Unique neural coding of crucial versus irrelevant plant odors in a hawkmoth
Figures
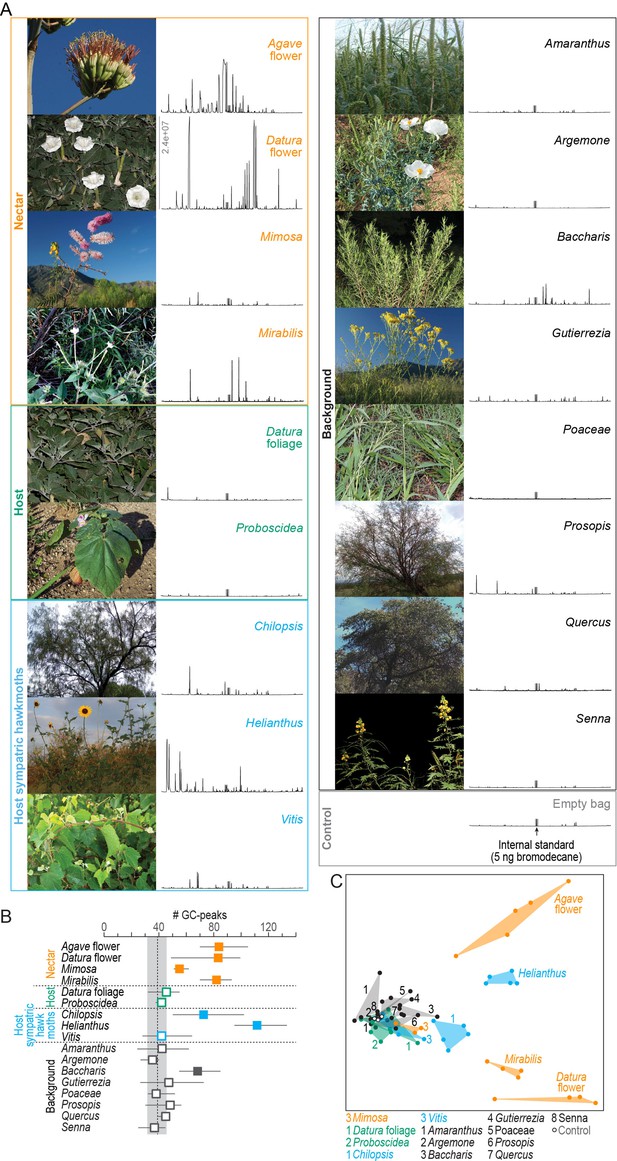
Chemical analysis of nocturnal headspaces collected from plants in the habitat of M. sexta in Southern Arizona.
(A) Representative photographs (left) and chromatographs (right) of each headspace collection. x-axis of chromatographs, retention time; y-axis, abundance, same scale for all headspaces, maximum abundance indicated in Datura flower headspace; gray bar, internal standard (5 ng 1-bromodecane). (B) Number of GC-peaks. Squares, average values of 3–5 individual plant samples; whiskers, range; dotted line and gray area, average and range of control values obtained from nocturnal collections in the same habitat with empty bags (n = 2), and with unused filter material (n = 1); open squares, within control range; filled squares, outside control range. (C) Non-metric multidimensional scaling plot (Bray–Curtis, 2D stress: 0.09) based on a nontargeted analysis (https://xcmsonline.scripps.edu; Tautenhahn et al., 2012) of 69 chromatograms (Figure 1—source data 1). Color code of plant samples as in (B).
-
Figure 1—source data 1
Related to Figure 1C.
XCMS analysis of 69 headspaces.
- https://cdn.elifesciences.org/articles/77429/elife-77429-fig1-data1-v1.xlsx
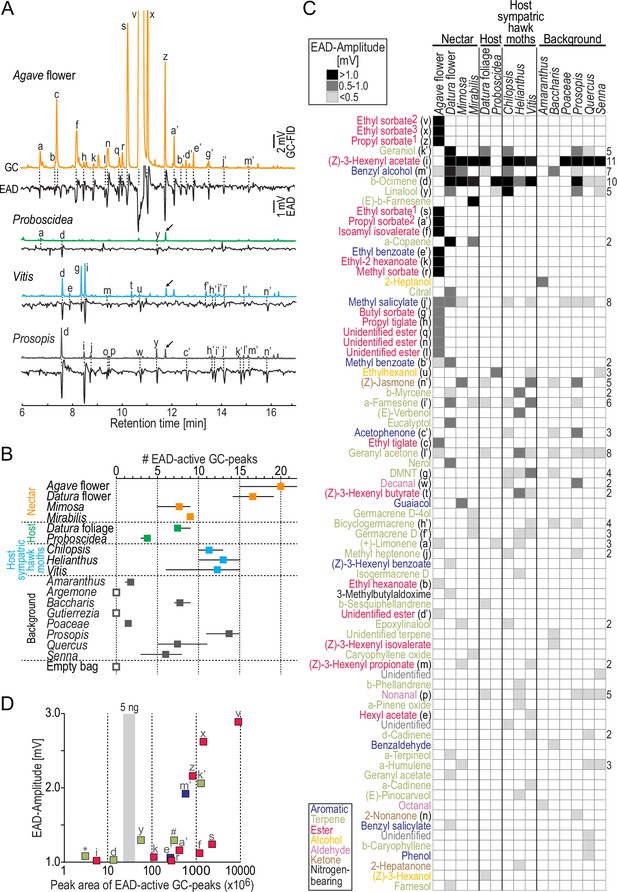
Antennal responses of M. sexta females to nocturnal headspaces of plants.
(A) Examples of gas chromatography-coupled electro-antennographic detection (GC-EAD) recordings after stimulation with four plant headspaces representing nectar sources (Agave flower), host plants (Proboscidea), host plants of sympatric hawkmoths (Vitis), and background plants (Prosopis). Upper traces, gas chromatograph-coupled flame ionization detection (GC-FID); lower traces, electro-antennographic detection (EAD) of female M. sexta. Letters indicate EAD-active GC-peaks (labeled in C) that evoked a response in at least three animals. Arrows, internal standard: 5 ng 1-bromodecane; in Agave flower, the internal standard co-eluted with GC-peak ‘z,’ and GC-peaks ‘v’ and ‘x’ are cropped. (B) Number of EAD-active GC-peaks per plant species. We stimulated the antennae (4–7 moths/headspace) with the same representative sample per headspace type. Filled squares, average values; whiskers, range; open squares, no active GC-peaks detected in three moths. Each moth was tested only once. (C) Antennal responses towards GC-peaks (rows) present in headspace (columns). Each cell in the heat map represents the median EAD amplitude of on average five moths (range: 4–7) per headspace. Rows are sorted by EAD amplitude (Figure 2—source data 1); magnitude of response is coded by shades of gray (see inset at top); empty cells, no response/GC fraction not present. Color code of compounds according to chemical class (see inset at bottom). Numbers next to ethyl sorbate and propyl sorbate label different enantiomers present in Agave flower and depict their order by retention time; DMNT, (E)–4,8-dimethyl-1,4,7-nonatriene. Numbers to the right of the heat map depict how often a given compound was present; rows without numbers indicate compounds found only in one headspace. (D) Effectiveness of the strongest antennal stimulants. x-axis, concentration of compounds derived from their peak area (logarithmic scale); y-axis, median EAD amplitudes ≥ 1 mV; gray vertical bar, range of peak areas of the internal standard 1-bromodecane (5 ng). For compounds present in more than one plant species, the lowest concentration eliciting a median EAD amplitude ≥ 1 mV was chosen; letters indicate compounds as in (C); *α-copaene; #(E)-β-farnesene. Peak area of ethyl sorbate2 (‘v’) shows lower limit of concentration as the GC seemed overloaded with this odor.
-
Figure 2—source data 1
Related to Figure 2C.
Gas chromatography-coupled electro-antennographic detection (GC-EAD) results from 80 antennae.
- https://cdn.elifesciences.org/articles/77429/elife-77429-fig2-data1-v1.xlsx
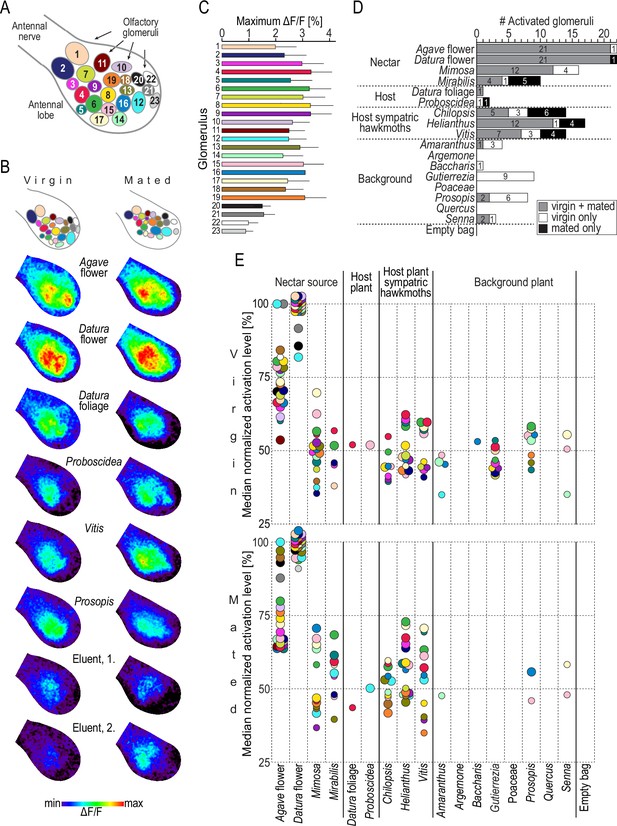
Headspace-evoked activity patterns in the antennal lobe of female M. sexta.
(A) Schematic of 23 olfactory glomeruli at the dorsal surface of the right antennal lobe. Entrance of the antennal nerve is in the upper-left corner. Numbers, glomeruli identification as in Bisch-Knaden et al., 2018. (B) Examples of in vivo calcium imaging recordings after stimulation with plant headspaces representing nectar sources (Agave flower, Datura flower), host plants (Datura foliage, Proboscidea), host plants of sympatric hawkmoths (Vitis), background plants (Prosopis), and the eluent dichloromethane (first and second stimulations at the beginning and end of the experiment). False-color-coded imaging results of the right antennal lobe in a virgin (left column) and a mated female (right column) normalized to their highest response (see color bar). Top row, schematic of individual antennal lobes, colors as in (A). (C) Maximum increase of fluorescence in 23 identified glomeruli. Graph depicts for each glomerulus (color code as in A) the average maximum responses (bars) and 1 standard deviation (whiskers) of 10 virgin and 10 mated females after stimulation with plant headspaces. In 69% of 460 cases (20 maximum values in 23 glomeruli), Datura flower was the headspace eliciting the maximum response, and in 17% it was Agave flower. (D) Number of activated glomeruli in the antennal lobe depending on female mating status. A glomerulus was scored as activated if its headspace-evoked response was different from the averaged response to the two stimulations with the eluent dichloromethane (p<0.01, Friedman test with Dunn’s multiple-comparisons test). For the identity of glomeruli activated by each plant headspace, see Table 2. (E) Activity levels evoked by plant headspace in individual glomeruli in the antennal lobe. Colored dots represent median normalized responses of activated glomeruli in 10 virgin (top) and 10 mated (bottom) females; color code of glomeruli as in (A). Only values of activated glomeruli are shown (small circles, p<0.01, large circles, p<0.001, Friedman test with Dunn’s multiple comparisons test, Figure 3—source data 1).
-
Figure 3—source code 1
Custom-written software for processing calcium imaging data in IDL (L3Harris Geospatial).
- https://cdn.elifesciences.org/articles/77429/elife-77429-fig3-code1-v1.zip
-
Figure 3—source data 1
Calcium imaging results from 10 virgin and 10 mated females (Figure 3E).
- https://cdn.elifesciences.org/articles/77429/elife-77429-fig3-data1-v1.xlsx
Tables
Headspace collections from plants at the Santa Rita Experimental Range in Arizona (US).
| Plant species (plant family), common name | Type of sample | Nectar source for adult M. sexta | Host plant for M. sexta larvae | Larval host plant for sympatric hawkmoths | Nocturnal pollination | |
|---|---|---|---|---|---|---|
| Agave palmeri (Asparagaceae), Palmer’s century plant | Flower | X | − | − | X | |
| Datura wrightii (Solanaceae), Sacred datura | Flower | Branch | X | X | X* | X |
| Mimosa dysocarpa (Fabaceae), Velvetpod | Flowering branch | X | − | − | X | |
| Mirabilis longiflora (Nyctaginaceae), Sweet four o'clock | Flowering branch | X | − | − | X | |
| Proboscidea parviflora (Martyniaceae), Devil’s claw | Flowering plant | − | X | − | − | |
| Chilopsis linearis (Bignoniaceae), Desert willow | Branch with seeds | − | − | X† | − | |
| Helianthus annuus (Asteraceae), Common sunflower | Flowering plant | − | − | X‡ | X | |
| Vitis arizonica (Vitaceae), Wild grape | Branch | − | − | X§ | − | |
| Amaranthus palmeri (Amaranthaceae), Carelessweed | Flowering plant | − | − | − | − | |
| Argemone pleiacantha (Papaveraceae), Prickly poppy | Flowering branch | − | − | − | − | |
| Baccharis salicifolia (Asteraceae), Seepwillow | Branch with buds | − | − | − | − | |
| Gutierrezia sarothrae (Asteraceae), Snakeweed | Flowering plant | − | − | − | − | |
| Poaceae spp,, Grass | Tuft of grass | − | − | − | − | |
| Prosopis velutina (Fabaceae), Velvet mesquite | Branch | − | − | − | − | |
| Quercus emoryi (Fagaceae), Emory oak | Branch | − | − | − | − | |
| Senna hirsuta v glaberrima (Fabaceae), Woolly Senna | Flowering plant | − | − | − | − | |
-
*
M. quinquemaculata.
-
†
M. rustica, M. florestan.
-
‡
M. muscosa.
-
§
Eumorpha achemon.
Headspace-activated glomeruli independent and dependent of mating status.
| Glomerulus | Response independent of mating status | Response only before mating | Response only after mating |
|---|---|---|---|
| 1* | Agave, Datura | Mirabilis, Helianthus, Gutierrezia | |
| 2 | Agave, Datura, Mirabilis, Helianthus | Mimosa, Vitis, Gutierrezia | |
| 3 | Agave, Datura, Mirabilis, Helianthus | Mimosa, Chilopsis, Vitis, Gutierrezia | |
| 4* | Agave, Datura, Mimosa, Mirabilis, Datura foliage, Chilopsis, Helianthus, Vitis | Gutierrezia | |
| 5 | Agave, Datura, Helianthus, Vitis | Mimosa, Chilopsis, Gutierrezia, Prosopis | Mirabilis |
| 6* | Agave, Datura, Mimosa, Mirabilis, Chilopsis, Helianthus, Vitis | Gutierrezia, Prosopis | |
| 7 | Agave, Datura, Helianthus | Mimosa, Vitis, Gutierrezia | |
| 8 | Agave, Datura, Mimosa, Chilopsis, Helianthus, Vitis | Gutierrezia | |
| 9 | Agave, Datura, Mimosa, Helianthus, Vitis | Chilopsis, Gutierrezia | |
| 10 | Agave, Datura | ||
| 11 | Agave, Datura | ||
| 12* | Agave, Datura, Mimosa | Amaranthus | Mirabilis, Proboscidea, Chilopsis, Helianthus, Vitis |
| 13* | Agave, Datura, Mimosa | Prosopis | Mirabilis, Chilopsis, Helianthus, Vitis |
| 14 | Agave, Datura, Mimosa, Amaranthus | Prosopis, Senna | Chilopsis, Helianthus |
| 15 | Agave, Datura, Mimosa, Chilopsis, Helianthus, Vitis, Prosopis, Senna | Proboscidea, Amaranthus | Mirabilis |
| 16 | Agave, Datura, Mimosa, Chilopsis, Helianthus, Prosopis | Amaranthus, Baccharis | Vitis |
| 17* | Agave, Datura, Mimosa, Helianthus, Vitis, Senna | Prosopis | Mirabilis, Chilopsis |
| 18* | Agave, Datura, Mimosa | Chilopsis, Helianthus | |
| 19* | Agave, Datura, Mimosa, Helianthus | Prosopis | Chilopsis, Vitis |
| 20* | Agave, Datura | ||
| 21* | Agave, Datura | ||
| 22 | Agave | ||
| 23 | Datura |
-
Font format depicts type of plant headspace: nectar source of M. sexta, host plant of M. sexta, host plant of sympatric hawk moths, background plant.
-
*
Glomerulus whose activation level is positively correlated with odor-guided behavior of virgin females in wind tunnel experiments (Bisch-Knaden et al., 2018).





