte subsets and pristane- and mineral oil-treated mice.
| Ly6C | CD138 | CD11b | Nr4a1 | CCR2 | Cx3Cr1 | CD62L | CD11c | LFA1 | Treml4 | TLR7 | TNFa | CD43 | CD115 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R1 (P)* | ++ | (−) | + | (−) | ++ | + | ++ | (−) | + | (−) | + | ++ | ++ | + |
| R2A (P) | + | (−) | + | + | + | + | + | + | ++ | + | + | ++ | + | + |
| R2B (P) | (−) | (−) | + | + | (−) | + | + | + | ++ | + | + | + | + | + |
| R3A (P) | + | ++ | ++ | ++ | + | ++ | (−) | + | ++ | ++ | ++ | ++ | + | ++ |
| R3B (P) | (−) | ++ | + | + | (−) | ++ | (−) | ++ | ++ | ++ | ++ | + | ++ | ++ |
| R1 (MO)* | ++ | (−) | + | (−) | n.d. | n.d. | n.d. | (−) | n.d. | (−) | + | + | + | n.d. |
| R3A (MO) | + | (−) | ++ | ++ | n.d. | n.d. | n.d. | + | n.d. | ++ | + | ++ | + | n.d. |
| R3B (MO) | (−) | (−) | + | + | n.d. | n.d. | n.d. | ++ | n.d. | ++ | + | + | + | n.d. |
-
*P, pristane; MO, mineral oil; n.d., not determined.
Murine monoclonal antibodies.
| Specificity (clone) | Fluorochrome* | Source |
|---|---|---|
| CD138 (281-2) | PE; APC; APC-Cy7 | BioLegend |
| CD11b (M1/70) | BV421, Pacific Blue | BioLegend |
| Ly6C (HK1.4) | APC-Cy7; Alexa Fluor 488 | BioLegend |
| Ly6G (1A8) | APC-Cy7; PE | BioLegend |
| CD43 | FITC | BioLegend |
| TREML4 | PE | BioLegend |
| CD115 | APC-Cy7 | BioLegend |
| F4/80 | PE, BV421 | BioLegend |
| CD62L | PE | BD Pharmingen |
| CD64 | PE | BD Pharmingen |
| CCR2 | FITC | BioLegend |
| Tim4 | APC, PE | BioLegend |
| CD11c | PE | BD Pharmingen |
| CX3CR1 (SA011F11) | FITC | BioLegend |
| LFA-1 | PE | BioLegend |
| NR4A1 | PE | Miltenyi |
| TLR7 | PE | BioLegend |
| CD86 | APC-Cy7 | BioLegend |
| TNFα | APC, PE | BioLegend |
-
*PE, phycoerythrin; APC, allophycocyanin; FITC, fluorescein isothiocyanate; BV, brilliant violet; APC-Cy7, APC/cyanine 7.





