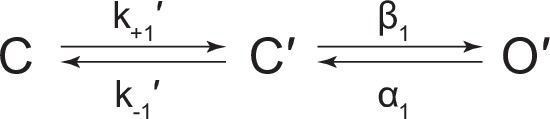Ancestral acetylcholine receptor β-subunit forms homopentamers that prime before opening spontaneously
Figures
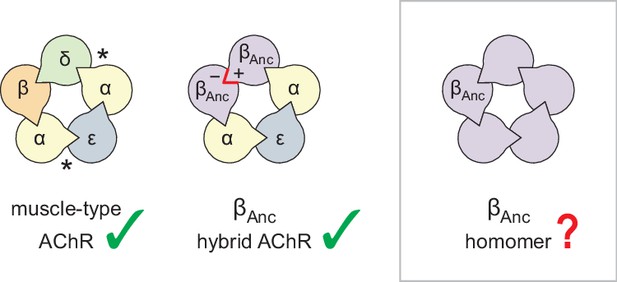
Subunit composition of heterologously expressed acetylcholine receptors (AChR).
Subunit stoichiometry and arrangement of the human adult muscle-type AChR (left), where the agonist-binding sites at the α–δ and α–ε subunit interfaces are indicated with asterisks (*). A reconstructed ancestral β-subunit (βAnc; purple) forms hybrid AChRs (middle) where βAnc substitutes for the human β-subunit (β; orange) and supplants the human δ-subunit (δ; green). The principal (+) and complementary (−) interfaces of βAnc must be compatible for two βAnc subunits to sit side-by-side (red highlight), which predicts that homomers formed from multiple βAnc subunits should be possible (right, boxed).
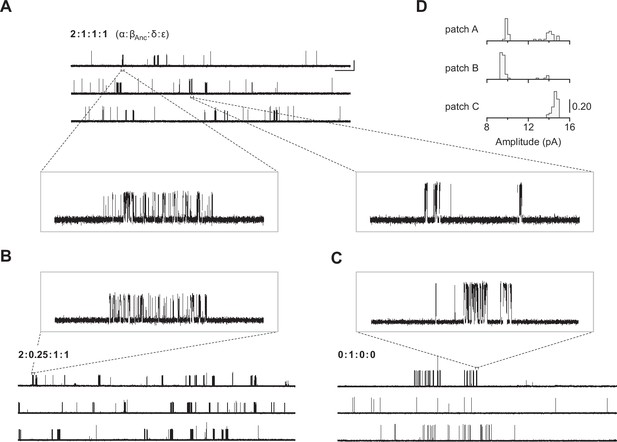
Single-channel recordings of βAnc-containing channels.
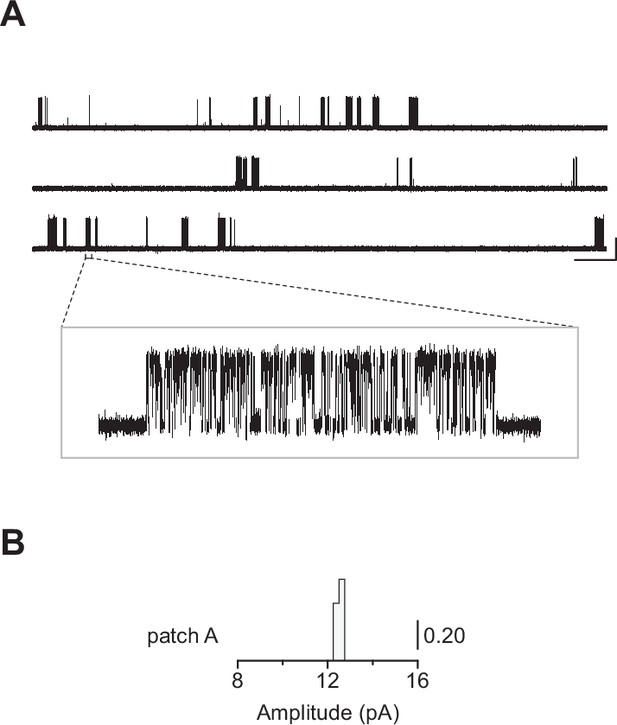
(A) Representative continuous recording from a cell-attached patch where cells were transfected with cDNAs encoding human muscle-type α-, δ-, and ε-subunits, and an additional cDNA encoding βAnc at a cDNA ratio of 2:1:1:1 (α:βAnc:δ:ε). (B) Same as in (A), but where cells were transfected with an altered 2:0.25:1:1 cDNA ratio, making the βAnc subunit limiting, or (C) where cells were transfected with only the cDNA encoding βAnc. In all cases openings are upward deflections, in the presence of 30 µM acetylcholine, and with an applied voltage of –120 mV. Continuous recordings are digitally filtered to 5 kHz, and the scale bar (2 s, 10 pA) in (A) applies to (B) and (C). Insets are digitally filtered to 10 kHz, with boxes representing scale bars (300 ms, 25 pA). (D) Event-based amplitude histograms for single-channel bursts from each of the patches shown in (A), (B), and (C). In each case, the height of the bins was normalised to the total number of bursts in each patch (A: 40; B: 50; C: 41), with the scale bar representing the indicated fraction (0.20) of the total bursts.
-
Figure 2—source data 1
Unrasterized version of Figure 2.
- https://cdn.elifesciences.org/articles/76504/elife-76504-fig2-data1-v2.eps
Single-channel recordings of the human adult muscle-type acetylcholine receptor exhibit homogeneous burst behaviour.
(A) Representative continuous recording from a cell-attached patch where cells were transfected with cDNAs encoding human muscle-type α-, β-, δ-, and ε-subunits at a cDNA ratio of 2:1:1:1 (α:β:δ:ε). Activity was recorded in the presence of 30 µM acetylcholine, with an applied voltage of –120 mV. Continuous trace (top) was digitally filtered to 5 kHz (scale bar = 2 s, 10 pA), while inset burst (boxed; bottom) was filtered to 10 kHz where box itself represents 300 ms and 25 pA. Openings are shown as upward deflections. (B) Event-based amplitude histogram for single-channel bursts for the patch shown in panel A. The height of the bins was normalised to the total number of bursts in the patch (29 total), with the scale bar representing 0.20 of the total number of bursts.
-
Figure 2—figure supplement 1—source data 1
Unrasterized version of Figure 2—figure supplement 1.
- https://cdn.elifesciences.org/articles/76504/elife-76504-fig2-figsupp1-data1-v2.eps
Spontaneous single-channel openings of βAnc homomers.
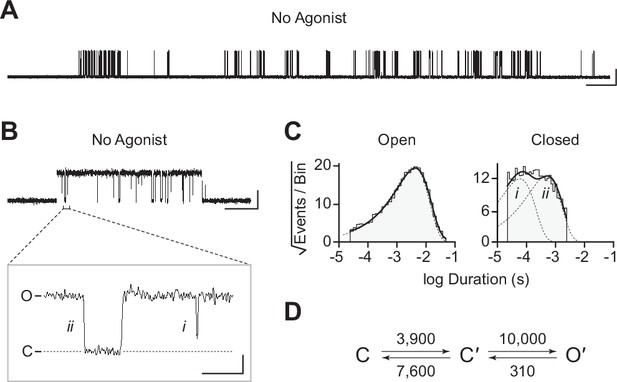
(A) Representative continuous recording of a cell-attached patch from cells transfected with a single cDNA encoding βAnc. Recording was made in the absence of acetylcholine and at an applied voltage of –120 mV. Data was digitally filtered to 5 kHz (scale bar = 2 s, 10 pA). (B) Single burst of openings from a homomeric βAnc channel, shown digitally filtered to 10 kHz (scale bar = 25 ms, 10 pA). Inset depicts (i) brief and (ii) long closings within bursts, where the former (i) are reminiscent of ‘nachschlag shuttings’ (scale bar = 1 ms, 5 pA). (C) Open and closed dwell duration histograms for the representative patch depicted in (B). Individual exponential components determined manually (dashed lines) and kinetic fits from MIL (solid lines) are overlaid. Global kinetic fitting was performed on three individual recordings, from two separate transfections. (D) The single-channel data fit a three-state scheme (Scheme 1), where C, C′, and O′ correspond to closed, closed-primed, and open-primed states. Rate constants with units s–1 are shown above and below corresponding arrow, with error estimates provided in Table 1.
-
Figure 3—source data 1
Source data for Figure 3.
Detected single-channel event durations of spontaneously opening βAnc homomers. Compressed file includes three TAC 4.3.3 event files (*.evt format) of the single-channel detections for the three recordings used in the presented kinetic analysis, as well as the associated R scripts (*.txt format) for defining and sorting bursts.
- https://cdn.elifesciences.org/articles/76504/elife-76504-fig3-data1-v2.zip
-
Figure 3—source data 2
Unrasterized version of Figure 3.
- https://cdn.elifesciences.org/articles/76504/elife-76504-fig3-data2-v2.eps
Kinetic fitting of alternate schemes describing spontaneous single-channel activity of βAnc homomers.
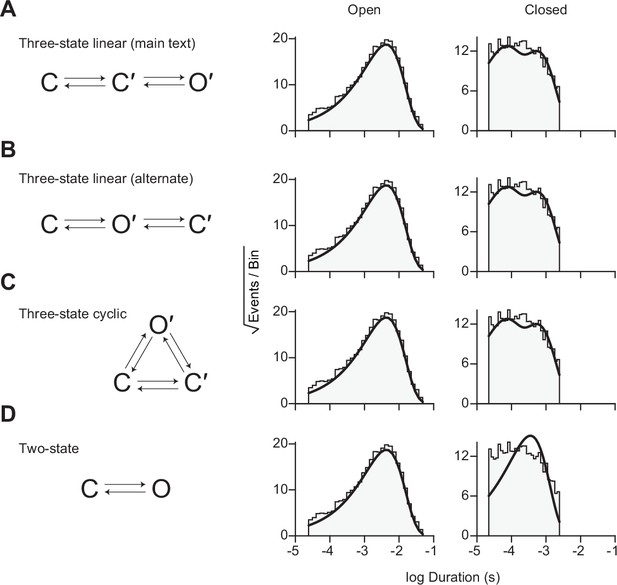
Each scheme is shown on the left with the same open and closed duration histograms shown on the right. In each case, the resulting fit of the same data using the various schemes is overlaid (solid black lines). (A) The three-state linear scheme described in the main text is reproduced here for comparison with (B) an alternate three-state linear scheme, as well as (C) the three-state cyclic scheme. (D) A two-state scheme with a single closed state and a single open state was also fit to validate the need for a second closed state.
Open-channel block of βAnc homomers by acetylcholine and 2-[(2,6-dimethylphenyl)amino]-N,N,N-trimethyl-2-oxoethaniminium chloride (QX-222).
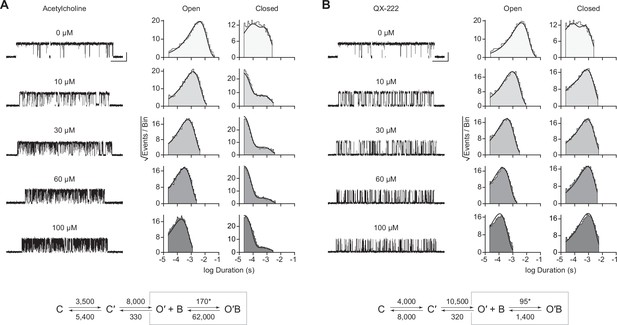
Representative single-channel activity of βAnc homomers in the presence of increasing concentrations of (A) acetylcholine and (B) QX-222. Openings are upward deflections. Recordings were obtained with an applied voltage of –120 mV. Data were filtered to 10 kHz (scale bars = 25 ms, 10 pA; applies to (A) and (B)). The sequence of dwells from each dataset, encompassing the full concentration range of the blocker, was globally fit to the same three-state scheme used for βAnc, where an additional fourth state corresponding to the open/blocked channel was added (Scheme 2). Global kinetic fits were performed on three individual recordings for each concentration of blocker, from at least two separate transfections, corresponding to 15 total patches for each global fit. Note that the recordings in the absence of blocker are the same for each dataset. Rate constants are overlaid on the scheme below each dataset, with error estimates presented in Table 1.
-
Figure 4—source data 1
Source data for Figure 4.
Detected single-channel event durations of spontaneously opening βAnc homomers in the absence (agonist-free) and presence of increasing concentrations of acetylcholine (ACh; 10 µM, 30 µM, 60 µM, and 100 µM) or 2-[(2,6-dimethylphenyl)amino]-N,N,N-trimethyl-2-oxoethaniminium chloride (QX-222; QX; 10 µM, 30 µM, 60 µM, and 100µM). Compressed file includes 27 TAC 4.3.3 event files (*.evt format) of the single-channel detections for the three recordings for each condition (fileA, fileB, and fileC in each case) in the presented kinetic analysis, as well as the associated R scripts (*.txt format) for defining and sorting bursts.
- https://cdn.elifesciences.org/articles/76504/elife-76504-fig4-data1-v2.zip
-
Figure 4—source data 2
Unrasterized version of Figure 4.
- https://cdn.elifesciences.org/articles/76504/elife-76504-fig4-data2-v2.eps
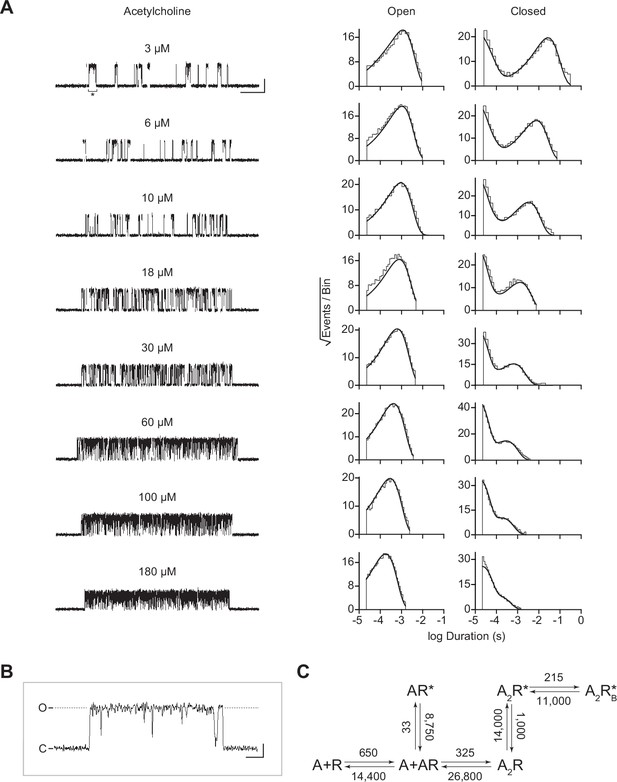
Single-channel kinetics of the human adult muscle-type acetylcholine receptor (AChR).
(A) Representative bursts from single-channel recordings of the wild-type AChR over a full acetylcholine concentration range (left). Recordings were acquired in the cell-attached patch configuration, with an applied voltage of –120 mV. Openings are upward deflections, bursts are filtered to 10 kHz, and the scale bar represents 25 ms and 10 pA. Corresponding open and closed duration histograms are presented for each concentration (right), with overlaid fits (solid line) from global fitting of the entire dataset. (B) Representative openings interrupted by ‘nachschlag shuttings’ in the presence of 3 µM acetylcholine (see asterisk in ‘A’), where ‘C’ represents the closed, baseline current, and ‘O’ represents the current through a single open conducting AChR. Scale bar in (B) represents 1 ms and 5 pA. (C) Modified del Castillo and Katz kinetic scheme for the muscle-type AChR, containing two agonist binding steps (Colquhoun and Sakmann, 1985), and a single blocked state flanking the doubly-liganded open state (Scheme 3). Estimates of the rate constants from global fitting of the entire dataset are shown, with error estimates presented in Table 2. Global kinetic fits were performed on three individual recordings for each concentration of acetylcholine, from at least two separate transfections, corresponding to 24 total patches for the entire global fit.
-
Figure 4—figure supplement 1—source data 1
Source data for Figure 4—figure supplement 1.
Detected single-channel event durations for the human adult muscle-type acetylcholine receptor in the presence of increasing concentrations of acetylcholine (ACh; 3 µM, 6 µM, 10 µM, 18 µM, 30 µM, 60 µM, 100 µM, and 180 µM). Compressed file includes 24 TAC 4.3.3 event files (*.evt format) of the single-channel detections for the three recordings for each acetylcholine concentration (fileA, fileB, and fileC in each case) in the presented kinetic analysis, as well as the associated R scripts (*.txt format) for defining and sorting bursts.
- https://cdn.elifesciences.org/articles/76504/elife-76504-fig4-figsupp1-data1-v2.zip
-
Figure 4—figure supplement 1—source data 2
Unrasterized version of Figure 4—figure supplement 1.
- https://cdn.elifesciences.org/articles/76504/elife-76504-fig4-figsupp1-data2-v2.eps
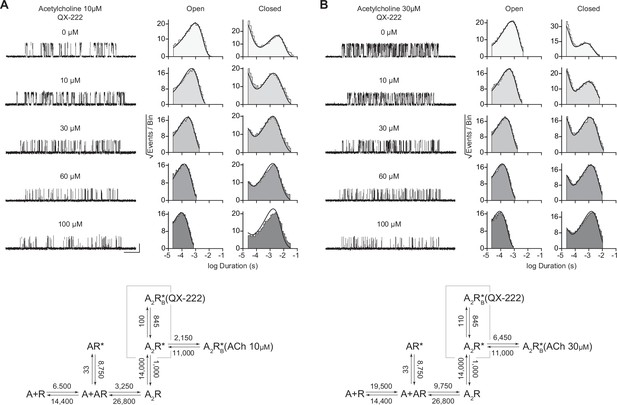
Kinetics of 2-[(2,6-dimethylphenyl)amino]-N,N,N-trimethyl-2-oxoethaniminium chloride (QX-222) block of the human adult muscle-type acetylcholine receptor.
Single-channel recordings were obtained with increasing concentrations of QX-222, while the concentration of agonist (acetylcholine [ACh]) was kept constant at either (A) 10 µM or (B) 30 µM. Single-channel recordings were obtained in the cell-attached configuration, with an applied voltage of –120 mV, where openings are upward deflections, and traces filtered to 10 kHz. Corresponding open and closed duration histograms are presented for all QX-222 concentrations with overlaid fits (solid line) from global fitting of the entire dataset. Kinetic scheme is the same presented in Figure 4—figure supplement 1, but with an additional open/blocked state (corresponding to QX-222 block) flanking the doubly-liganded open state (Scheme 4). All rates, except those describing QX-222 block, were fixed to that at the indicated acetylcholine concentration as estimated from original QX-222-free fits in Figure 4—figure supplement 1. For both concentrations of acetylcholine, estimates of the rate constants from global fitting of each dataset are shown, with error estimates presented in Table 2. Global kinetic fits were performed on three individual recordings for each concentration of QX-222 at both concentrations of ACh, and from at least two separate transfections, corresponding to 15 total patches for each global fit.
-
Figure 4—figure supplement 2—source data 1
Source data for Figure 4—figure supplement 2.
Detected single-channel event durations for 2-[(2,6-dimethylphenyl)amino]-N,N,N-trimethyl-2-oxoethaniminium chloride (QX-222) block of the human adult muscle-type acetylcholine receptor. Recordings were in the presence of either 10 µM or 30 µM acetylcholine, and increasing concentrations of QX-222 (QX222; 10 µM, 30 µM, 60 µM, and 100 µM). Compressed file includes 30 TAC 4.3.3 event files (*.evt format) of the single-channel detections for three recordings for each condition (fileA, fileB, and fileC in each case) included in the kinetic analysis, as well as the associated R scripts (*.txt format) for defining and sorting bursts.
- https://cdn.elifesciences.org/articles/76504/elife-76504-fig4-figsupp2-data1-v2.zip
-
Figure 4—figure supplement 2—source data 2
Unrasterized version of Figure 4—figure supplement 2.
- https://cdn.elifesciences.org/articles/76504/elife-76504-fig4-figsupp2-data2-v2.eps
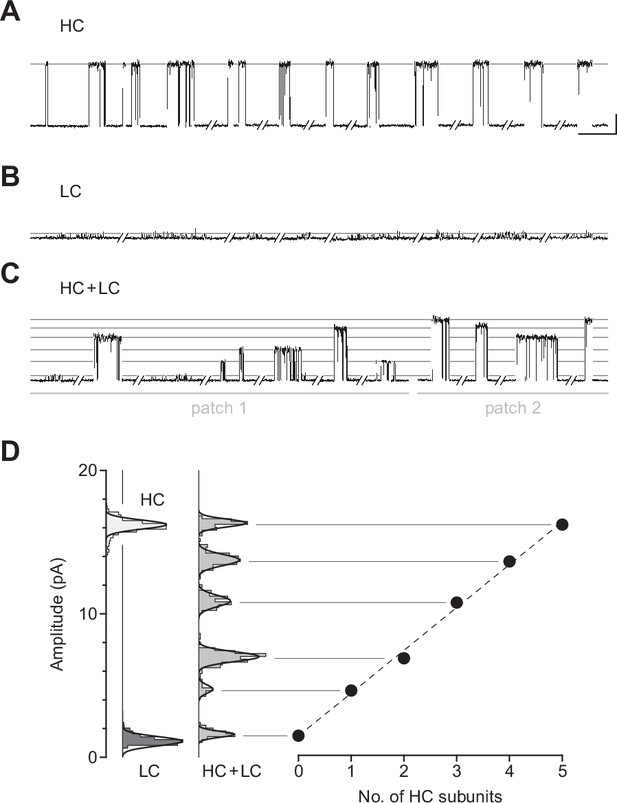
Electrical fingerprinting to determine subunit stoichiometry of βAnc homomers.
Representative single-channel activity from cells transfected with (A) cDNA encoding the wild-type high-conductance (HC) βAnc subunit, or (B) a mutant low-conductance (LC) βAnc variant harbouring substitutions that reduce single-channel amplitude. (C) Cotransfection of cDNAs encoding HC and LC βAnc variants led to patches (two shown) with heterogeneous amplitudes. (D) The amplitudes segregate into six well-defined amplitude classes (total of 495 bursts combined from the two patches in (C)), where the highest and lowest amplitude classes match that of the all-HC (1569 bursts) and all-LC classes (883 bursts), respectively. Plot of the mean amplitude of each class as a function of the presumed number of incorporated HC subunits (error bars = standard deviations of the mean but are smaller than the points themselves). Recordings were obtained with an applied voltage of –120 mV, and traces were digitally filtered to 1 kHz to facilitate amplitude detection (scale bar = 50 ms, 5 pA; applies to (A), (B), and (C)).
-
Figure 5—source data 1
Unrasterized version of Figure 5.
- https://cdn.elifesciences.org/articles/76504/elife-76504-fig5-data1-v2.eps
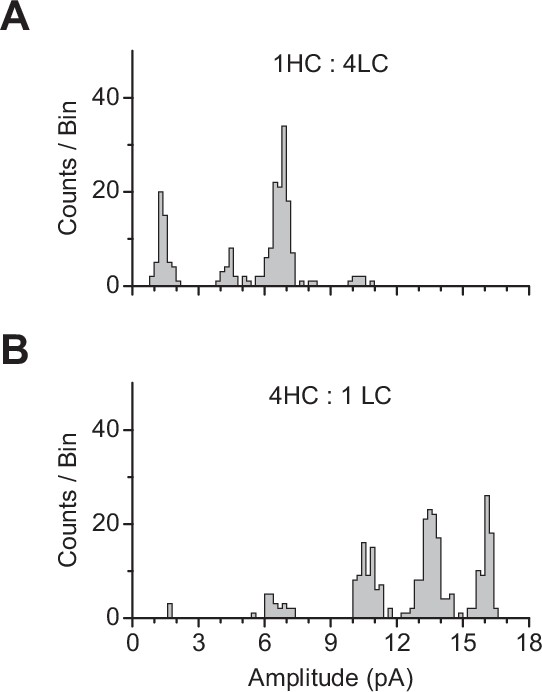
Event-based amplitude histograms derived from a representative single patch where cells were cotransfected with cDNAs encoding for high-conductance (HC) and low-conductance (LC) variants of βAnc at a ratio (wt:wt) of (A) 1HC:4LC (208 total bursts) or (B) 4HC:1LC (287 total bursts).
In each case, cotransfection of HC and LC βAnc subunits led to a distribution of amplitudes that segregated into distinct amplitude classes, where the proportion of events in each class was biased by the relative proportion of each type of cDNA transfected. These two patches with overlapping amplitude classes were combined to produce the plot in Figure 5D.
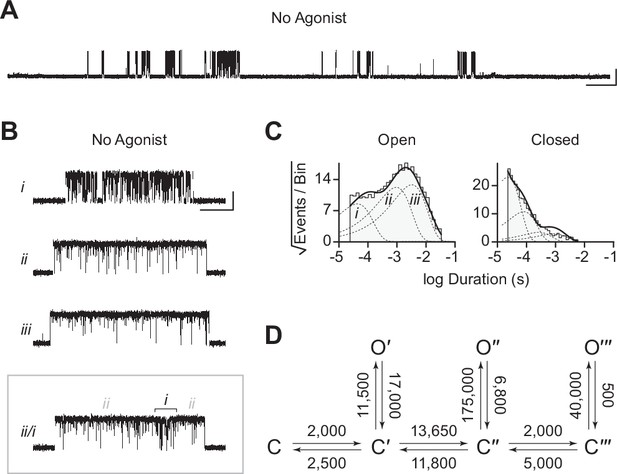
Spontaneous single-channel openings of homomers formed from an alternate ancestral β-subunit (βAncS).
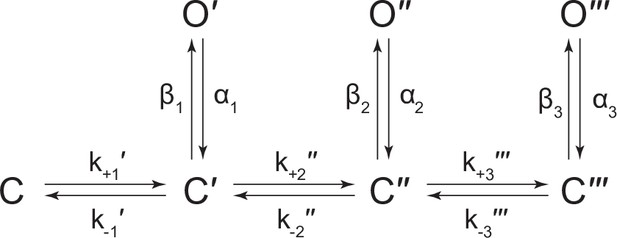
(A) Representative continuous recording of a cell-attached patch from cells transfected with a single cDNA encoding βAncS. Recording was made in the absence of acetylcholine, at an applied voltage of –120 mV, where spontaneous openings are upward deflections. Data was digitally filtered to 5 kHz (scale bar = 2 s, 10 pA). (B) Bursts from homomeric βAncS channels, each exhibiting one of three different types (i, ii, iii) of openings (scale bar = 25 ms, 10 pA). The boxed burst at the bottom is an example of a single burst that contains more than one type of opening (ii/i). (C) Open and closed dwell duration histograms for the representative patch depicted in (B). Individual exponential components determined manually (dashed lines) and kinetic fits from MIL (solid lines) are overlaid. Global kinetic fitting was performed on three individual recordings, from two separate transfections. The exponential components (i, ii, iii) in the open duration histogram correspond to the different types of openings observed within the bursts in panel (B). (D) The three-state scheme in Figure 3 (Scheme 1) was expanded to include additional priming steps (‘singly’, ‘doubly’, and ‘triply’ primed), each with their own connected open state (Scheme 5). Rate constants are shown, with error estimates provided in Table 3.
-
Figure 6—source data 1
Source data for Figure 6.
Detected single-channel event durations of spontaneously opening βAncS homomers. Compressed file includes three TAC 4.3.3 event files (*.evt format) of the single-channel detections for the three recordings used in the presented kinetic analysis, as well as the associated R scripts (*.txt format) for defining and sorting bursts.
- https://cdn.elifesciences.org/articles/76504/elife-76504-fig6-data1-v2.zip
-
Figure 6—source data 2
Unrasterized version of Figure 6.
- https://cdn.elifesciences.org/articles/76504/elife-76504-fig6-data2-v2.eps
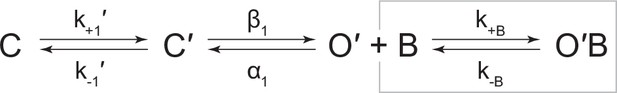
Kinetic scheme describing single-channel activity of βAnc homomers in the presence of acetylcholine or 2-[(2,6-dimethylphenyl)amino]-N,N,N-trimethyl-2-oxoethaniminium chloride (QX-222).
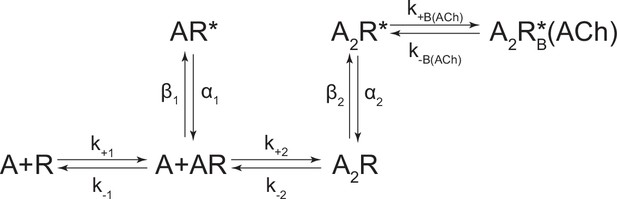
Kinetic scheme describing single-channel activity of the human adult muscle-type acetylcholine (ACh) receptor.
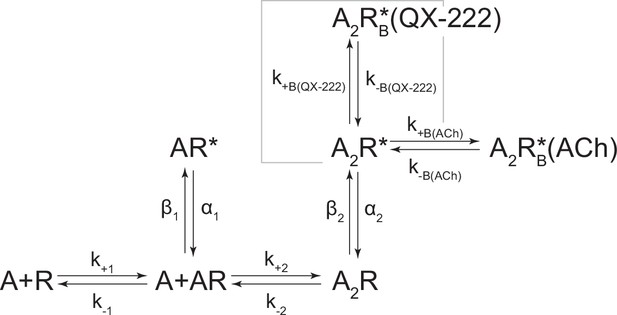
Kinetic scheme describing single-channel activity of the human adult muscle-type acetylcholine (ACh) receptor in the presence of 2-[(2,6-dimethylphenyl)amino]-N,N,N-trimethyl-2-oxoethaniminium chloride (QX-222).

Multiple sequence alignment of βAnc and βAncS with the human β-subunit (βHs).
Positions aligning with those previously reported as being important for spontaneous openings (blue) and homomeric assembly (orange) in other pentameric ligand-gated ion channels (pLGICs) are highlighted. Sites implicated in both spontaneous opening and homomeric assembly are highlighted in yellow. In each case, if the residue implicated is conserved across all three subunits the highlight is faded. Corresponding references for each position are indicated (a–n), where a: Torres and Weiss, 2002; b: Beckstead, 2002; c: Miller et al., 2008; d: Nayak et al., 2012; e:Purohit and Auerbach, 2009; f: Taylor et al., 1999; g: Sexton et al., 2021; h: Alves et al., 2011; i: Hannan and Smart, 2018; j: Pan et al., 1997; k: Mukhtasimova et al., 2009; l: Ohno et al., 1995; m: Grosman and Auerbach, 2000; n: Engel et al., 1996.
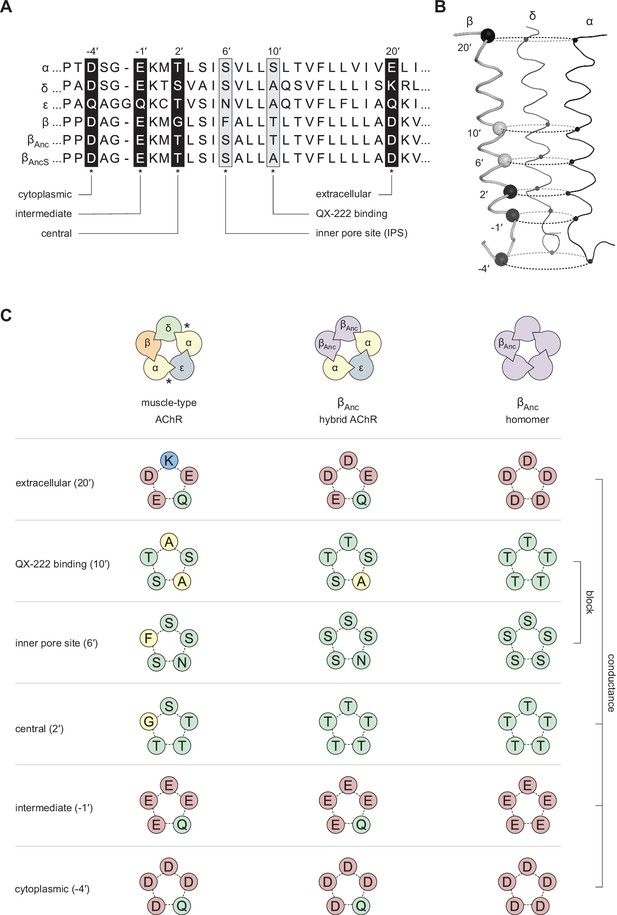
Primary and tertiary structure of the M2 segments from the adult human muscle-type AChR subunits, as well the two reconstructed ancestral β-subunits (βAnc and βAncS).
(A) Multiple sequence alignment of residues comprising the pore-lining M2 helix, highlighting positions discussed in the main text and known to influence single-channel conductance (black boxes; –4′, –1′, 2′, and 20′), or open-channel block (grey boxes; 6′ and 10′). (B) Tertiary structure of the AChR pore. M2 segments corresponding to the residues shown in panel “A” are mapped onto the Cα backbone of the Torpedo AChR (PDB: 6UWZ). The two subunits in the front (α and ε) have been hidden for illustrative purposes, and positions of highlighted residues in “A” are mapped onto the β-subunit with their Cα shown as black and grey spheres. (C) Analogous residues from each subunit form concentric rings, where the chemical properties of amino acids in each ring influence single-channel conductance or open-channel block. Residues contributed to each ring by individual subunits in the heteropentameric human adult muscle-type AChR (left) and βAnc-containing hybrid AChR (middle), as well as in βAnc homompentamers (right) are shown. Acidic, basic, polar, and nonpolar residues are colored red, blue, green, and yellow, respectively.
Tables
Single-channel kinetics of spontaneously opening βAnc homomers.
| Homomer | k+1′ | k-1′ | K′ | β1 | α1 | Θ1 | k+B | k-B | KB(μM) |
|---|---|---|---|---|---|---|---|---|---|
| No agonist (3 patches) | 3900 (110) | 7600 (450) | 0.51 | 10,000 (300) | 310 (3) | 32.26 | N/A | N/A | N/A |
| Acetylcholine (15 patches) | 3500 (85) | 5400 (265) | 0.65 | 8000 (200) | 330 (3) | 24.24 | 170* (1.8) | 62,000 (450) | 364.71 |
| QX-222 (15 patches) | 4000 (110) | 8000 (440) | 0.50 | 10,500 (300) | 320 (3) | 32.81 | 95* (0.5) | 1400 (7) | 14.74 |
| Acetylcholine (Constrained) (15 patches) | 3900 | 7600 | 0.51 | 10,000 | 310 | 32.26 | 170* (1.5) | 60,800 (410) | 357.65 |
| QX-222 (Constrained) (15 patches) | 3900 | 7600 | 0.51 | 10,000 | 310 | 32.26 | 95* (0.5) | 1400 (6.5) | 14.74 |
-
Note: Rate constants were estimated from fitting Scheme 1 or Scheme 2 presented in Figures 3 and 4, respectively. Data were globally fit (number of patches indicated in each case) over a range of acetylcholine/2-[(2,6-dimethylphenyl)amino]-N,N,N-trimethyl-2-oxoethaniminium chloride (QX-222) concentrations, with rate constants and associated errors (parentheses) estimated by MIL (see Materials and methods). Priming (K′), gating (θ1), and blocking (KB) equilibrium constants represent k+1′/k-1′, β1/α1, and k-B/k+B, respectively. Association rate constants (*); (k+B) are presented in units of μM–1·s–1, while remaining rate constants are presented in units of s–1. Constrained rates (presented in italicized) were held constant at the values determined from the agonist-free dataset, while the blocking rate constants were estimated.
Kinetics of acetylcholine (ACh) activation and 2-[(2,6-dimethylphenyl)amino]-N,N,N-trimethyl-2-oxoethaniminium chloride (QX-222) block of human adult muscle-type acetylcholine receptors (AChRs).
| WT | k+1 | k-1 | K1(μM) | k+2 | k-2 | K2(μM) | β1 | α1 | Θ1 | β2 | α2 | Θ2 | k+B (ACh) | k-B(ACh) | KB(ACh)(μM) | k+B(QX-222) | k-B(QX-222) | KB(QX-222)(μM) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACh (24 pat.) | 650* (35) | 14,400 (1000) | 22.12 | 325* (20) | 26,500 (400) | 81.54 | 33 (2.5) | 8750 (650) | 3.77 E-03 | 14,000 (450) | 1000 (10) | 14 | 215* (3) | 110,000 (800) | 511.63 | N/A | N/A | N/A |
| QX-222 (10μM ACh) (15 pat.) | 6500 | 14,400 | 2.21 | 3250 | 26,500 | 8.15 | 33 | 8750 | 3.77 E-03 | 14,000 | 1000 | 14 | 2150 | 110,000 | 51.16 | 100* (0.5) | 845 (5.5) | 8.45 |
| QX-222 (30μM ACh) (15 pat.) | 19,500 | 14,400 | 0.74 | 9750 | 26,500 | 2.72 | 33 | 8750 | 3.77 E-03 | 14,000 | 1000 | 14 | 6450 | 110,000 | 17.05 | 110* (0.5) | 845 (4.5) | 7.68 |
-
Note: Rate constants were estimated from fitting Scheme 3 or Scheme 4 presented in Figure 4—figure supplements 1 and 2, respectively. Where ‘A’ represents agonist, and ‘R’, ‘R*’, and ‘R*B’ represent the closed, open, and open-blocked states of the human adult muscle-type acetylcholine receptor (AChR). Data were globally fit (number of patches indicated in each case) over a range of 2-[(2,6-dimethylphenyl)amino]-N,N,N-trimethyl-2-oxoethaniminium chloride (QX-222) concentrations with fixed concentration of acetylcholine (ACh: 10 μM or 30 μM), with rate constants and associated errors (parentheses) estimated with MIL (see Materials and methods). Apparent binding (Kn), apparent gating (θn), and blocking (KB) equilibrium constants represent k-n/k+n, βn/αn, and k-B/k+B, respectively. Association rate constants (k+1, k+2, and k+B) are presented in units of μM–1·s–1, while remaining rate constants are presented in units of s–1. Constrained rates (italicized) were held constant to the rates estimated from fitting of wild-type (WT) in the absence of QX-222, allowing the QX-222 blocking rates to be estimated independently.
Single-channel kinetics of spontaneously opening βAncS homomers.
| k+1′ | k-1′ | β1 | α1 | Θ1 | k+2′′ | k-2′′ | β2 | α2 | Θ2 | k+3′′′ | k-3′′′ | β3 | α3 | Θ3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| βAncS (3 pat.) | 2000 (80) | 2500 (140) | 11,500 (450) | 17,000 (800) | 0.676 | 13,650 (400) | 14,800 (2000) | 175,000 (17,000) | 6800 (1400) | 25.74 | 2000 (300) | 5000 (450) | 40,000 (1550) | 500 (15) | 80 |
-
Note: Rate constants were estimated from fitting Scheme 5 in Figure 6 (and below). Data were globally fit (three individual patches from two separate transfections) with rate constants and associated errors (parentheses) estimated within MIL (see Materials and methods). Gating equilibrium (θn) constants represent βn/αn. Rate constants are presented as s–1.
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Cell line (Homo sapiens) | BOSC 23 | ATCC | CRL11270 (discontinued) | Modified Homo sapiens embryonic kidney cells |
| Recombinant DNA reagent | pRBG4 – AChR α1 | Provided by Steven M. Sine (Mayo Clinic) | Homo sapiens CHRNA1 (Accession: NM_000079.4) | |
| Recombinant DNA reagent | pRBG4 – AChR β1 | Provided by Steven M. Sine (Mayo Clinic) | Homo sapiens CHRNB1 (Accession: NM_000747.3) | |
| Recombinant DNA reagent | pRBG4 – AChR δ | Provided by Steven M. Sine (Mayo Clinic) | Homo sapiens CHRND (Accession: NM_000751.3) | |
| Recombinant DNA reagent | pRBG4 – AChR ε | Provided by Steven M. Sine (Mayo Clinic) | Homo sapiens CHRNE (Accession: NM_000080.4) | |
| Recombinant DNA reagent | pRBG4 – AChR βAnc | Custom gene synthesis | Construct originating from: PMID:28689969 | |
| Recombinant DNA reagent | pRBG4 – AChR βAncS | Custom gene synthesis | Construct originating from: PMID:33579823 | |
| Recombinant DNA reagent | pGreenLantern | Provided by Steven M. Sine (Mayo Clinic) | ||
| Commercial assay or kit | Q5 DNA polymerase | New England Biolabs, inc | M0491 | PCR |
| Sequence-based reagent | SDM_AChRBAncLC_F | This paper | Mutagenesis primer | AGAACGCTGAAGAGAGACTGGCAGTACGTGGCCAT |
| Sequence-based reagent | SDM_AChRBAncLC_R | This paper | Mutagenesis primer | ATAGTCCTCTCTTTTCTGCAGCTGCTCAGCGAT |
| Chemical compound, drug | Acetylcholine Chloride | Sigma | A9101-10VL | Purity: 99% |
| Chemical compound, drug | QX-222 | Tocris | 1043/10 | Purity:>98% |
| Software, algorithm | TAC 4.3.3 | Bruxton (https://www.bruxton.com/legacy.html) | Single-channel recording, detection, and analysis | |
| Software, algorithm | R | https://www.r-project.org/ | Open-source statistical computing software | |
| Software, algorithm | scbursts | https://cran.r-project.org/web/packages/scbursts/index.html | R Package – single-channel burst analysis | |
| Software, algorithm | extreme-values | https://cran.r-project.org/web/packages/extremevalues/index.html | R Package – outlier detection | |
| Software, algorithm | MASS | https://cran.r-project.org/web/packages/MASS/index.html | R Package – function and statistical analysis | |
| Software, algorithm | xlsx | https://cran.r-project.org/web/packages/xlsx/index.html | R Package – read and write excel files |
Additional files
-
Supplementary file 1
Multiple sequence alignment in FASTA format used to identify residues that differ between βAnc, βAncS, and the human β-subunit, and that also align with residues shown to be important for homomeric pLGIC assembly (Alves et al., 2011; Hannan and Smart, 2018; Sexton et al., 2021; Taylor et al., 1999) and spontaneous activity (Beckstead, 2002; Engel et al., 1996; Grosman and Auerbach, 2000; Miller et al., 2008; Mukhtasimova et al., 2009; Nayak et al., 2012; Ohno et al., 1995; Pan et al., 1997; Purohit and Auerbach, 2009; Torres and Weiss, 2002).
- https://cdn.elifesciences.org/articles/76504/elife-76504-supp1-v2.txt
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/76504/elife-76504-transrepform1-v2.pdf