c-Myc plays a key role in IFN-γ-induced persistence of Chlamydia trachomatis
Figures

Interferon-gamma (IFN-γ) induces depletion of c-Myc and impairs chlamydial growth.
(A) HeLa 229 cells with an anhydrotetracycline (AHT)-inducible expression of shc-Myc were infected with Chlamydia trachomatis (Ctr) at multiplicity of infection (MOI) 1. The infected cells were either left untreated or treated with 100 ng/mL AHT to deplete c-Myc 8 hr before infection. After 24 hr of infection, AHT was removed to release c-Myc expression, and the restoration of inclusion formation was tested. Cells were either fixed with 4% PFA after 36 hpi and immunostained for Ctr (cHSP60: green) and DNA (DAPI: blue) or lysed and analysed by (B) Western blot in order to analyse the rescue. Additionally, infected cells were lysed to infect freshly plated HeLa 229 shc-Myc cells at 24 hpi and analysed via Western blot to investigate the formation of infectious progenies. The panel shows the image of one representative blot (n=3). cHSP60 indicates Chlamydia infection and β-Actin staining serves as the loading control (n=3). (C) Inclusions and cells were counted and the results are shown as bar diagram. One-way ANOVA was used for analysis. *** indicates p-value <0.001, **** indicates a p-value <0.0001. (D) HeLa 229 cells were either left untreated or were pre-treated for 2 hr with 10 ng/mL IFN-γ or 1 unit of penicillin, infected with Ctr at MOI 1 for different time intervals and lysed for Western blot analysis. Bacterial load (cHSP60) and c-Myc levels were determined, and β-Actin served as loading control (n=3). (E) HeLa 229 cells were either left untreated or were pre-treated for 2 hr with 10 ng/mL of IFN-γ and infected with Chlamydia (Ctr) at MOI 1 and lysed at 30 hr to perform Western blot analysis. Phosphorylated form of c-Myc at serine 62 (pc-Myc [Ser62]) and threonine 58 (pc-Myc [Thr58]), c-Myc, and Chlamydia (cHSP60) were detected and quantified (Fc) (n=3). (F) HeLa 229 cells were either left untreated or treated with 10 ng/mL of IFN-γ and infected with Ctr. The cells were lysed and relative mRNA levels of c-Myc were determined by qPCR. GAPDH was used for normalization (n=3). One-way ANOVA was used for analysis. *** indicates p-value <0.001, **** indicates a p-value <0.0001. (G) Cartoon depicting IFN-γ signalling. IFN-γ binds to the IFN-γ receptor which results in the phosphorylation of STAT1. pSTAT1 binds to the IFN-γ-activated sequence (GAS) sequence and thus blocks c-Myc transcription. IFN-γ can also induce indoleamine-2,3-dioxygenase (IDO) and thereby the degradation of L-tryptophan. (H) HeLa 229 cells were either left untreated or were pre-treated for 2 hr with 10 ng/mL of IFN-γ and infected with Chlamydia (Ctr) at MOI 1 and lysed at 30 hpi to study STAT1 signalling after Ctr infection (n=3). (I) HeLa 229 cells were transfected with siRNA against STAT1 (+) or control (-) for 48 hr and then infected with Ctr for 24 hr. The cells were lysed and analysed by Western blot to investigate the STAT1 signalling and infectivity of Ctr (n=3). (J) HeLa 229 cells were either left untreated or were pre-treated for 2 hr with 10 ng/mL of IFN-α and infected with Ctr at MOI 1 and lysed at 36 hpi and analysed via Western blot. For all Western blots shown in A-J, Chlamydia load (cHSP60) and the respective host cell protein levels were quantified by normalization to β-Actin and indicated as fold change (Fc). Image of Western blots was taken from one of a total of at least three blots of biological replicates.
-
Figure 1—source data 1
Complete and cutted membranes of all Western blots from Figure 1.
- https://cdn.elifesciences.org/articles/76721/elife-76721-fig1-data1-v1.pdf

Interferon-gamma (IFN-γ) induces c-Myc depletion and impairs chlamydial growth.
(A) HeLa 229 NTC SCC5 cells with an anhydrotetracycline (AHT)-inducible expression of a control short hairpin RNA (shRNA) were infected with Chlamydia trachomatis (Ctr) at a multiplicity of infection (MOI) of 1. The infected cells were either left untreated or treated with 100 ng/mL AHT 8 hr before infection to show that AHT has no effect on Ctr. After 24 hr of infection, AHT was removed to have the same conditions as in the shc-Myc cell line (Figure 1A). Cells were fixed with 4% PFA after 36 hpi and immunostained for Ctr (cHSP60: green) and DNA (DAPI: blue). The panel shows representative images (n=3). (B) Additionally, infected cells were lysed to infect freshly plated HeLa 229 NTC SCC5 cells to investigate the formation of infectious progenies. Inclusions and cells were counted, and the results are shown as bar diagram. One-way ANOVA was used for analysis (ns, not significant). (C) An infectivity assay was performed in HeLa 229 cells to test for infectious progenies after addition of IFN-γ (10 ng/mL) or penicillin (1 unit). Lysates were subjected to Western blot analysis and bacterial load (cHSP60) was quantified by normalizing to β-Actin levels. (D) The number of inclusions and the number of the cells were counted to plot the graph. One-way ANOVA was used for analysis. **** indicates a p-value <0.0001. (E/F) HeLa 229 cells (E) or human Fimb cells (F) were treated with different doses of IFN-γ (10–50 ng/mL) and either left uninfected or infected with Ctr for 30/24 hr. The cells were lysed and analysed via Western blotting (n=3). (G) HeLa 229 cells were treated with 10 or 50 ng/mL of IFN-γ and infected with Ctr D serovar. After 48 hr the cells were lysed with glass beads and the supernatant was used to infect freshly plated HeLa 229 cells. After 30 hr the cells were lysed and analysed via Western blotting. Bacterial load was determined by quantifying cHSP60 levels and β-Actin serves as loading control. (H) The number of inclusions and the number of the cells were counted to plot the graph. One-way ANOVA was used for analysis. *** indicates a p-value <0.001. (I) Infectivity assay of the experiment shown in Figure 1H. The cells were lysed at 48 hpi and dilutions of the resulting Chlamydia-containing supernatants were added onto fresh HeLa 229 cells, which were then lysed at 30 hpi for Western blot analysis (n=3). (J) The number of inclusions and the number of the cells were counted to plot the graph. Paired t-test was used for analysis. * indicates a p-value <0.05. (K) HeLa 229 cells were either left untreated or treated with 10 ng/mL of IFN-γ and infected with Ctr for 30 hr. 12 hpi media was changed for reactivation. The cells were lysed and relative mRNA levels of TrpB were determined by qPCR. 16SrRNA was used for normalization (n=3). (L) HeLa 229 cells with an AHT-inducible expression of shc-Myc were either left untreated or induced with 100 ng/mL AHT and infected with Ctr MOI 1 for 30 hr. 12 hpi media was changed for reactivation. The cells were lysed and relative mRNA levels of TrpB were determined by qPCR. 16SrRNA was used for normalization (n=3). Western blots shown in A-L were quantified by normalizing the Chlamydia load (cHSP60) and the respective host cell protein levels to β-Actin and the result was indicated as fold change (Fc). Image of Western blots was taken from one of a total of at least three blots of biological replicates (n=3).
-
Figure 1—figure supplement 1—source data 1
Complete and cutted membranes of all Western blots from Figure 1—figure supplement 1.
- https://cdn.elifesciences.org/articles/76721/elife-76721-fig1-figsupp1-data1-v1.pdf

Interferon-gamma (IFN-γ) induces depletion of c-Myc and impairs chlamydial growth in human fallopian tube organoids.
(A) Layout of the infectivity assay performed in human fallopian tube organoids. Organoids (see Materials and methods) were infected with Chlamydia trachomatis (Ctr) and treated with or without IFN-γ for 6 days and lysed using glass beads. Dilutions of the supernatants were used to infect freshly plated HeLa 229 cells. (B) Human fallopian tube organoids were infected with Ctr and were either left untreated or treated with IFN-γ for 6 days. The organoids were fixed with 4% PFA and immunostained for DNA (DAPI: blue), Ctr (cHSP60: green), Cytokeratin (magenta), and F-actin (Phalloidin, cyan). The panel shows representative images from organoids derived from five patients. (C) The infected organoids from (B) were lysed using glass beads, and dilutions of the supernatant were used to infect freshly plated HeLa 229 cells. The number of inclusions as shown in (F) were counted from five different patients and mean ± SD was depicted in the graph (n=5). * indicates p-value <0.05. (D) Human fallopian tube organoids were infected with Ctr and treated with or without IFN-γ for 6 days or IFN-γ was removed after 2 days for a 4-day recovery. Infected organoids were lysed using glass beads, and dilutions of the supernatants were used to infect freshly plated HeLa 229 cells. The number of inclusions and the number of the cells were counted to plot the graph. Paired t-test was used for analysis. * indicates a p-value <0.05. (E) Human fallopian tube organoids were infected with Ctr and treated with IFN-γ or left untreated for 6 days. The organoids were lysed in ×2 Laemmli buffer and analysed by Western blotting. Chlamydia load (cHSP60) and STAT1 protein levels were quantified by normalization to β-Actin and indicated as fold change (Fc). Shown are representative Western blots of at least three independent experiments (n=3).
-
Figure 2—source data 1
Complete and cutted membranes of all Western blots from Figure 2.
- https://cdn.elifesciences.org/articles/76721/elife-76721-fig2-data1-v1.pdf
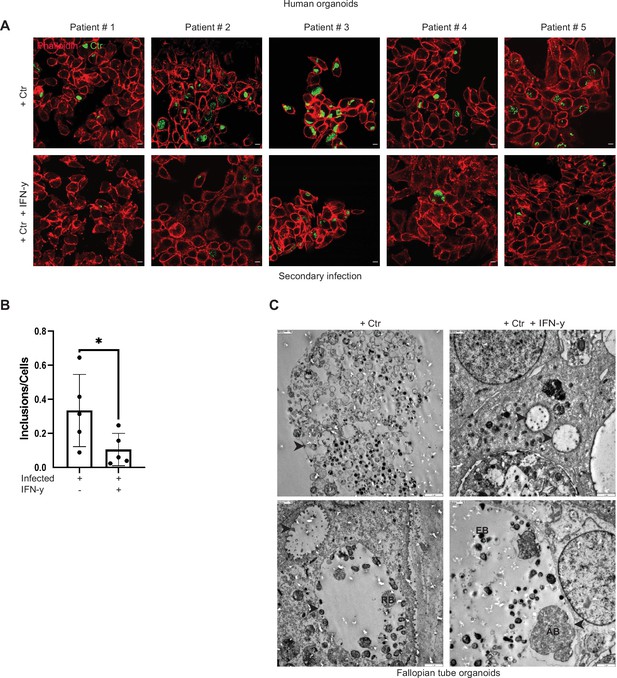
Interferon-gamma (IFN-γ)-induced persistence of Chlamydia trachomatis (Ctr) in human organoids.
(A) Human fallopian tube organoids were infected with Ctr and treated with or without IFN-γ for 6 days. The infected organoids were lysed using glass beads, and dilutions of the supernatant were used to infect freshly plated HeLa 229 cells for an infectivity assay. Cells were fixed with 4% PFA after 30 hpi and immunostained for Ctr (cHSP60: green) and actin (Phalloidin: red). The data from five different patients are presented here. (B) The number of inclusions and the number of the cells were counted to plot the graph. Paired t-test was used for analysis. * indicates a p-value <0.05. (C) Infected and treated with or without IFN-γ human fallopian tube organoids were pelleted after 6 days and analysed by electron microscopy. Elementary bodies (EB), reticulate bodies (RB), and aberrant bodies (AB) are indicated. Arrowheads point to inclusions and to one AB in the IFN-γ-treated sample. Scale bar 2 µm.

Expression of c-Myc rescues Chlamydia from persistence.
(A) WII-U2OS cells were induced with 100 ng/mL anhydrotetracycline (AHT) for 2 hr. Cells were left untreated or were pre-treated for 2 hr with 10 ng/mL interferon-gamma (IFN-γ), infected with Chlamydia (Ctr) at multiplicity of infection (MOI) 1 and lysed at 30 hpi for Western blot analysis (n=3). (B) The infected cells from (A) were lysed to infect freshly plated HeLa 229 cells. The numbers of inclusions were counted from the different conditions shown in (A). The mean ± SD are shown in the graph. **** indicates a p<0.0001. (C) For the same culture conditions as in (A), an infectivity assay was performed. The HeLa 229 cells were fixed with 4% PFA after 30 hpi and immunostained for Ctr (cHSP60: green) and actin (Phalloidin: blue) in order to analyse the rescue. (D) From the experiment shown in (E) the infected/ IFN-γ-treated organoids were lysed with glass beads and different dilutions of the supernatant was used to infect freshly plated HeLa 229 cells. The number of inclusions were counted from three different experiments and mean ± SD are shown in the graph. **** indicates a p-value <0.0001. (E) The organoids from Figure 3—figure supplement 1 were infected with Ctr for 6 days with and without IFN-γ, were lysed using glass beads, and dilutions of the supernatant were used to infect freshly plated HeLa 229 cells for an infectivity assay. Cells were fixed with 4% PFA after 30 hpi and immunostained for Ctr (cHSP60: green) and actin (Phalloidin: red) in order to analyse the rescue (n=3).
-
Figure 3—source data 1
Complete and cutted membranes of all Western blots from Figure 3.
- https://cdn.elifesciences.org/articles/76721/elife-76721-fig3-data1-v1.pdf
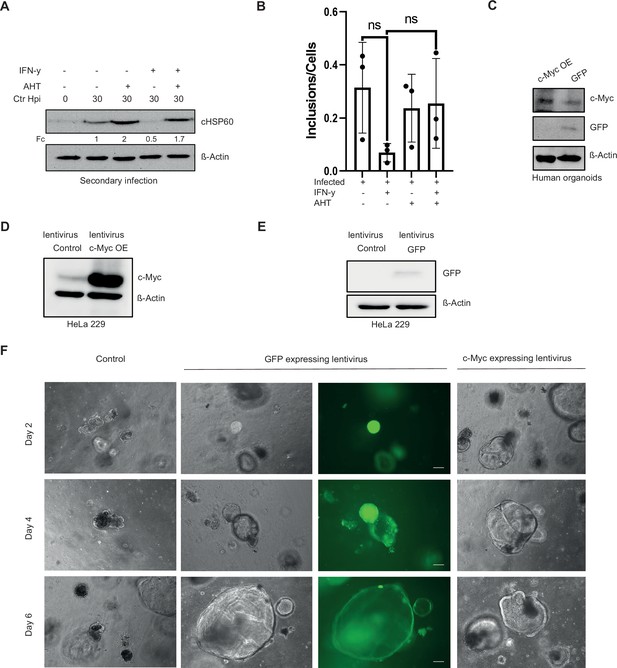
Expression of c-Myc rescues Chlamydia from persistence.
(A) For the same culture conditions as in Figure 3A, an infectivity assay was performed. The cells were lysed at 48 hpi and dilutions of the resulting Chlamydia-containing supernatant were added onto fresh HeLa 229 cells, which were then lysed at 30 hpi to examine infectious progenies. The cHSP60 detect Chlamydia infection and the β-Actin serves as loading control (n=3). (B) WII-U2OS cells were left untreated or were pre-treated for 2 hr with 10 ng/mL IFN-γ, infected with Chlamydia (Ctr) at multiplicity of infection (MOI) 1 and after 24 hr of IFN-γ treatment induced with 100 ng/mL anhydrotetracycline (AHT). The cells were lysed at 48 hpi and dilutions of the resulting Chlamydia-containing supernatant were added onto fresh WII-U2OS cells to examine infectious progenies. Number of inclusions were counted of three different experiments to plot the graph. (C) Human fallopian tube organoids were transduced with either lentivirus expressing c-Myc or GFP as control (see Materials and methods). The puromycin selected organoids were lysed and analysed via Western blotting. β-Actin serves as loading control. (D/E) HeLa 229 cells were transduced with lentivirus expressing c-Myc (D) or GFP (E). The cells were analysed by Western blotting. β-Actin serves as the loading control. (F) The virus preparations from (D) and (E) were used to infect human organoids derived from fallopian tube. The organoids were selected for puromycin. The images show organoids under selection 2, 4, and 6 days, respectively.
-
Figure 3—figure supplement 1—source data 1
Complete and cutted membranes of all Western blots from Figure 3—figure supplement 1.
- https://cdn.elifesciences.org/articles/76721/elife-76721-fig3-figsupp1-data1-v1.pdf

L-tryptophan activates the pGSK3β-c-Myc axis and rescues chlamydial infection.
(A) HeLa 229 cells were treated with interferon-gamma (IFN-γ) and different doses of L-tryptophan (W) (10–100 ng/mL). After 30 hr the cells were lysed and used for Western blot analysis (n=3). (B) HeLa 229 cells were infected with Ctr at multiplicity of infection (MOI) 1 for 30 hr and treated with IFN-γ and/or indole. The cells were lysed and analysed by Western blotting for c-Myc, pGSK3β, and cHSP60 levels (n=3). (C) HeLa 229 cells with an anhydrotetracycline (AHT)-inducible expression of shc-Myc were either left uninfected or infected with Chlamydia at MOI 1 for 30 hr. Additionally, cells were treated with L-tryptophan and/or 100 ng/mL AHT to deplete c-Myc. The cells were further analysed via Western blotting (n=3). (D) Cartoon left side: active phosphatidylinositol-3-kinase (PI3K) with inactive glycogen synthase kinase-3 (GSK3β) leading to c-Myc stabilization. Right side: IFN-γ binding to its receptor, activates PI3K and serine-threonine protein kinase (AKT) and induces the dephosphorylation and activation of GSK3β, leading to c-Myc depletion. Chlamydia infection activates the PI3K and MEK/ERK pathway. (E) HeLa 229 cells were either left uninfected or infected with Ctr and treated with IFN-γ with or without L-tryptophan (W). The cells were analysed by Western blotting 30 hpi. cHSP60 shows the intensity of chlamydial infection and β-Actin serves as loading control (n=3). Western blots shown in A-E were quantified by normalizing the Chlamydia load (cHSP60 or OmpA) and the respective host cell protein levels to β-Actin and the result was indicated as fold change (Fc). Image of Western blots was taken from one of a total of at least three blots of biological replicates (n=3).
-
Figure 4—source data 1
Complete and cutted membranes of all Western blots from Figure 4.
- https://cdn.elifesciences.org/articles/76721/elife-76721-fig4-data1-v1.pdf
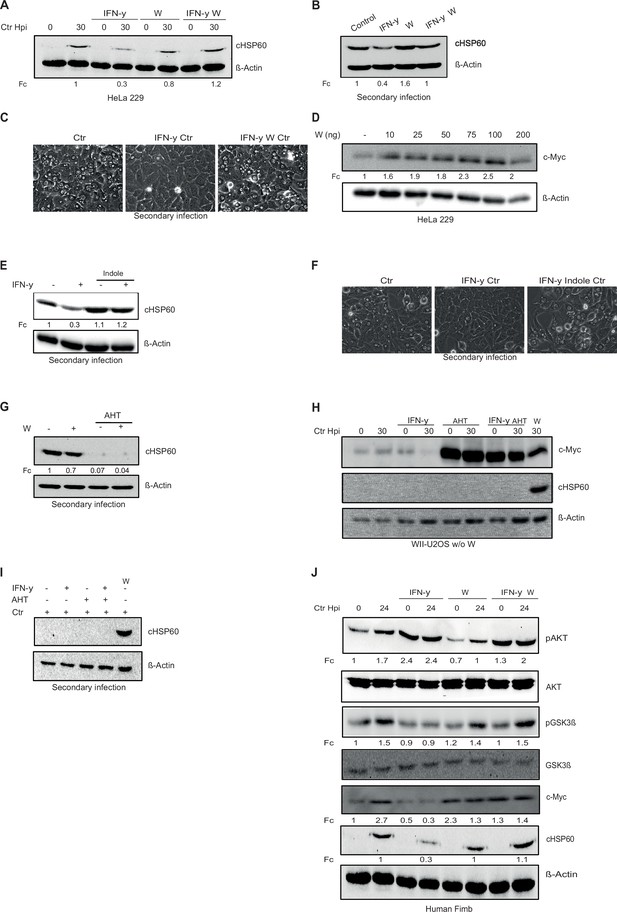
L-tryptophan activates the pGSK3β-c-Myc axis and rescues chlamydial infection.
(A) HeLa 229 cells were either left uninfected or infected with Chlamydia (Ctr) and treated with interferon-gamma (IFN-γ) with or without L-tryptophan (W). After 30 hpi, the cells were analysed via Western blotting (n=3). (B) HeLa 229 cells were infected with Ctr at multiplicity of infection (MOI) 1 for 24 hr and or treated with IFN-γ and L-tryptophan (W). The cells were lysed, and the supernatant was used to infect freshly plated HeLa 229 cells and bacterial load was determined by Western blotting for cHSP60. (C) Light microscopy pictures of (B). (D) HeLa 229 cells were either left untreated or were treated with different amounts of L-tryptophan (W). After 24 hr the cells were lysed and analysed by Western blotting. (E) The cells from Figure 4B were lysed and the supernatant was used to infect freshly plated HeLa 229 cells to assess the infectivity of the progeny. (F) Light microscopy pictures of (E). (G) HeLa 229 cells with anhydrotetracycline (AHT)-inducible expression of shc-Myc cells were treated with 100 ng/mL AHT for 2 hr. Cells were left untreated or were pre-treated for 2 hr with 100 ng/mL L-tryptophan (W), infected with Ctr at MOI 1. The cells were lysed, the supernatant was used to infect fresh cells, and lysed 30 hpi to study infectivity. (H) WII-U2OS cells with inducible expression of c-Myc were left untreated or treated with 100 ng/mL AHT and 10 ng/mL IFN-γ for 2 hr. Cells were grown without L-tryptophan and infected with Ctr at MOI 1 and lysed at 30 hpi for Western blot analysis. (I) Cells from the experiment shown in (H) were used for infectivity assay. (J) Cells from human fimbriae (human Fimb) were either left uninfected or infected with Ctr and treated with IFN-γ with or without L-tryptophan (W). The cells were analysed by Western blotting 24 hpi. cHSP60 shows the intensity of chlamydial infection and β-Actin serves as loading control (n=3). Western blots shown in A-J were quantified by normalizing the Chlamydia load (cHSP60) and the respective host cell protein levels to β-Actin and the result was indicated as fold change (Fc). Image of Western blots was taken from one of a total of at least three blots of biological replicates (n=3).
-
Figure 4—figure supplement 1—source data 1
Complete and cutted membranes of all Western blots from Figure 4—figure supplement 1.
- https://cdn.elifesciences.org/articles/76721/elife-76721-fig4-figsupp1-data1-v1.pdf

Influence of stabilized c-Myc on L-tryptophan (Trp) uptake and metabolites.
(A) HeLa 229 cells were infected with Chlamydia (Ctr) for various time points. The cells were analysed via Western blotting for the levels of LAT1 (n=3). (B) HeLa 229 cells were either left untreated or were pre-treated for 2 hr with 10 ng/mL of IFN-γ, infected with Ctr at multiplicity of infection (MOI) 1 and lysed at 24 hpi to examine LAT1 regulation via Western blotting. (C) c-Myc overexpression in WII-U2OS cells was induced with 100 ng/mL anhydrotetracycline (AHT) for 2 hr. Cells were left untreated or were pre-treated for 2 hr with 10 ng/mL IFN-γ, infected with Chlamydia (Ctr) at MOI 1 and lysed at 24 hpi for Western blot analysis (n=3). (D) HeLa 229 cells were either left untreated or were pre-treated for 2 hr with 10 ng/mL of IFN-γ and/or 10 µM IDO inhibitor, infected with Ctr at MOI 1 and lysed at 36 hpi to examine c-Myc regulation via Western blotting. (E) After 48 hpi cells from (D) were lysed and used to infect freshly plated HeLa 229 cells to analyse progeny via Western blot. (F) The number of inclusions and the number of the cells of (E) were counted to plot the graph. One-way ANOVA was used for analysis. * indicates a p-value <0.05, *** indicates a p-value <0.001. (G) WII-U2OS cells were either left uninfected or infected with Ctr at MOI 1 for 30 hr. These cells were either left untreated or treated with just 10 ng/mL IFN-γ and/or 100 ng/mL AHT to induce expression of c-Myc. The cells were extracted, and metabolites were analysed by LC-MS. Data are presented as mean ± SD of triplicate wells. ** indicates a p-value <0.01, *** indicates a p-value <0.001. The intracellular levels of tryptophan are shown. (H) WII-U2OS cells were either left uninfected or infected with Ctr at MOI 1 for 30 hr. The infected cells were either left untreated or treated with just 10 ng/mL IFN-γ and 100 ng/mL AHT to induce overexpression of c-Myc. The media, in which the cells were grown, was extracted and metabolites were analysed by LC-MS. Data are presented as mean ± SD of triplicate wells. ** indicates a p-value <0.01, *** indicates a p-value <0.001. The levels of tryptophan present in medium are shown. (I) WII-U2OS cells were either left uninfected or infected with Ctr at MOI 1 for 30 hr. The infected cells were either left untreated or treated with just 10 ng/mL IFN-γ and 100 ng/mL AHT to induce expression of c-Myc. The cells were extracted, and metabolites were analysed by LC-MS. Data are presented as mean ± SD of triplicate wells. **** indicates a p-value <0.0001. The intracellular levels of kynurenine are shown. Western blots shown in Figure 5 were quantified by normalizing the Chlamydia load (cHSP60) and the respective host cell protein levels to β-Actin and the result was indicated as fold change (Fc). Image of western blots was taken from one of a total of at least three blots of biological replicates (n=3).
-
Figure 5—source data 1
Complete and cutted membranes of all Western blots from Figure 5.
- https://cdn.elifesciences.org/articles/76721/elife-76721-fig5-data1-v1.pdf
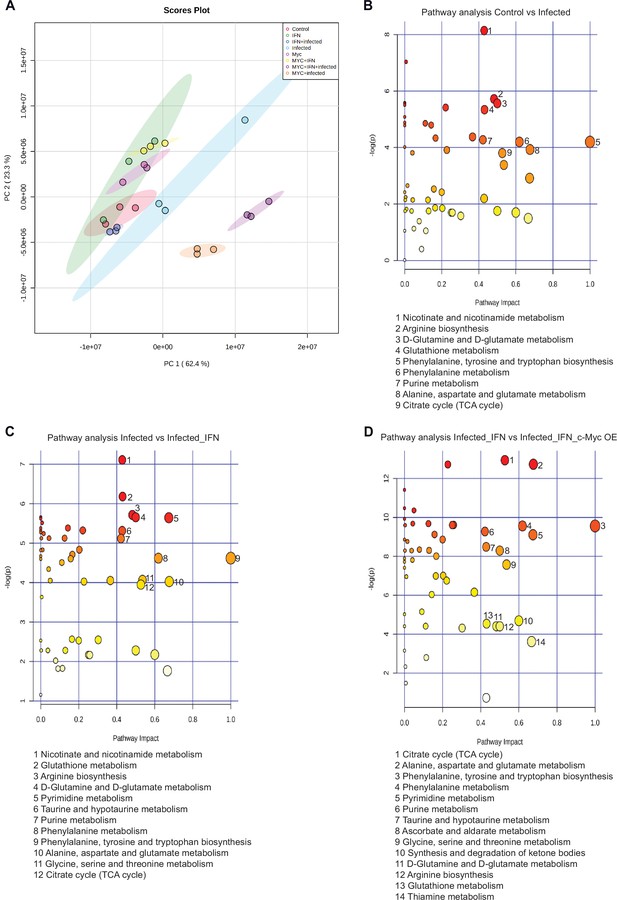
Pathway analysis.
(A.–D) WII-U2OS cells were either left uninfected or infected with Chlamydia trachomatis (Ctr) at multiplicity of infection (MOI) 1 for 30 hr. The infected cells were either left untreated or treated with just 10 ng/mL interferon-gamma (IFN-γ) and 100 ng/mL anhydrotetracycline (AHT) to induce expression of c-Myc. The cells were extracted, and metabolites were analysed by LC-MS. Quality controls and data normalization were performed and a principal component analysis (PCA) (A), and pathway analysis (control vs. infected (B), infected vs. infected IFN-γ treated (C), infected IFN-γ treated vs. infected IFN-γ treated and c-Myc expressed (D)) are shown here. FDR <0.05, Impact >0.4.

Tricarboxylic acid (TCA) intermediates and nucleosides can overcome interferon-gamma (IFN-γ)-induced persistence.
(A) A heatmap with hierarchical clustering of all metabolites detected by LC-MS analysis of (Figure 5G). (B/C) WII-U2OS cells were either left uninfected or infected with Chlamydia trachomatis (Ctr) at multiplicity of infection (MOI) 1 for 30 hr. The infected cells were either left untreated or treated with just 10 ng/mL IFN-γ and 100 ng/mL anhydrotetracycline (AHT) to induce overexpression of c-Myc. The cells were extracted, and metabolites were analysed by LC-MS. Data are presented as mean ± SD of triplicate wells. Intracellular levels of metabolites like citrate, aconitate, α-ketoglutarate, glutamate (B), or nucleosides (adenosine, guanosine, cytidine, and uridine) (C) were determined and quantified. Statistical analysis was performed using MetaboAnalyst 4.0. Significantly changed metabolite levels were determined by ANOVA with subsequent FDR correction, where a p value of 0.05 was considered statistically significant (*) and Tukey HSD was applied as post hoc test. (D) Cells were left untreated or were pre-treated for 2 hr with 10 ng/mL IFN-γ, 4 mM α-ketoglutarate (DMKG), or 100 µM nucleosides, infected with Ctr at MOI 1 and lysed at 30 hpi for Western blot analysis (n=3). The α-ketoglutarate was supplied as cell-permeable dimethyl ester. (E) For the same culture conditions as in (D), an infectivity assay was performed. The cells were lysed at 48 hpi and dilutions of the resulting Chlamydia containing supernatant were added onto freshly plated WII-U2OS cells, which were then lysed at 30 hpi to examine infectious progeny via Western blotting (n=3). Inclusions and cells were counted, and the results are shown as bar diagram. One-way ANOVA was used for analysis. * indicates a p-value <0.05, *** indicates a p-value <0.001. (F) HeLa 229 cells were either infected with Chlamydia or infected and treated with IFN-γ and nucleosides (uridine/cytosine or adenosine/guanosine) were added. The cells were lysed at 48 hpi and dilutions of the resulting Chlamydia containing supernatant were added onto freshly plated HeLa 229 cells, which were then lysed at 30 hpi to examine infectious progenies via Western blot (n=3). Inclusions and cells were counted, and the results are shown as bar diagram. One-way ANOVA was used for analysis. * indicates a p-value <0.05, *** indicates a p-value <0.001, **** indicates a p-value <0.0001. (G) HeLa 229 cells were left untreated or were pre-treated for 2 hr with 10 ng/mL IFN-γ before 4 mM citrate and Ctr at an MOI 1 were added. The cells were lysed at 48 hpi and dilutions of the resulting Ctr containing supernatant were added onto freshly plated HeLa 229 cells, which were then lysed at 24 hpi to examine infectious progenies via Western blot (n=3). Inclusions and cells were counted, and the results are shown as bar diagram. One-way ANOVA was used for analysis. *** indicates a p-value <0.001. (H) Human Fimb cells were left untreated or were pre-treated for 2 hr with 10 ng/mL IFN-γ, 4 mM α-ketoglutarate (DMKG), infected with Ctr at MOI 1. The cells were lysed at 48 hpi and dilutions of the resulting Chlamydia containing supernatant were added onto freshly plated human Fimb cells, which were then lysed at 24 hpi to examine infectious progenies via Western blot (n=3). Inclusions and cells were counted, and the results are shown as bar diagram. One-way ANOVA was used for analysis. ** indicates a p-value <0.01, *** indicates a p-value <0.001. Western blots shown in Figure 6 were quantified by normalizing the Chlamydia load (cHSP60) and the respective host cell protein levels to β-Actin and the result was indicated as fold change (Fc). Image of Western blots was taken from one of a total of at least three blots of biological replicates (n=3).
-
Figure 6—source data 1
Complete and cutted membranes of all Western blots from Figure 6.
- https://cdn.elifesciences.org/articles/76721/elife-76721-fig6-data1-v1.pdf
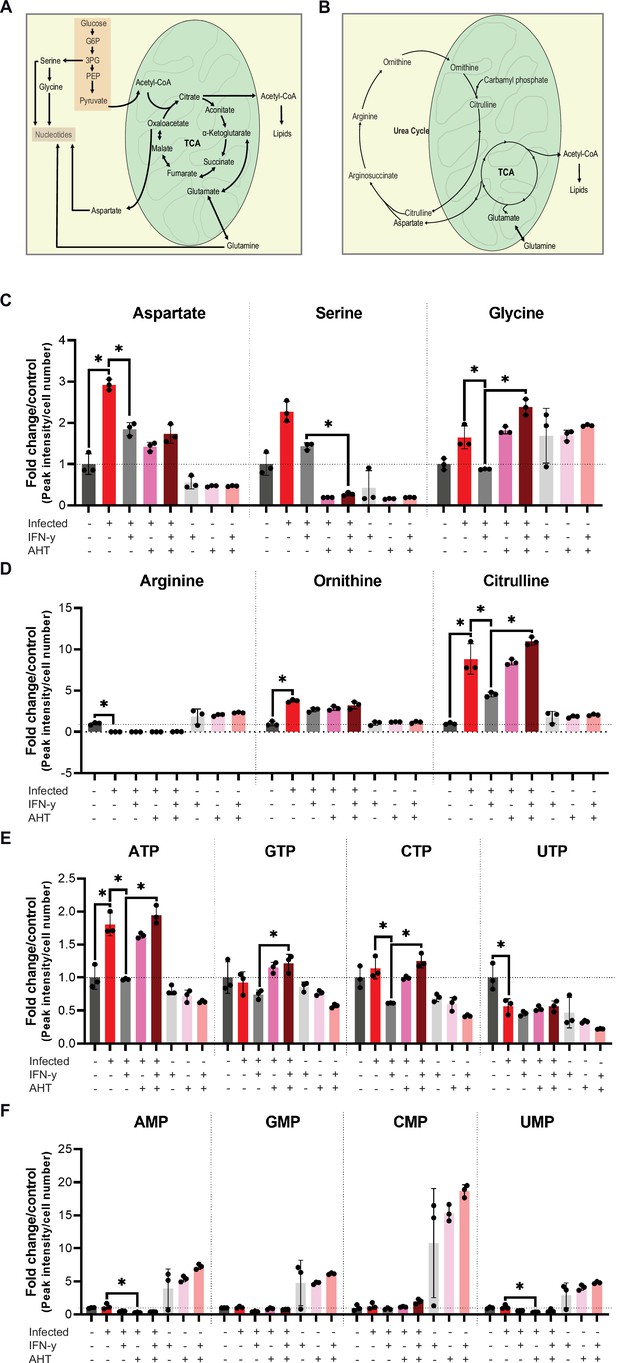
Urea cycle intermediates.
(A) Cartoon depicting tricarboxylic acid (TCA) cycle and nucleotide biosynthesis in human cells. (B) Cartoon depicting the flux of aspartate from the TCA cycle. Oxaloacetate from TCA is converted into aspartate by mitochondrial malate dehydrogenase. The glutamate-aspartate antiporter transports aspartate into the cytosol where it is shuttled into the urea cycle. (C–F) WII-U2OS cells were either left uninfected or infected with Chlamydia trachomatis (Ctr) at multiplicity of infection (MOI) 1 for 30 hr. The infected cells were either left untreated or treated with just 10 ng/mL interferon-gamma (IFN-γ) and 100 ng/mL anhydrotetracycline (AHT) to induce overexpression of c-Myc. The cells were extracted, and metabolites were analysed by LC-MS. Data are presented as mean ± SD of triplicate wells. Statistical analysis was performed using MetaboAnalyst 4.0. Significantly changed metabolite levels were determined by ANOVA with subsequent FDR correction, where a p value of 0.05 was considered statistically significant (*) and Tukey HSD was applied as post hoc test. The levels of aspartate, serine, and glycine (C), arginine, ornithine, citrulline (D), nucleotide triphosphate (ATP, GTP, CTP, and UTP) (E), and nucleotide monophosphate (AMP, GMP, CMP, and UMP) (F) are shown.

The chlamydial Trp synthase operon is not involved in the rescue from persistence by c-Myc expression and tricarboxylic acid (TCA) metabolite supply.
(A) Western blot of wildtype Chlamydia trachomatis (Ctr) and TrpBA mutant to demonstrate the loss of TrpA and TrpB. (B) HeLa 229 cells were infected with Ctr or TrpBA mutant at multiplicity of infection (MOI) 1 for 48 hr and treated with 10 ng/mL IFN-γ and/or 50 µM indole. The cells were lysed, and the supernatant was used to infect freshly plated HeLa 229 cells to assess the infectivity of the progeny. The number of inclusions and the number of the cells were counted to plot the graph. One-way ANOVA was used for analysis. ** indicates p-value <0.01. (C) WII-U2OS cells were induced with 100 ng/mL anhydrotetracycline (AHT) for 2 hr. Cells were left untreated or were pre-treated for 2 hr with 10 ng/mL IFN-γ, infected with Ctr or TrpBA mutant at MOI 1 and lysed at 44 hpi for infecting freshly plated WII-U2OS cells to check infectious progeny. The number of inclusions and the number of the cells were counted to plot the graph. One-way ANOVA was used for analysis. * indicates a p-value <0.05, ** indicates p-value <0.01, *** indicates a p-value <0.001. (D) HeLa 229 cells were infected with Ctr or TrpBA mutant at MOI 1 for 48 hr and treated with 10 ng/mL IFN-γ and/or 4 mM α-ketoglutarate (DMKG). The cells were lysed, and the supernatant was used to infect freshly plated HeLa 229 cells to assess the infectivity of the progeny. Inclusions and cells were counted, and the results are shown as bar diagram. One-way ANOVA was used for analysis. * indicates p-value <0.05, **** indicates a p-value <0.0001.
-
Figure 7—source data 1
Complete and cutted membranes of all Western blots from Figure 7.
- https://cdn.elifesciences.org/articles/76721/elife-76721-fig7-data1-v1.pdf
Additional files
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/76721/elife-76721-transrepform1-v1.pdf
-
Source data 1
Inclusion Forming Units (IFU).
- https://cdn.elifesciences.org/articles/76721/elife-76721-data1-v1.xlsx
-
Source data 2
Metabolic profiling.
- https://cdn.elifesciences.org/articles/76721/elife-76721-data2-v1.xlsx





