Human hippocampal responses to network intracranial stimulation vary with theta phase
Figures
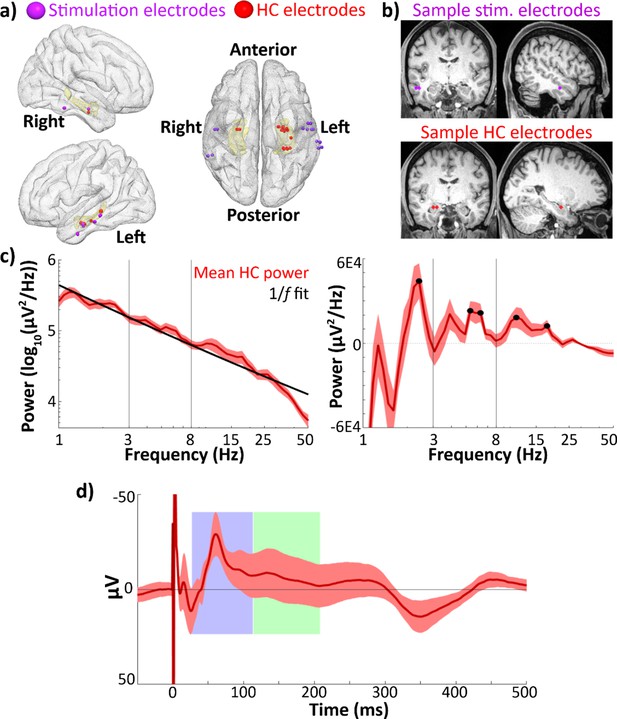
Hippocampal recordings and evoked response.
(a) Group-level electrode localization for lateral temporal stimulating electrodes (n = 14) and hippocampal recording electrodes (n = 18) plotted on an MNI template (Holmes et al., 1998). Amygdala and hippocampus are highlighted in yellow. Electrodes are enlarged ~500% for visualization purposes. Note: Imaging was unavailable in one subject (see Materials and methods: Electrode localization). Hippocampal recording electrodes did not align to the template brain hippocampus in one subject and are not shown in this image. (b) Locations of lateral temporal stimulating electrodes (top) and recording electrodes in hippocampus (bottom) in one sample participant. (c) Mean power spectral densities across all hippocampal recording electrodes (n = 23). Power was assessed during a recorded pre-stimulation rest period. Left: Mean power spectral density (red) and estimated 1/f fit (black). Right: Mean 1/f-corrected power spectral density across hippocampal recording electrodes. Significant peaks in oscillatory power marked in black. Error bars indicate ±1 standard error of the mean (SEM) across electrodes. (d) Phase-balanced grand average hippocampal evoked potential (EP) (i.e., 0°, 90°, 180°, and 270° phase bins contribute equally to the average) elicited by lateral temporal stimulation. Error bars indicate ±1 SEM across hippocampal recording electrodes. Identified early and late negative components are highlighted in blue and green. The y-axis shows negative values in the upwards direction to emphasize the negative-going EP components of interest.
Rotating view of the group-level electrode localization shown in Figure 1a.
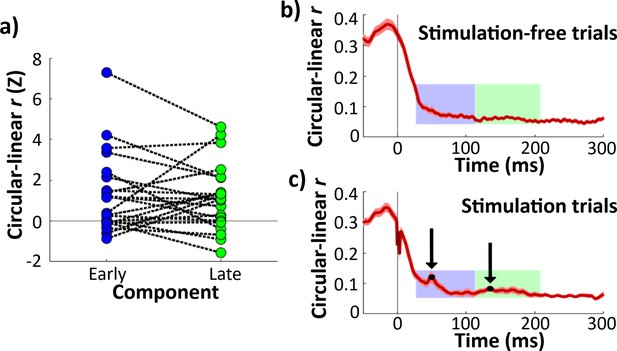
Continuous theta phase predicted response amplitude during early and late evoked-potential (EP) components.
(a) Z-scored circular–linear correlation r for the relationship between hippocampal theta phase at stimulation onset and EP component amplitude. Each line represents one electrode. Z-scores are shown for early (left) and late (right) components. Horizontal line shows chance-level circularity. Both early and late components showed significant theta circularity (**p < 0.01). (b) Circular–linear r plotted for each timepoint for stimulation-free trials, Error bars indicate ±1 standard error of the mean (SEM) across hippocampal recording electrodes. Early and late component timecourses highlighted in blue and green for reference. (c) As (b), for stimulation trials. Local maxima denoted in black.
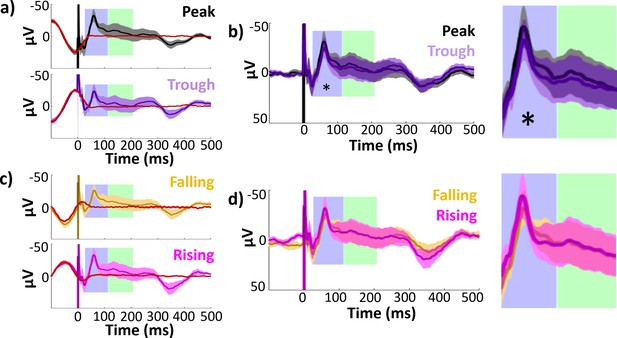
Hippocampal evoked potentials (EPs) and isolated responses binned according to theta phase at stimulation delivery.
Early and late component timecourses highlighted in blue and green for reference. (a) Mean hippocampal EPs elicited by lateral temporal stimulation at theta peak (black) and trough (purple), alongside phase-matched stimulation-free trials (red; see Materials and methods: Comparison of stimulation trials to phase-matched stimulation-free trials). Error bars indicate ±1 standard error of the mean (SEM) across recording electrodes. Theta oscillation is visible around stimulation at t = 0. The isolated response was obtained by subtracting the mean phase-matched stimulation-free trial from the EP. (b) Isolated hippocampal evoked responses to peak and trough stimulation. The non-evoked oscillatory component is abolished in the isolated response. Asterisk indicates isolated response components with significant amplitude differences across phase bins at 180° intervals (i.e., peak versus trough or rising versus falling trials; *p ≤ 0.05). Left: Full timecourse. Right: Enlarged panels showing component timecourses. (c, d) As in (a) and (b), for stimulation at theta falling (yellow) versus rising (magenta) phase angles.
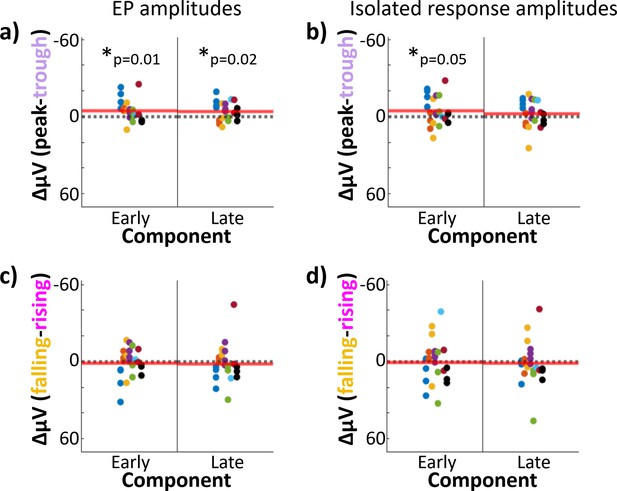
Stimulation at theta peak versus trough produced differences in early-component amplitude.
Hippocampal component amplitudes plotted by theta phase of stimulation. Each dot represents one electrode; dot color indicates participant-of-origin. Red line indicates the mean difference across electrodes. Difference between hippocampal evoked-potential (EP) component amplitudes elicited by lateral temporal stimulation delivered at peak versus trough (a) and falling versus rising (c) phases. Asterisk indicates components with significant amplitude differences across phase bins at 180° intervals (i.e., peak versus trough or rising versus falling trials; *p ≤ 0.05). Left: Early component amplitude difference. Right: Late component amplitude difference. (b, d) As in (a) and (c), but for the isolated hippocampal response (i.e., EP minus phase-matched stimulation-free trials).
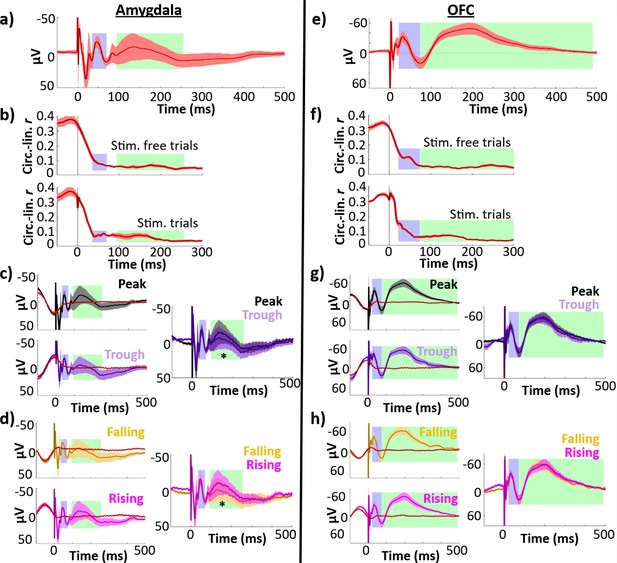
Control regions showed less temporal specificity in their phase–amplitude relationships and no difference in peak versus trough or falling versus rising early response amplitudes.
Left column, amygdala (n = 9 electrodes); right column, OFC (n = 22 electrodes). Early (blue) and late (green) components are highlighted. Error bars indicate ±1 standard error of the mean (SEM) across electrodes. (a, e) Phase-balanced grand average evoked potential (EP). (b, f) Phase–amplitude circular–linear r plotted for each timepoint in the peri-stimulation period for stimulation trials (top) and stimulation-free trials (bottom). (c, g) Left: Mean EPs elicited by lateral temporal stimulation at local theta peak (black) and trough (purple), alongside phase-matched stimulation-free trials (red; see Materials and methods: Comparison of stimulation trials to phase-matched stimulation-free trials). The isolated response was obtained by subtracting the mean phase-matched stimulation-free trial from the EP. Right: Isolated evoked responses to peak and trough stimulation. Asterisk indicates isolated response components with significant amplitude differences across phase bins at 180° intervals (i.e., peak versus trough or rising versus falling trials; *p ≤ 0.05). (d, h) As in (c, g), for stimulation at local theta falling (yellow) versus rising (magenta) phase angles.
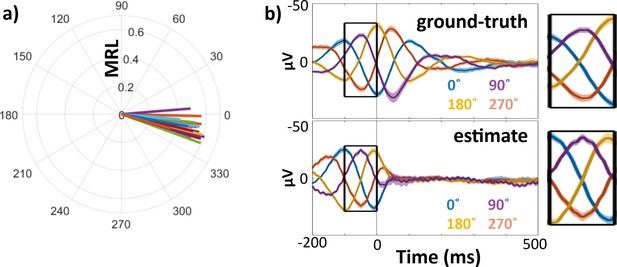
Phase estimation method yielded consistent, low-magnitude offset to ground-truth phase in pseudotrial analysis.
Comparison of ground-truth and estimated phase angles from pseudotrials (stimulation-free resting data with added model stimulation artifact and EP). Validation was performed across all hippocampal channels included in the main EP analysis (n = 23). (a) Mean phase angle and mean resultant length (MRL) of trialwise distance between ground-truth and estimated phases. Each line represents one hippocampal channel. (b) Left: Mean stimulation-free validation trials binned according to ground-truth (top) and estimated (bottom) phase at mock stimulation onset. Line color indicates bin center (0°, 90°, 180°, and 270°). Right: enlarged panels from −100 to 0 ms, showing similarity of theta phase at t = 0 across ground-truth (top) and estimated (bottom) bins.
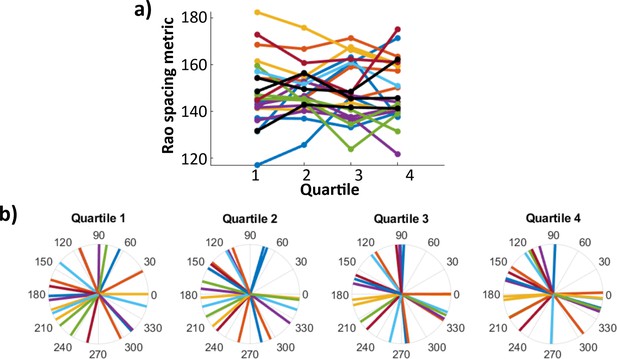
Theta-phase distribution of stimulation pulses did not change according to order in the experimental session.
Color indicates participant of origin. (a) Rao’s spacing test statistic u for stimulation phase angles in first, second, third, and fourth quartiles of the experimental session. Each dot is one hippocampal electrode (n = 23). (B) Mean stimulation phase angle for each hippocampal electrode across quartiles. No significant differences are found in either distribution uniformity or directionality according to order in the experimental session.
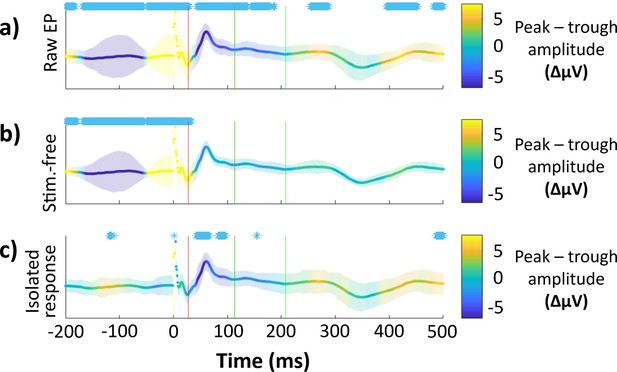
Effect of peak versus trough stimulation on hippocampal isolated response amplitude is temporally specific to components.
The difference between peak and trough amplitude at each timepoint is colorized and plotted on top of the grand average hippocampal EP. Purple indicates greater negativity for peak stimulation trials, yellow indicates greater negativity for trough stimulation. Error bars indicate ±1 standard error of the mean (SEM) of the peak − trough amplitude difference (a.u.). Timepoints where p < 0.05 (uncorrected, two-tailed) for non-zero amplitude difference between peak and trough are marked (*) for visualization. Component boundaries are marked with vertical lines (early: red, late: green). Plotted for: (a) Raw EPs (i.e., stimulation trials). (b) Stimulation-free trials only. (c) Isolated evoked response (i.e., stimulation trials minus stimulation-free trials).
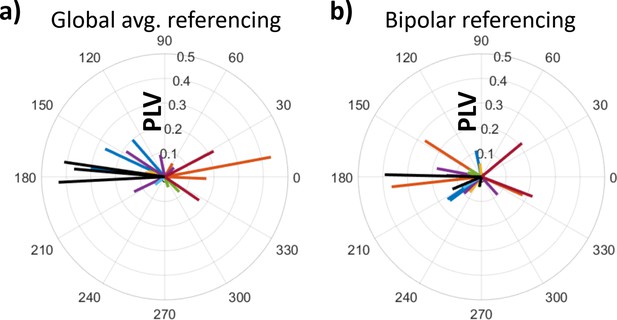
Distributions of phase offsets between lateral temporal and hippocampal theta were uniform across electrodes.
(a) Distribution of theta-phase offsets between lateral temporal cortex and hippocampus under global average referencing scheme (as in Figures 1—4). Dashed line indicates circular-mean phase angle across electrodes. Each line represents one hippocampal electrode. Color indicates participant-of-origin for each electrode. Electrode line length indicates phase-locking value. (b) As (a), under bipolar referencing scheme wherein data from each electrode were referenced to the adjacent lateral contact on the same depth electrode.
Tables
Participant characteristics.
| Sex | Age | Hemisphere of electrodes | # hippocampal recording electrodes analyzed | Pulses delivered | Stimulation protocol |
|---|---|---|---|---|---|
| F | 28 | Right | 3 | 1721 | 0.5 and 1 Hz |
| F | 29 | Left | 3 | 1576 | 0.5 and 1 Hz |
| F | 30 | Left | 3 | 1170 | 0.5 and 1 Hz |
| F | 44 | Left | 4 | 241 | 0.5 Hz |
| F | 55 | Left | 1 | 2566 | 0.5 and 1 Hz |
| F | 47 | Left | 3 | 1036 | 0.5 and 1 Hz |
| M | 31 | Left | 4 | 1766 | 0.5 and 1 Hz |
| M | 29 | Right | 2 | 982 | 0.5 and 1 Hz |





