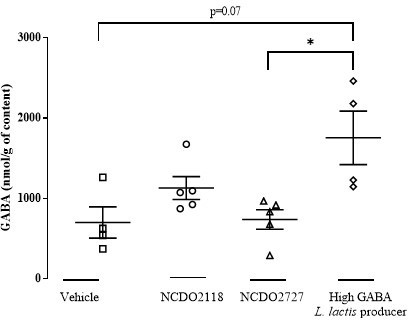
Lactococcus lactis NCDO2118 exerts visceral antinociceptive properties in rat via GABA production in the gastro-intestinal tract
Figures
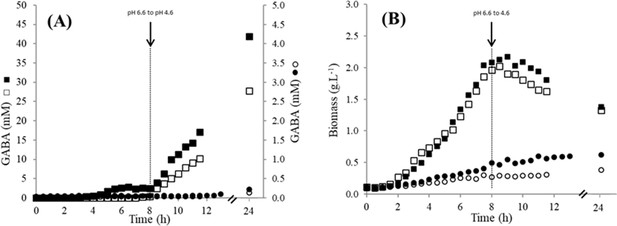
Growth and GABA production of the different Lactococcus lactis strains.
(A) γ-Aminobutyric acid (GABA) production (mM); (B) evolution of biomass (g/L) during growth of Lactococcus lactis NCDO2118 (□, ■) or NCDO2727 (○, ●) in M17 supplemented with glutamate (8 g/L), arginine (5 g/L), glucose (45 g/L), and NaCl (300 mM). Two independent duplicates were performed.
-
Figure 1—source data 1
Table of biomass and GABA concentrations for L. lactis NCDO2118 and NCDO2727 strains.
- https://cdn.elifesciences.org/articles/77100/elife-77100-fig1-data1-v1.xlsx
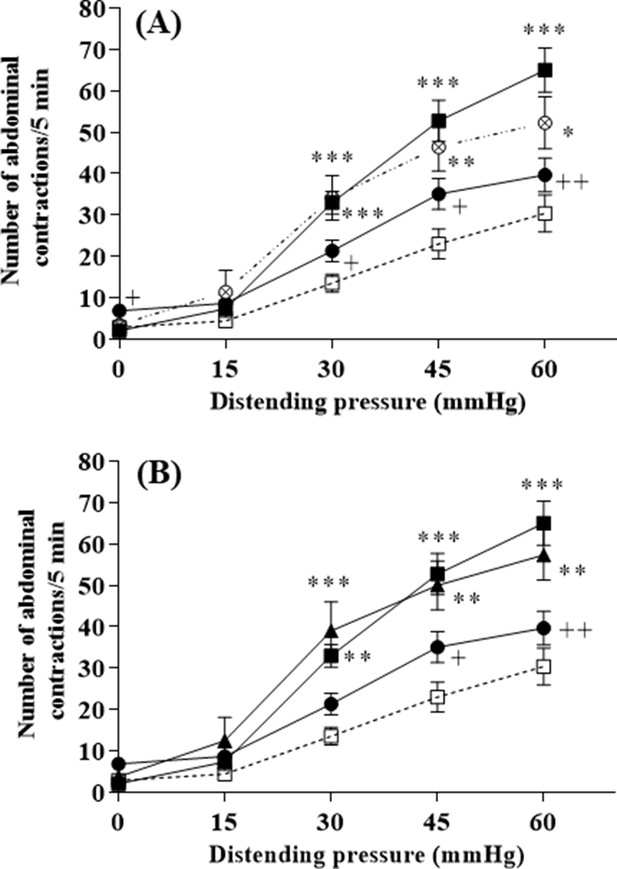
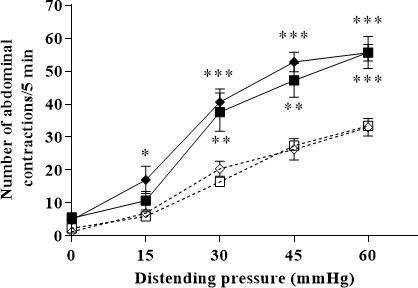
L. lactis effect on visceral hypersensitivity induced by stress in response to colorectal distension.
(A) Effect of 10-day oral administration of γ-aminobutyric acid (GABA)-producing Lactococcus lactis NCDO2118 (109 CFU per day) in presence or absence of glutamate on PRS-induced visceral hypersensitivity at all the distension pressures of colorectal distension (CRD; from 15 to 60 mmHg). Data are expressed as means ± SEM (n=9 for the ‘vehicle’ group [ ] and ‘PRS + vehicle’ group [
] and ‘PRS + vehicle’ group [ ]; n=7 for ‘PRS + NCDO2118’ group [
]; n=7 for ‘PRS + NCDO2118’ group [ ]; n=12 for the ‘PRS + NCDO2118 + glutamate’ group [
]; n=12 for the ‘PRS + NCDO2118 + glutamate’ group [ ]); (B) Effect of 10-day oral administration of GABA-producing L. lactis NCDO2118 and low GABA-producing L. lactis NCDO2727 (109 CFU per day) in presence of glutamate on PRS-induced visceral hypersensitivity at all the distension pressures of CRD. Data are expressed as means ± SEM (n=9 for the ‘vehicle’ group [
]); (B) Effect of 10-day oral administration of GABA-producing L. lactis NCDO2118 and low GABA-producing L. lactis NCDO2727 (109 CFU per day) in presence of glutamate on PRS-induced visceral hypersensitivity at all the distension pressures of CRD. Data are expressed as means ± SEM (n=9 for the ‘vehicle’ group [ ] and ‘PRS + vehicle’ group [
] and ‘PRS + vehicle’ group [ ]; n=12 for the ‘PRS + NCDO2118 + glutamate’ group [
]; n=12 for the ‘PRS + NCDO2118 + glutamate’ group [ ]; n=8 for the ‘PRS + NCDO2727 + glutamate’ group [
]; n=8 for the ‘PRS + NCDO2727 + glutamate’ group [ ]). *p<0.05, **p<0.01, ***p<0.001 vs. basal values for animals treated with vehicle. +p<0.05, ++p<0.01 vs. values for stressed animals treated with vehicle.
]). *p<0.05, **p<0.01, ***p<0.001 vs. basal values for animals treated with vehicle. +p<0.05, ++p<0.01 vs. values for stressed animals treated with vehicle.
-
Figure 2—source data 1
Effect of 10-day oral administration of GABA-producing L. lactis NCDO2118 on PRS-induced visceral hypersensitivity.
- https://cdn.elifesciences.org/articles/77100/elife-77100-fig2-data1-v1.txt
-
Figure 2—source data 2
Effect of 10-day oral administration of GABA-producing L. lactis NCDO2118 and low GABA-producing L. lactis NCDO2727 on PRS-induced visceral hypersensitivity.
- https://cdn.elifesciences.org/articles/77100/elife-77100-fig2-data2-v1.txt
-
Figure 2—source data 3
Incidence of 10-day oral administration of L. lactis NCDO2118 in presence of glutamate on basal visceral sensitivity.
- https://cdn.elifesciences.org/articles/77100/elife-77100-fig2-data3-v1.txt
-
Figure 2—source data 4
Incidence of 10-day oral administration of glutamate on basal visceral sensitivity and PRS-induced visceral hypersensitivity.
- https://cdn.elifesciences.org/articles/77100/elife-77100-fig2-data4-v1.txt
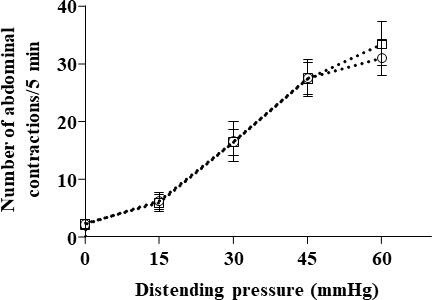
Incidence of 10-day oral administration of L. lactis NCDO2118 in presence of glutamate on basal visceral sensitivity at all the distension pressures of CRD (from 15 to 60 mmHg).
Data are expressed as means ± SEM (n=10 for the “vehicle” group ( ); n=12 for “NCDO2118+glutamate” group (
); n=12 for “NCDO2118+glutamate” group ( )). No statistical difference was found between the two groups (unpaired t test).
)). No statistical difference was found between the two groups (unpaired t test).
Incidence of 10-day oral administration of glutamate on basal visceral sensitivity and PRS-induced visceral hypersensitivity at all the distension pressures of CRD (from 15 to 60 mmHg).
Data are expressed as means ± SEM (n=9 for the “vehicle” group ( )) and “PRS+vehicle” group (
)) and “PRS+vehicle” group ( ); n=10 for “vehicle+glutamate” group (
); n=10 for “vehicle+glutamate” group ( ); n=10 for the “PRS+vehicle+glutamate” group (
); n=10 for the “PRS+vehicle+glutamate” group ( ). * P<0.05, **P<0.01, *** P<0.001 vs. basal values for animals treated with vehicle.
). * P<0.05, **P<0.01, *** P<0.001 vs. basal values for animals treated with vehicle.

The anti-nociceptive effect of L. lactis NCDO2118.
(A) Effect of 10-day oral administration of γ-aminobutyric acid (GABA)-producing Lactococcus lactis NCDO2118 and non-GABA producing NCDO2118 ΔgadB mutant (109 CFU per day) on PRS-induced visceral hypersensitivity at all the distension pressures of colorectal distension (CRD; from 15 to 60 mmHg). Data are expressed as means ± SEM (n=9 for the ‘vehicle’ group [ ] and ‘PRS + vehicle’ group [
] and ‘PRS + vehicle’ group [ ]; n=12 for the ‘PRS + NCDO2118 + glutamate’ group [
]; n=12 for the ‘PRS + NCDO2118 + glutamate’ group [ ]; n=13 for the ‘PRS + NCDO2118 ΔgadB + glutamate’ group [
]; n=13 for the ‘PRS + NCDO2118 ΔgadB + glutamate’ group [ ]); (B) Effect of 10-day oral administration of GABA-producing L. lactis NCDO2118 (109 CFU per day) on PRS-induced visceral hypersensitivity at all the distension pressures of CRD in presence or not of GABAB receptor antagonist SCH-50911 (3 mg/kg bw, IP). Data are expressed as means ± SEM (n=9 for the ‘vehicle’ group [
]); (B) Effect of 10-day oral administration of GABA-producing L. lactis NCDO2118 (109 CFU per day) on PRS-induced visceral hypersensitivity at all the distension pressures of CRD in presence or not of GABAB receptor antagonist SCH-50911 (3 mg/kg bw, IP). Data are expressed as means ± SEM (n=9 for the ‘vehicle’ group [ ] and ‘PRS + vehicle’ group [
] and ‘PRS + vehicle’ group [ ]; n=12 for the ‘PRS + NCDO2118 + glutamate’ group [
]; n=12 for the ‘PRS + NCDO2118 + glutamate’ group [ ]; n=7 for the ‘PRS + NCDO2118 + glutamate + SCH-50911’ group [
]; n=7 for the ‘PRS + NCDO2118 + glutamate + SCH-50911’ group [ ]). *p<0.05, **p<0.01, ****p<0.0001 vs. basal values for animals treated with vehicle. +p<0.05, +++p<0.001 vs. values for stressed animals treated with vehicle. $p<0.05 vs. for stressed animals treated with NCDO2118 + glutamate.
]). *p<0.05, **p<0.01, ****p<0.0001 vs. basal values for animals treated with vehicle. +p<0.05, +++p<0.001 vs. values for stressed animals treated with vehicle. $p<0.05 vs. for stressed animals treated with NCDO2118 + glutamate.
-
Figure 3—source data 1
Effect of 10-day oral administration of GABA-producing L. lactis NCDO2118 and non-GABA producing NCDO2118 delta-gadB mutant on PRS-induced visceral hypersensitivity.
- https://cdn.elifesciences.org/articles/77100/elife-77100-fig3-data1-v1.txt
-
Figure 3—source data 2
Effect of 10-day oral administration of GABA-producing L. lactis NCDO2118 on PRS-induced visceral hypersensitivity in presence or not of GABAB receptor antagonist SCH-50911.
- https://cdn.elifesciences.org/articles/77100/elife-77100-fig3-data2-v1.txt
-
Figure 3—source data 3
Table of biomass and GABA concentrations for L. lactis NCDO2118 and its ΔgadB mutant.
- https://cdn.elifesciences.org/articles/77100/elife-77100-fig3-data3-v1.xlsx
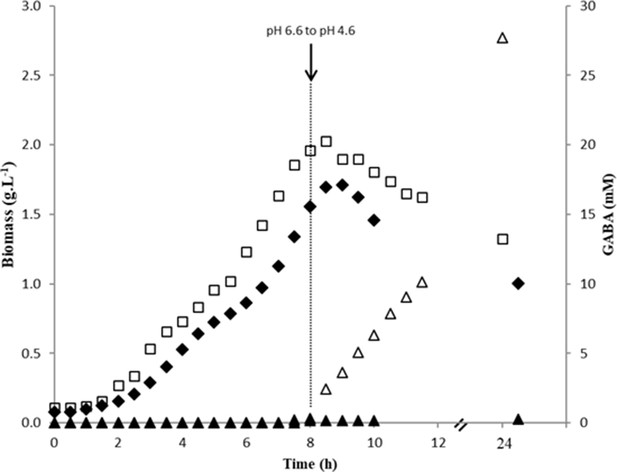
Evolution of biomass (g/L) during growth of Lactococcus lactis NCDO2118 ΔgadB mutant (♦) and evolution of γ-aminobutyric acid (GABA) production (▲) compared to L. lactis NCDO2118 (biomass □ and GABA ∆) in M17 supplemented with glutamate (8 g/L), arginine (5 g/L), glucose (45 g/L), and NaCl (300 mM).
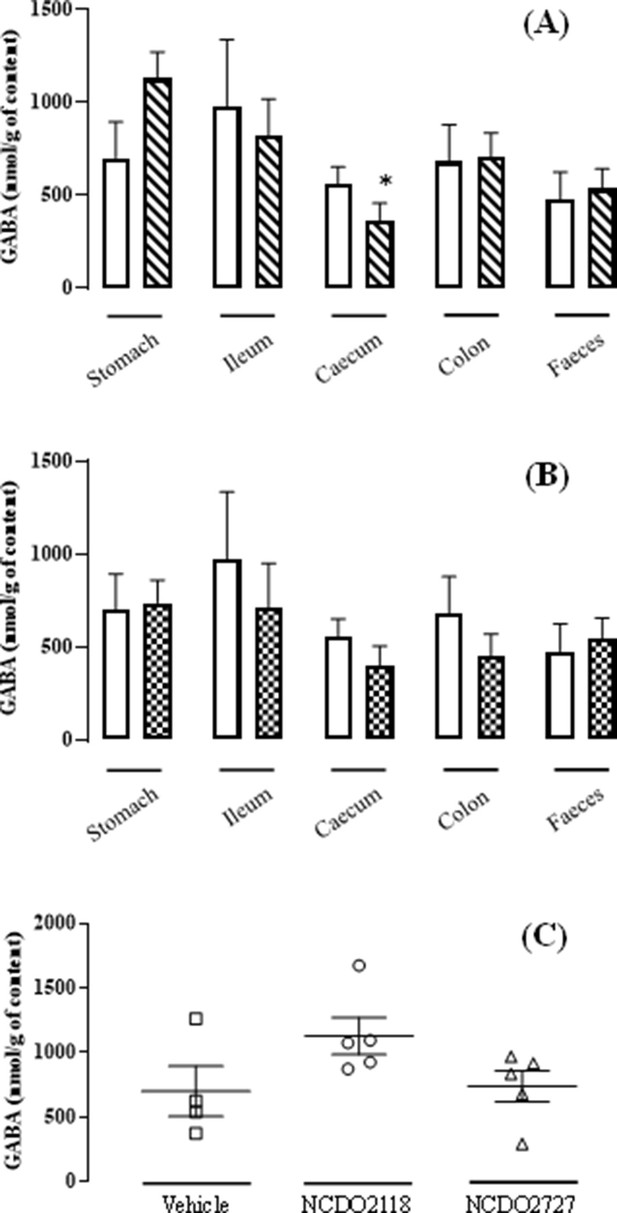
L. lactis effect on the GABA production all along the gastro-intestinal tract.
(A) Variation of the γ-aminobutyric acid (GABA) concentration all along the gastro-intestinal tract for the group ‘NCDO2118 + glutamate’ ( ) vs. the group ‘vehicle’ (
) vs. the group ‘vehicle’ ( ) after a 10-day daily oral administration at 109 CFU per day. (B) Variation of the GABA concentration all along the gastro-intestinal tract for the group ‘NCDO22727 + glutamate’ (
) after a 10-day daily oral administration at 109 CFU per day. (B) Variation of the GABA concentration all along the gastro-intestinal tract for the group ‘NCDO22727 + glutamate’ ( ) vs. group ‘vehicle’ (
) vs. group ‘vehicle’ ( ) after a 10-day daily oral administration at 109 CFU per day. (C) Variation of the GABA concentration in the gastric content for the group ‘vehicle’ (
) after a 10-day daily oral administration at 109 CFU per day. (C) Variation of the GABA concentration in the gastric content for the group ‘vehicle’ ( ), the group ‘NCDO2118 + glutamate’ (
), the group ‘NCDO2118 + glutamate’ ( ), and the group ‘NCDO2727 + glutamate’ (
), and the group ‘NCDO2727 + glutamate’ ( ) after a 10-day daily oral administration at 109 CFU per day. Data are expressed as means ± SEM. Non-parametric Kruskal-Wallis test, supplemented by Dunn’s multiple comparison test, revealed no statistical differences between samples except caecum vs. stomach for the NCDO2118 strain (*p<0.05).
) after a 10-day daily oral administration at 109 CFU per day. Data are expressed as means ± SEM. Non-parametric Kruskal-Wallis test, supplemented by Dunn’s multiple comparison test, revealed no statistical differences between samples except caecum vs. stomach for the NCDO2118 strain (*p<0.05).
-
Figure 4—source data 1
Variation of the GABA concentration all along the gastro-intestinal tract for NCDO2118+glutamate.
- https://cdn.elifesciences.org/articles/77100/elife-77100-fig4-data1-v1.txt
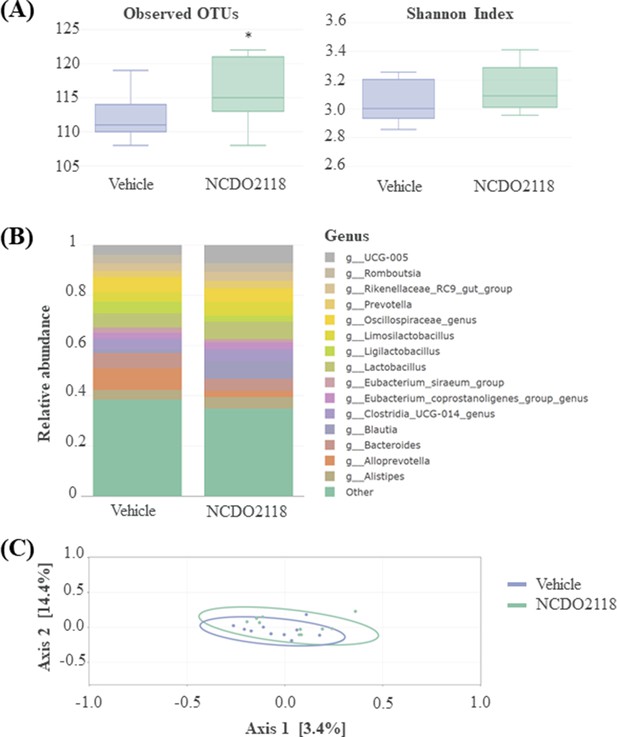
Overview of the faecal microbiota after a 10-day daily oral administration of γ-aminobutyric acid (GABA)-producing L. lactis NCDO2118 (109 CFU per day) in presence of glutamate vs. ‘vehicle’ group.
(A) Alpha diversity within faecal samples. Two measures are shown: mean observed number of OTUs per sample, an estimate of richness (left panel), and Shannon Index, indicating the evenness of the sample (right sample). One-way ANOVA followed by the post hoc pairwise Tukey’s test revealed no statistical differences between samples (p>0.05). (B) Top 15 dominant bacterial genera in faecal samples; (C) MDS ordination plot of the Bray-Curtis distance between samples, a representation of phylogenetic similarity. PERMANOVA revealed no statistical differences between samples (p>0.05).
Additional files
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/77100/elife-77100-transrepform1-v1.docx
-
Supplementary file 1
Specific glutamate decarboxylase activity (µmol/min.mg) of the two Lactococcus lactis strains after 7 or 24 hr of growth in M17 supplemented with glutamate (8 g/L), arginine (5 g/L), and NaCl (300 mM; n=3 for each of the two cultures replicates in bioreactor).
- https://cdn.elifesciences.org/articles/77100/elife-77100-supp1-v1.docx
-
Supplementary file 2
In vitro kinetics of γ-aminobutyric acid (GABA) production by Lactococcus lactis NCDO2118 and L. lactis NCDO2727.
GABA production rates (µmol/min) were estimated when bacteria or control vehicle, were in presence of 0.2% (w/v) glutamate at 37°C and pH=4.4, either in 100 mM acetate buffer or with gastric content of naive rat. Two independent replicates were performed.
- https://cdn.elifesciences.org/articles/77100/elife-77100-supp2-v1.docx
-
Supplementary file 3
Non-parametric comparison of taxa relative abundancies between conditions.
Highlighted lines show conditions with significant differences in relative abundancies after Kruskal-Wallis test (p<0.07). Purple highlight indicates that the taxon is more abundant in the faecal microbiota of vehicle-treated animals, green highlight indicates that the taxon is more abundant in the faecal microbiota of NCDO2118-treated animals.
- https://cdn.elifesciences.org/articles/77100/elife-77100-supp3-v1.docx
-
Supplementary file 4
List of primers.
Primers used for the inactivation of gadB in Lactococcus lactis NCDO2118.
- https://cdn.elifesciences.org/articles/77100/elife-77100-supp4-v1.docx