Dopaminergic regulation of vestibulo-cerebellar circuits through unipolar brush cells
Figures
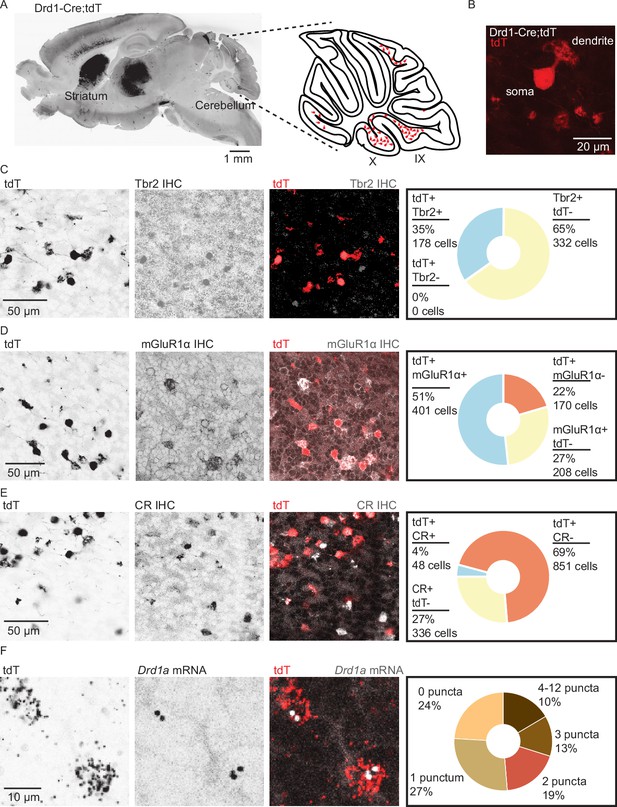
Dopamine type 1a receptor transcripts are expressed in mGluR1+ unipolar brush cells.
(A) Left: A whole brain parasagittal section from a Drd1-Cre;tdTomato (tdT) reporter cross. Right: A cartoon view of a parasagittal slice of the cerebellar vermis. tdT expressing unipolar brush cells (UBCs) are abundant in lobules IX/X of the vermis. Scale bar, 1 mm. (B) A confocal image of a tdT+ UBC in the cerebellum of a Drd1-Cre mouse, showing the characteristic dendritic brush morphology of a UBC. Scale bar, 20 µm. (C) Left: Confocal images of immunofluorescent labeling of Eomesodermin (Tbr2) in lobules IX/X of the cerebellar vermis. Right: Pie chart describing overlap of cerebellar tdT+ cells and Tbr2. All tdT+ cells express Tbr2, and a subpopulation (~35%) of Tbr2 cells expresses tdT (n = 2 mice, 510 cells). Inverted grayscale, single-channel images; in merged images, red represents tdT signal, gray represents antibody labeling. Scale bar, 50 µm. (D) Left: Similar to C, but for metabotropic glutamate receptor type 1 (mGluR1). Right: Out of all tdT+ UBCs, 70% expressed mGluR1; 66% of mGluR1+ UBCs were positive for tdT (n = 2 mice, 779 cells). Scale bar, 50 µm. (E) Left: Similar to C−D, but for calretinin (CR). Right: little overlap was observed between CR and tdT+ UBCs: 5.3% of tdT+ UBCs express CR, and 12.5% of CR+ express tdT (n = 2 mice, 1235 cells). Scale bar, 50 µm. (F) Left: Sample confocal images of fluorescent in situ hybridization, labeling tdT, and Drd1a transcripts in cerebellar UBCs. Inverted grayscale lookup table (LUT) is used for single-channel images. In merged images, red represents tdT signal, gray represents Drd1 labeling. Right: The majority (~76%) of tdT+ UBCs express dopamine type 1a receptor (Drd1a) transcripts (n = 4 mice, 2553 cells). Scale bar, 10 µm.
-
Figure 1—source data 1
Numerical data for graphs in Figure 1.
- https://cdn.elifesciences.org/articles/76912/elife-76912-fig1-data1-v2.xlsx
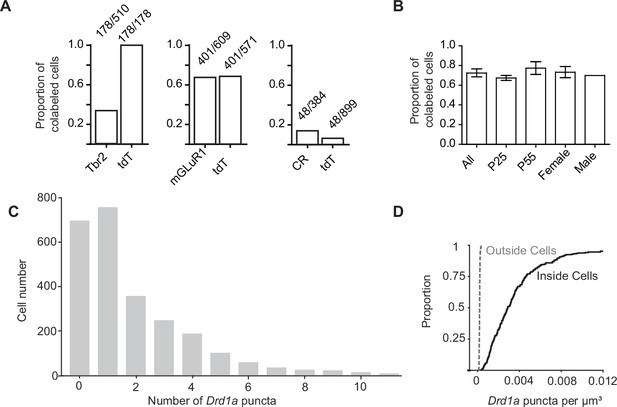
Co-localization of tdTomato expressing unipolar brush cells with unipolar brush cell subtype markers and dopamine type 1a receptor mRNA.
(A) The proportion of cells co-labeled by known immunohistochemical markers of unipolar brush cells (UBCs) subtypes and tdTomato (tdT) in Drd1-Cre; tdT mouse line. Left: A subset of eomesodermin+ (Tbr2) UBCs were co-labeled by tdT, while all tdT+ cells expressed Tbr2 protein. Center: tdT+ and metabotropic glutamate receptor type 1 (mGluR1) expression show substantial overlap. Right: Few calretinin+ (CR) UBCs express tdT. Ratios represent the population of co-labeled cells divided by the total number of cells expressing a given marker. (B) A comparison of tdT+ UBCs between two age groups and across sex show similar proportions of UBCs that express dopamine type 1a receptor (Drd1a) transcripts (n = 4 mice total, P25 n = 2, P55 n = 2, female n = 3, male n = 1). (C) A histogram of the number of Drd1a puncta per UBC tdT+ soma (n = 4 mice, 2553 cells). D. 3D quantification of Drd1a puncta per unit volume shows 40-fold enrichment within the somata of UBCs expressing tdT, compared with regions outside tdT+ ROIs.
-
Figure 1—figure supplement 1—source data 1
Numerical data for graphs in Figure 1—figure supplement 1.
- https://cdn.elifesciences.org/articles/76912/elife-76912-fig1-figsupp1-data1-v2.xlsx
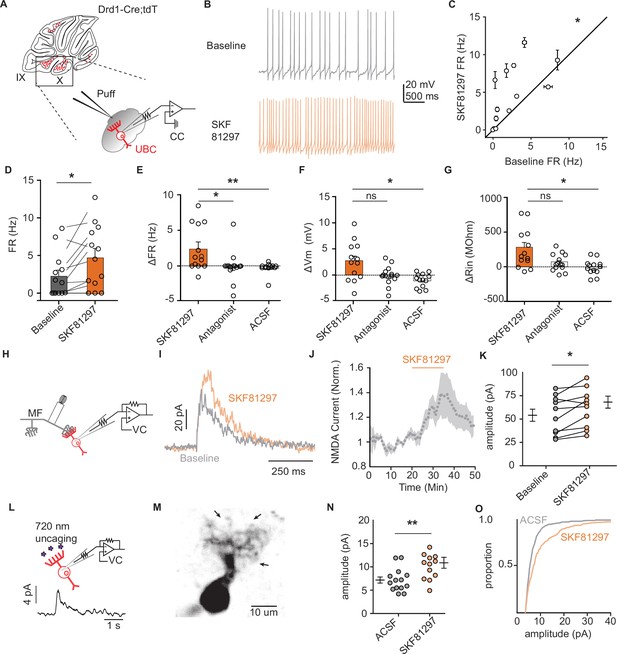
Dopamine type 1 receptor activation increases the firing rate and NMDAR currents in unipolar brush cells.
(A) Diagram of experimental setup with tdTomato (tdT+) unipolar brush cells (UBCs) in whole-cell current clamp configuration. FR was measured during baseline period, followed by application of Drd1 agonist SKF81297 (500 µM) with 300-ms long puffs from a proximally located pipette. (B) Membrane voltage traces from an example cell which increased its firing rate (FR) in response to SKF81297 application. (C, D) Group comparison, mean FRs during the baseline period, and application of SKF81297 (n = 13 cells, paired Wilcoxon signed rank test, p=0.02, *p<0.05). (E-G) Changes in firing rate (ΔFR) in E, resting membrane potential (ΔmV) in F, and input resistance (ΔRin) in G after drug application. Comparisons are between three independent datasets. SKF81297: puff application of Drd1 agonist. Antagonist: SKF81297 was applied in the presence of Drd1 blockers 1 µM SKF83566, 1 µM SCH39166, and 1 µM SCH23390. Artificial cerebrospinal fluid (ACSF): puff application ACSF (Kruskal-Wallis test, FR p=0.0044, Vm p=0.0024, Rin p=0.02; Dunn’s multiple comparison test, *p<0.05, **p<0.01). (H) Schematic of experimental setup. Voltage clamp recordings of tdT+ UBCs, paired with stimulation of mossy fiber inputs to evoke NMDA receptor mediated currents (intertrial interval [ITI], 5 s), in the presence of blockers for AMPA, GABA, and glycine receptors (1 µM NBQX, 1 µM gabazine, and 1 µM strychnine). (I) Sample traces show an increase in NMDAR current amplitudes during the application of SKF81297. (J) Trace of normalized peak amplitude of NMDAR currents across cells (n = 10 cells). Shaded region, ± SEM. (K) Summary plot of average evoked N-Methyl-D-aspartate receptor (NMDAR) current amplitudes before and after application of 10 µM SKF81297 (n = 10 cells, paired t-test, p=0.0251). (L) Top: Schematic of two-photon MNI-L-glutamate uncaging experiments. TdT+ UBCs were voltage clamped and imaged using a two-photon laser scanning microscope. Laser pulses (725 nm) directed near the dendritic brush (25 mW, 1 ms, 60 s ITI) were used to evoke NMDAR mediated currents. Bottom: Example current trace. (M) Two-photon imaging Z-projection of a tdT+ UBC. Arrows represent separate uncaging site locations. Scale bar, 10 µm. (N) NMDAR current amplitudes in ACSF vs 10 µM SKF81297 (unpaired t-test, p=0.0068, **p<0.01). Each data point is the average amplitude of NMDAR currents evoked by glutamate uncaging from one cell (ACSF n = 14 cells, SKF81297 n = 13 cells). (O) Cumulative distribution of uncaging-evoked NMDAR current peak amplitudes for ACSF (14 cells, 466 individual trials) and SKF81297 (13 cells, 349 individual trials) groups.
-
Figure 2—source data 1
Numerical data for graphs in Figure 2.
- https://cdn.elifesciences.org/articles/76912/elife-76912-fig2-data1-v2.xlsx
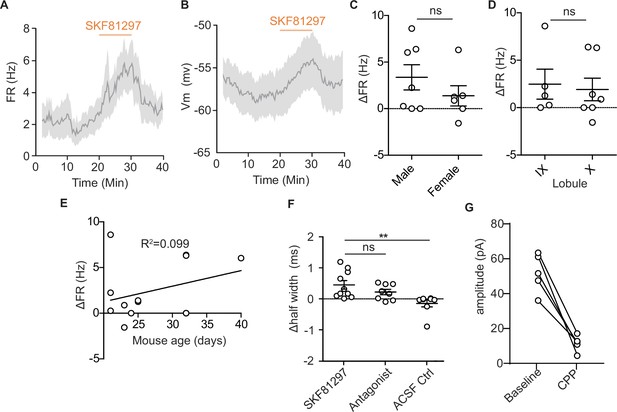
Characterization of dopamine type 1 receptor activation effects on unipolar brush cell firing and post-synaptic currents.
(A) Time course of changes in firing rate (FR) in response to SKF81297 application. Lines represent the mean across cells (n = 13), shaded regions are ± SEM. (B) Time course of changes in membrane potential in response to SKF81297 application. Lines represent the mean across cells (n = 13), shaded regions are ± SEM. (C) Comparison of changes in FR in response to SKF81297 application shows no difference across sex (unpaired t-test, Mann-Whitney U test, p=0.42). (D) Comparison of changes in FR in response to SKF81297 application across lobules IX and X of the vermis (unpaired t-test, Mann-Whitney U test, p=0.62). (E) Comparison of changes in FR in response to SKF81297 application across age, P20-P40 (R2 = 0.099, ns). (F) Comparison of changes in action potential half-width shows an increase with application of SKF81297, but not artificial cerebrospinal fluid (ACSF; Kruskal-Wallis test, p=0.0026, Dunn’s multiple comparison test, *p<0.05, ** p<0.01). (G) N-methyl-D-aspartate receptor currents modulated by Drd1 activation are largely blocked by flow in of 10 µM CPP.
-
Figure 2—figure supplement 1—source data 1
Numerical data for graphs in Figure 2—figure supplement 1.
- https://cdn.elifesciences.org/articles/76912/elife-76912-fig2-figsupp1-data1-v2.xlsx
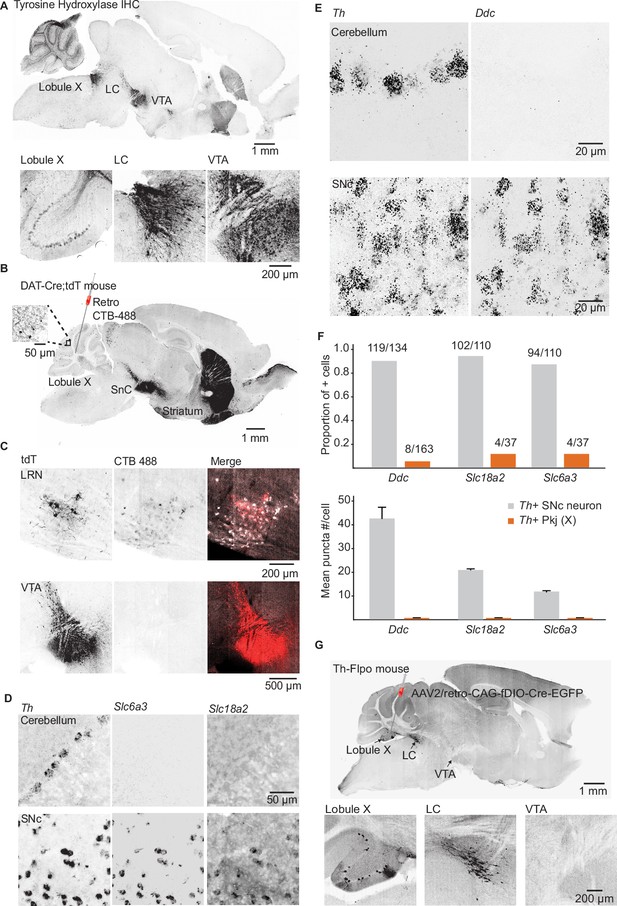
Interrogating potential dopamine sources for cerebellar lobules IX/X.
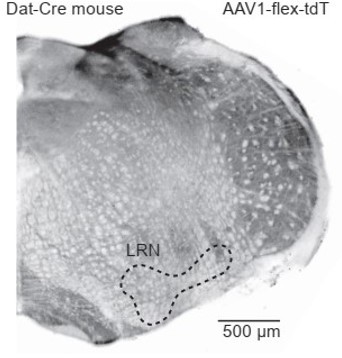
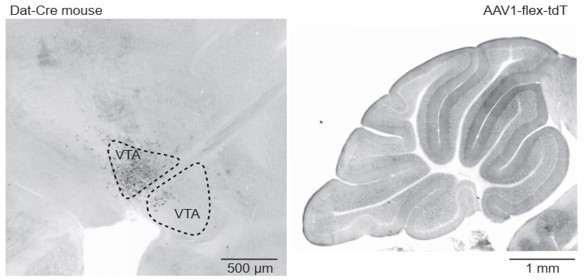
(A) Top: Parasagittal slice of a mouse brain immunolabeled for tyrosine hydroxylase (TH). Three hypothesized sources of dopamine include Purkinje (Pkj) cells in lobule X of the vermis, locus coeruleus (LC) neurons, and ventral tegmental area (VTA) dopamine neurons. Scale bar, 1 mm. Bottom: Close-up of regions of interest. Scale bar, 200 µm. (B) Example retrograde labeling experiment with CTB-488 injected into the cerebellum of a Dat-Cre;tdTomato (tdT) reporter mouse (n = 3 mice). Scale bar, 1 mm. Inset: tdTomato expressing cerebellar fibers are morphologically consistent with mossy fiber terminals and are restricted to the granule layer. Scale bar, 50 µm. (C) Close-up images from a retrograde CTB-488 injection. No labeling is observed in the tdT+ neurons in the VTA, in contrast to tdT+ neurons in the lateral reticular nucleus (LRN), a glutamatergic pre-cerebellar brainstem nucleus. Scale bars, 200 µm and 500 µm. (D) Top: Confocal images of fluorescent in situ hybridization labeling for tyrosine hydroxylase (Th) and dopa decarboxylase (Ddc) transcripts in Pkj cells in lobule X. Bottom, images of substantia nigra pars compacta (SNc) neurons co-labeled for Th and Ddc transcripts (n = 2 mice). Scale bar, 20 µm. (E) Comparison of TH+ Pkj cells and SNc neurons co-labeled for dopamine active transporter (Slc6a3) and vesicular monoamine transporter type 2 (Slc18a2) (n = 2 mice). Scale bar, 50 µm. (F) Quantification of transcripts involved in synthesis and release of dopamine. Top: Few T h+ Pkj cells express transcripts for Ddc, Slc18a2, or Slc6a3. Bottom: The few Th+ Pkj cells that express Ddc, Slc18a2, and Slc6a3 express low transcript numbers in comparison to SNc neurons (n = 2 mice). Gray bars represent counts from SNc dopamine neurons, red bars represent Th+ Pkj cells. (G) Top: Parasagittal section from a Th-Flpo mouse injected with retrograde Flp-dependent virus expressing green fluorescent protein (GFP) (AAV2/retro-CAG-fDIO-Cre-EGFP). Scale bar, 1 mm. Bottom: Close-up of regions of interest. No GFP labeling of VTA/SNc neurons was observed, in contrast to LC neurons and local Th+ Pkj cells that were retrogradely labeled by GFP (n = 2 mice). Scale bar, 200 µm.
-
Figure 3—source data 1
Numerical data for graphs in Figure 3.
- https://cdn.elifesciences.org/articles/76912/elife-76912-fig3-data1-v2.xlsx
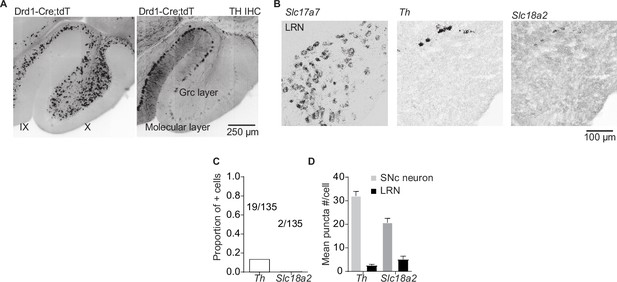
Potential dopamine sources for vestibulo-cerebellum.
(A) Example images from the cerebellum of dopamine type 1 receptor (Drd1)-Cre;tdTomato (tdT) mouse, immunolabeled for tyrosine hydroxylase (TH), showing TH+ Purkinje cells and tdT+ unipolar brush cells in lobules IX/X of the cerebellar vermis. Scale bar, 250 µm. (B) Lateral reticular nucleus (LRN) neurons, labeled by the Slc17a7 probe show little overlap with Th or vesicular monoamine transporter type 2 (Slc18a2). Scale bar, 100 µm. (C) Quantification of transcripts involved in synthesis and release of dopamine. Top: Few LRN neurons express transcripts for Slc18a2 or Th. (D) The few LRN neurons (n = 3 mice) that express Slc18a2 and Th express low transcript numbers in comparison to substantia nigra pars compacta (SNc) neurons (n = 2 mice). Gray bars represent counts from SNc dopamine neurons, black bars represent LRN cells.
-
Figure 3—figure supplement 1—source data 1
Numerical data for graphs in (Figure 3—figure supplement 1).
- https://cdn.elifesciences.org/articles/76912/elife-76912-fig3-figsupp1-data1-v2.xlsx
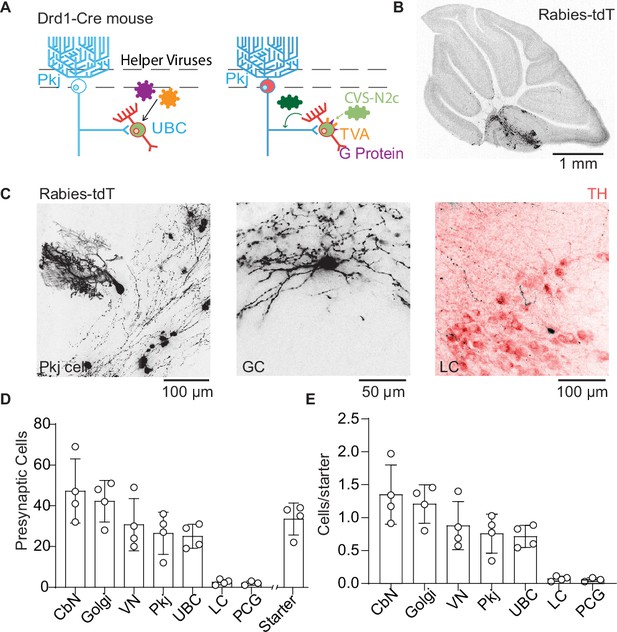
Trans-synaptic tracing of inputs to dopamine type 1 receptor expressing unipolar brush cells.
(A) Schematic of viral targeting for trans-synaptic modified rabies virus tracing. Helper adeno-associated viruses (AAVs) expressing two Cre-dependent constructs, tumor virus receptor A (TVA), and glycoprotein. After 4–6 weeks of helper virus expression in unipolar brush cells (UBCs), we injected modified rabies (CVS-N2cΔG). The rabies virus enters UBCs expressing the TVA receptor. Once expressed in a UBC along with the glycoprotein, the virus can create a functional capsid and travel pre-synaptically. (B) Representative image of a sagittal cerebellar slice. Cells infected with the rabies virus express tdTomato (tdT). Scale bar, 1 mm. (C) Example cells pre-synaptic to Drd1+ UBCs expressing tdT. Left: Purkinje (Pkj) cell, Center: Golgi cell (GC). Right: locus coeruleus (LC) neuron expressing tdT from the rabies virus, with TH antibody labeling. Scale bars, 50 and 100 µm. (D) Number of cells labeled by CVS-N2cΔG rabies virus (n = 4 mice). In addition to UBCs, Pkj cells, and Golgi cells, we found tdT-labeled cells in the cerebellar nuclei (CbN), vestibular nuclei (VN), pontine central gray (PCG), and LC. Starter cells indicate the number of UBCs co-expressing green fluorescent protein (GFP) and tdTomato (tdT). (E) Quantification of labeled pre-synaptic cells normalized to the number of starter UBCs.
-
Figure 4—source data 1
Numerical data for graphs in Figure 4.
- https://cdn.elifesciences.org/articles/76912/elife-76912-fig4-data1-v2.xlsx
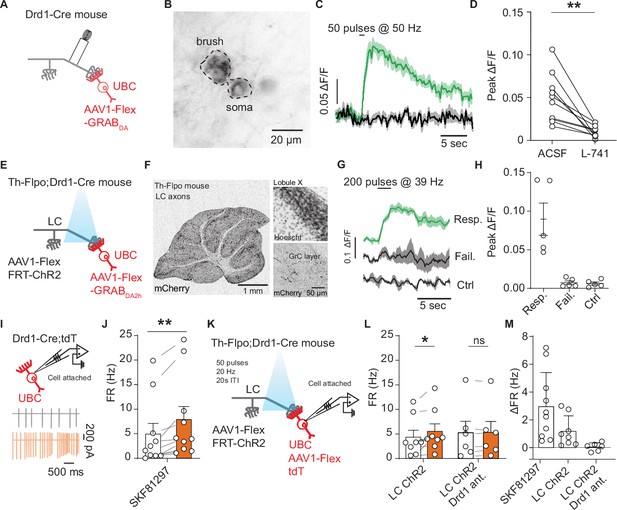
Locus coeruleus fibers release dopamine in the cerebellum activating unipolar brush cell dopamine type 1 receptors.
(A) Schematic of experimental setup, Cre-dependent GRABDA2h expressed in unipolar brush cells (UBCs), with a monopolar electrode placed in the white matter of lobule X. (B) Two-photon image of UBCs expressing Cre-dependent GRABDA2h in a Drd1-Cre cerebellum. (C) Sample trace from one cell (five trial average) in response to electrical stimulation before and after application of Drd2 antagonist (1 µM L-741,626). Shaded regions, SEM. (D) Group data of peak amplitude of ΔF/F in response to 50 Hz stimulation of axonal cerebellar input before and after flow in of Drd2 antagonist (1 µM L-741,626) (n = 10 cells, paired t-test, p=0.002, **p<0.01). (E) Experimental design using dual virus injection to express ChR2 (Flpo-dependent) in locus coeruleus (LC) axons and GRABDA2h (Cre-dependent) in UBCs. Light pulses were delivered using 460 nm LED (2 ms pulse width, 200 pulses, 39 Hz). (F) Anterograde labeling of axons from the LC to the cerebellum. Left: Injection of AAV1-FLEX-FRT-ChR2-mCherry into the LC shows axons spreading broadly across all layers of the cerebellum (n = 2 mice). Scale bar, 1 mm. Right: Confocal images show LC axons in molecular and granular layer of lobule X. Scale bar, 50 µm. (G) Sample traces of changes in GRABDA2h fluorescence in response to optical activation of ChR2+ LC axons from a single cell. The three traces correspond to trials with an evoked response, trials with no response, and control trials where no light stimulation was given. Lines represent the mean, shaded regions are ± SEM. (H) Summary data from several experiments, 5/20 cells responded to optogenetic activation (six acute slices, n = 2 mice). Error bars represent SEM. (I) Diagram of experimental setup, with tdTomato+ (tdT) UBCs recorded in a cell-attached recording configuration. Inset: Traces from an example cell show action potentials before (black) and after (orange) bath application of 10 µM SKF81297. (J) Summary data from cell-attached recordings before (white) and after (orange) the application of a Drd1 agonist (n = 10 cells, paired Wilcoxon signed rank test, p=0.002, **p<0.01). (K) Schematic of cell-attached recordings paired with selective optogenetic activation of LC fibers. After a baseline period, light stimuli were delivered every 20 s (460 nm, 50 pulses, 20 Hz, 1 ms pulse width). (L) Mean firing rates (FRs) before (white) and during (orange) optogenetic stimulation period. Right, same but with Drd1 antagonist in the bath. (M) Comparison of changes in FR in response to SKF81297 bath application, optogenetic activation of LC fibers, and optogenetic LC activation in the presence of Drd1 antagonist.
-
Figure 5—source data 1
Numerical data for graphs in Figure 5.
- https://cdn.elifesciences.org/articles/76912/elife-76912-fig5-data1-v2.xlsx
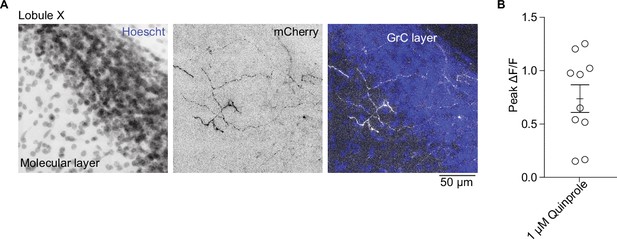
Locus coeruleus fiber location in the cerebellum and validation of GRABDA2h.
(A) Enlarged images of locus coeruleus (LC) axons in the granular and molecular layer of lobule X after injection of AAV1-CAG-FLEXFRT-ChR2(H134R)-mCherry into the LC of Th-Flpo mouse. Grayscale lookup table for left and middle panels; for overlay image, inverted grayscale (mCherry) and blue (Hoechst). Also included in Figure 5F. Scale bar, 50 µm. (B) Peak fluorescence change in GRABDA2h expressing unipolar brush cells (UBCs) after a flow-in of Drd2 agonist (1 µM quinpirole).
-
Figure 5—figure supplement 1—source data 1
Numerical data for graphs in Figure 5—figure supplement 1.
- https://cdn.elifesciences.org/articles/76912/elife-76912-fig5-figsupp1-data1-v2.xlsx
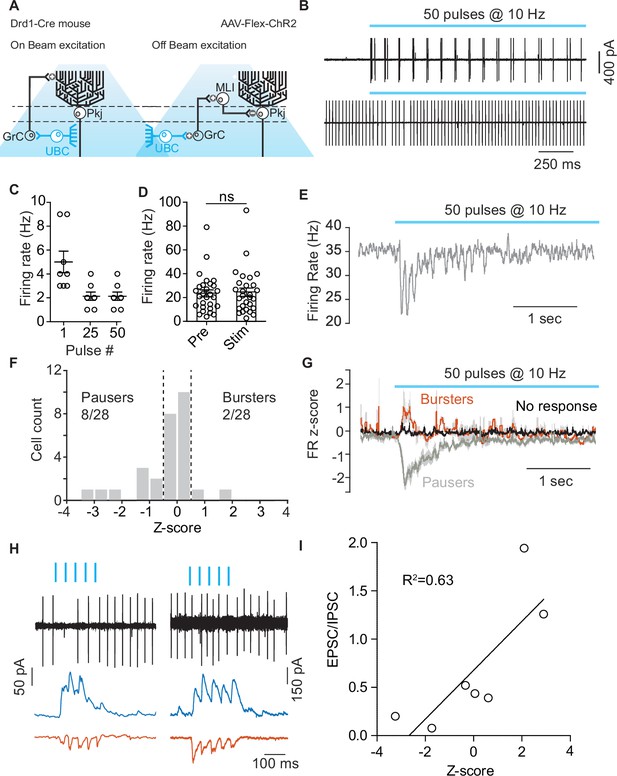
Optogenetic activation of unipolar brush cells can modulate Purkinje cell activity.
(A) Diagram of experimental setup highlighting the two pathways (On-beam, Off-beam) for unipolar brush cells (UBCs) to modulate Purkinje (Pkj) cell firing. Cre-dependent AAV5-EF1a-DIO-ChR2-eYFP was expressed in UBCs using a dopamine type 1 receptor (Drd1)-Cre mouse line. Pkj cells were recorded in cell-attached and/or whole-cell voltage clamp configuration. (B) Top: Example trace from a cell-attached recording of a ChR2+ UBC which increased its firing rate (FR) during light stimulation (460 nm, 10 Hz, 50 pulses, 1 ms pulse width). Bottom: Cell-attached recording from a Pkj cell. Recordings were done in the same slice, but not paired. (C) The number of action potentials evoked in ChR2+ UBCs by a train of light pulses in response to the 1st, 25th, and 50th individual pulse (n = 8 cells). (D) A comparison of average FR of Pkj cells for trials before optogenetic stimulation of UBCs (Pre) and for trials with optogenetic activation (Stim) shows no significant difference. (E) The average FR (five trials) of a single Pkj cell during the activation of nearby UBCs. Example cell transiently decreased its FR in response to UBC activation. (F) Histogram of average z-scores of FRs during the 500 ms period following the first optogenetic pulse. Dashed lines indicate cut-off for categorization of response as either ‘Burster’ z-score >0.5 or ‘Pauser’ z-score <–0.5. (G) Average z-score of FRs across cells. ‘Pausers’ (n = 8 cells) indicates data for cells that had z-score <–0.5, ‘Bursters’ (n = 2 cells) indicates data for cells that had z-score >0.5, ‘No response’ (n = 20 cells) include all other cells. Shaded regions represent SEM. (H) Cell-attached and post-synaptic current recordings from example Pkj cells; left ‘Pauser’, right ‘Burster’. Top traces show cell-attached recordings before break-in. After break-in inhibitory post-synaptic currents (IPSCs) (blue trace) and excitatory post-synaptic currents (EPSCs) (red trace) were recorded. (I) FR z-score after optogenetic activation of UBCs plotted against EPSC/IPSC total charge ratio. Data show a correlation between relative inhibitory input and pausing in Pkj cells (linear regression, R2 = 0.63, F1,5 = 8.82, p=0.0312).
-
Figure 6—source data 1
Numerical data for graphs in Figure 6.
- https://cdn.elifesciences.org/articles/76912/elife-76912-fig6-data1-v2.xlsx
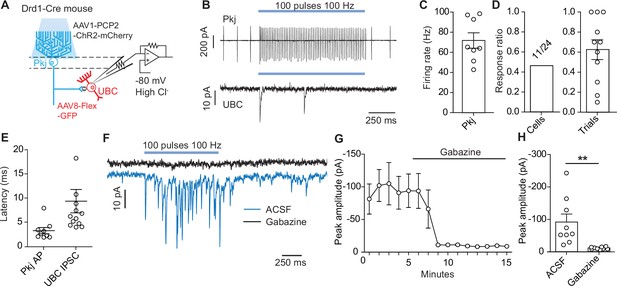
Optogenetic activation of Purkinje cells evokes short-latency inhibitory post-synaptic currents in unipolar brush cells.
(A) Diagram of experimental setup. Using a dopamine type 1 receptor (Drd1)-Cre mouse line a Cre-dependent green fuorescent protein (GFP) was expressed in unipolar brush cells (UBCs), Purkinje (Pkj) cells were transfected with AAV1-Pcp2-ChR2-mCherry. UBCs recorded in whole-cell voltage clamp configuration. Experiments were done with 5 µM NBQX, 2 µM CPP, 1 µM Strychnine, 1 µM CGP54626, and 2 µM LY354740 in the bath. (B) Top: Example trace from a cell-attached recording of a mCherry+ Pkj cell which increased its firing rate during light stimulation (460 nm, 100 pulses, 100 Hz, 0.5 ms pulse width). Bottom: Voltage clamp recording of UBC inhibitory post-synaptic currents (IPSCs). Recordings were done in the same slice but not paired. Viral incubation, 3 weeks. (C) Average firing rate of Pkj cells during light stimulation (n = 8 cells). (D) Ratio of successful IPSC responses. Left (cells): The proportion of cells with IPSCs evoked by optogenetic stimulation of Pkj cells (11/24, n = 24 cells). Right (trials): Ratio of trials in which IPSCs were successfully evoked. Each data point represents the mean from each cell (n = 11 cells). (E) The average latency to the first action potential in a Pkj cell (n = 9 cells) or first evoked IPSC in a UBC (n = 11 cells). Latency is calculated from the time of first pulse of 460 nm LED light. Each data point represents the mean from a single cell (5–10 trials). (F) Example data from one UBC shows a near complete inhibition of IPSCs (blue trace) after the application of 10 µM gabazine (black trace). Viral incubation, 5 weeks. (G) Time course of reduction of IPSC amplitude by gabazine (n = 9 cells). (H) Population data comparing peak IPSC amplitude before and after gabazine application (n = 9 cells, paired t-test, p=0.001).
-
Figure 7—source data 1
Numerical data for graphs in Figure 7.
- https://cdn.elifesciences.org/articles/76912/elife-76912-fig7-data1-v2.xlsx
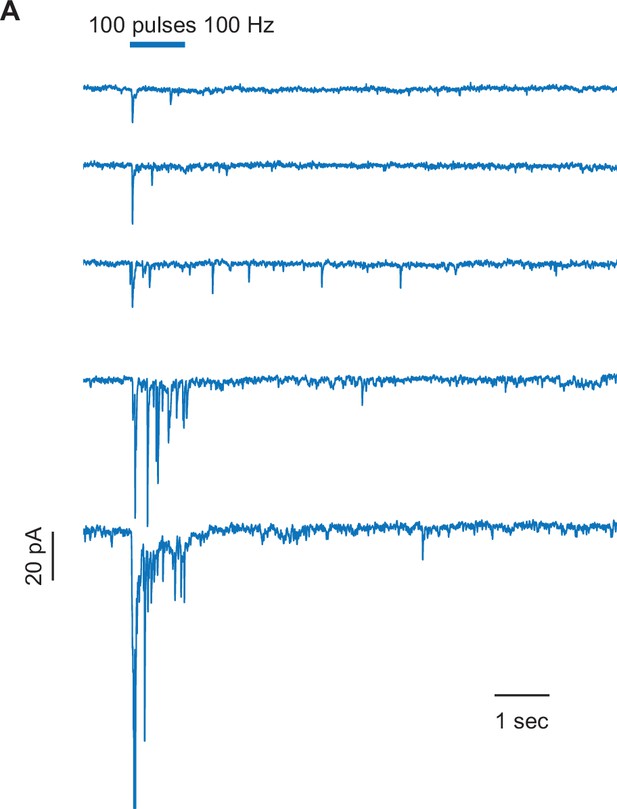
Inhibitory post-synaptic currents in unipolar brush cells in response to Purkinje cell optogenetic activation.
(A) Single trial example traces from five different unipolar brush cells (UBCs) show variability in the amplitude of response across cells, on the background of few spontaneous inhibitory post-synaptic currents (IPSCs), after optogenetic stimulation of Purkinje (Pkj) cells. Viral incubation time for each trace, sequentially: 22, 23, 29, 35, 39 days. Scale bar, 20 pA, 1 s.
-
Figure 7—figure supplement 1—source data 1
Numerical data for graphs in Figure 7—figure supplement 1.
- https://cdn.elifesciences.org/articles/76912/elife-76912-fig7-figsupp1-data1-v2.xlsx