Prefrontal-amygdalar oscillations related to social behavior in mice
Figures
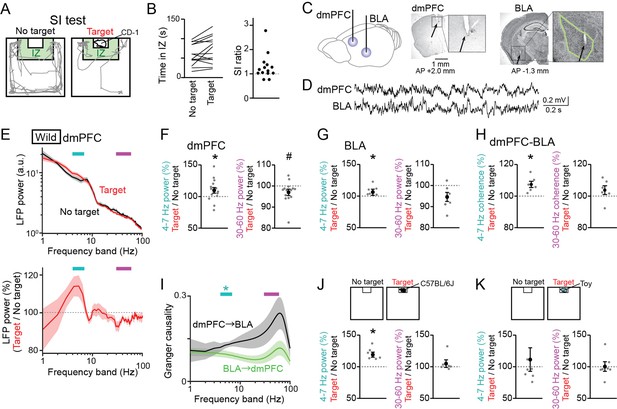
Changes in dorsal medial prefrontal cortex (dmPFC) and basolateral amygdala (BLA) Local field potential (LFP) signals in a social interaction (SI) test.
(A) A SI test with an interaction zone (IZ; labeled in green). Movement trajectories (gray lines) of a wild-type mouse are superimposed. (B) (Left) Occupancy time in the IZ. Each line indicates an individual mouse (n = 14 wild-type mice). (Right) SI ratios computed from the occupancy time. Each dot represents an individual mouse. (C) (Left) LFPs were recorded from the dmPFC and BLA. (Right) Histological confirmation of electrode locations (arrows). The dotted boxes are magnified in the right panels. The green line shows the contour of the BLA. The details of electrode locations are shown in Figure 1—figure supplement 1. (D) Typical LFP signals from the dmPFC and BLA. (E) (Top) Comparison of dmPFC LFP power spectrograms between the target (red) and no target (black) sessions averaged over all mice (n = 14 mice). Original datasets from individual mice are shown in Figure 1—figure supplement 2A. Data are presented as the mean ± SEM. Cyan and magenta bars above represent 4–7 Hz and 30–60 Hz bands, respectively. (Bottom) The percentages of LFP power at individual frequency bands in the target session relative to those in the no target session. The percentages were computed in individual mice and were averaged over all mice. (F) The percentages of dmPFC 4–7 Hz (left) and 30–60 Hz (right) LFP power averaged over an entire period of the target session relative to those of the no target session (n = 14 mice). Data are presented as the mean ± SEM. Each gray dot represents an individual data points. * and # represent a significant increase and decrease in the target session, respectively (p<0.05, paired t-test vs no target). (G) Same as F but for the BLA (n = 6 mice). (H) Same as F but for dmPFC-BLA coherence (n = 6 mice). (I) Spectral granger causality averaged over dmPFC-BLA electrode pairs. (n = 6 mice). *p<0.05, Mann-Whitney U test followed by Bonferroni correction. (J, K) Same as F but when an unfamiliar C57BL/6J mouse was used as a target mouse (J) or a toy mouse was placed in the cage instead of a target mouse (K).
-
Figure 1—source data 1
Individual data for Figure 1.
- https://cdn.elifesciences.org/articles/78428/elife-78428-fig1-data1-v1.xlsx
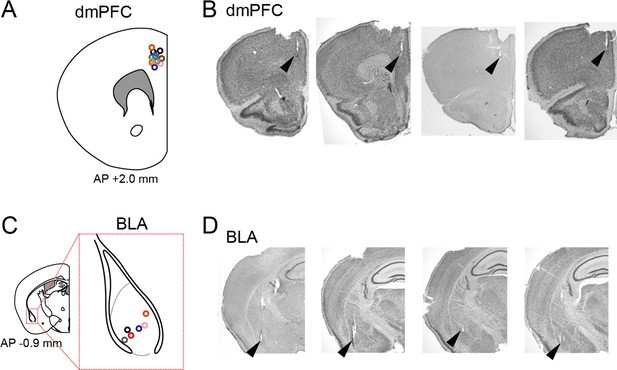
Confirmation of recording sites.
(A) Superimpositions of recording sites for all electrodes in wild-type mice (from 14 mice shown in Figure 1) indicated by circles on the dorsal medial prefrontal cortex (dmPFC). (B) Typical pictures of cresyl violet-stained sections with the arrowheads indicating the tips of electrode tracks. (C) Same as A but for the basolateral amygdala (BLA) (from 6 mice shown in Figure 1). The gray dotted line represents the border to define the BLA region. (D) Same as B but for the BLA.
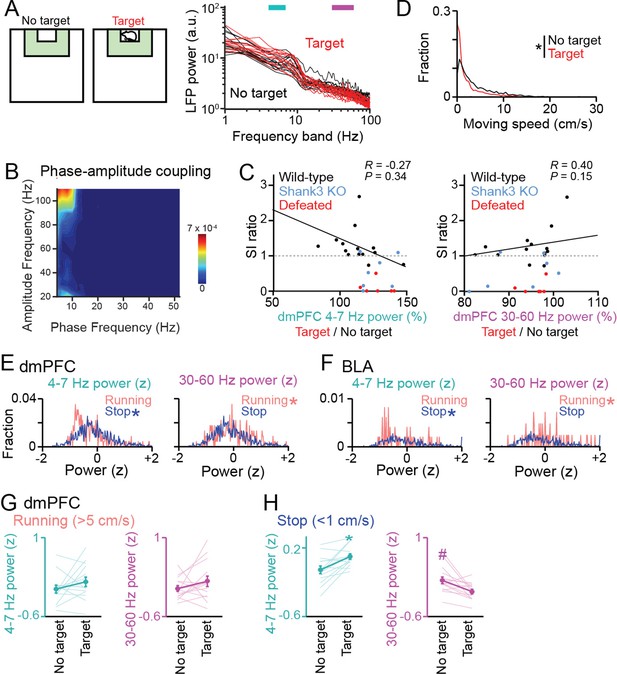
Datasets for local field potential (LFP) power analyses in Figure 1E–G.
(A) Supplementary datasets for Figure 1E. Original dorsal medial prefrontal cortex (dmPFC) LFP power spectrograms between the target (red) and no target (black) sessions for individual mice shown in Figure 1E (n = 14 wild-type mice). Each line shows each mouse. (B) Phase-amplitude coupling measures for a dmPFC LFP trace in the target session in a typical mouse, shown by contour plots of modulation index for all pairs of high (y-axis, gamma-range) and low (x-axis, theta-range) frequencies. Warm colors indicate stronger modulation. No pronounced coupling was observed. (C) Relationship between the percentages of dmPFC LFP power changes in the target session (corresponding with Figures 1F and 3B) and SI ratios (corresponding with Figure 1B). Each dot represents each mouse (n = 14 wild-type (black), 7 Shank3 knockout (KO) (blue), and 6 defeated (red) mice). In wild-type mice, no significant correlations were found between these variables (Black line). (D) Comparison of moving speed distributions between the no target and target sessions (n = 2086 frames; Z = 20.20, p=9.0 × 10-89, Mann-Whitney U test). (E, F) Comparisons of distributions of LFP power between running (>5 cm/s) and stop (<1 cm/s) periods in the target session (dmPFC, n = 254, and 1,008 frames; 4–7 Hz: Z = 4.93, p=8.26 × 10-7; 30–60 Hz: Z = 2.13, p=0.033; BLA, n = 58, and 519 frames; 4–7 Hz: Z = 2.42, p=0.016; 30–60 Hz: Z = 2.00, p=0.045, Mann-Whitney U test). Asterisks represent significantly higher power. (G) Supplementary datasets for Figure 1F. Comparison of z-scored dmPFC 4–7 Hz (cyan) and 30–60 Hz (magenta) power specifically computed from running periods between the target and no target sessions (n = 14 mice). LFP power at each frequency band was z-scored based on the average and SD of LFP power at each frequency band in an entire period including the no target and target sessions. Data are presented as the mean ± SEM. Each thin line represents each mouse. p>0.05, paired t-test. (H) Same as G but specifically computed from stop periods. * and # represent a significant increase and decrease in the target session, respectively (p<0.05, paired t-test vs no target).
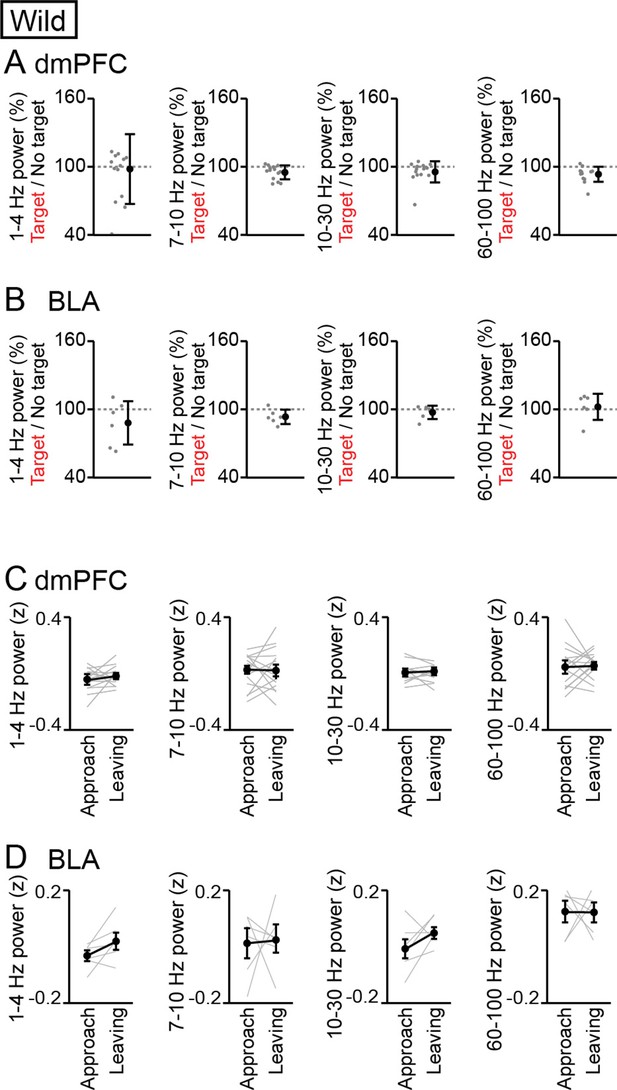
Analyses for the frequency bands other than 4–7 Hz and 30–60 Hz.
(A, B) Supplementary datasets for Figure 1F and G. For the frequency bands of 1–4 Hz, 7–10 Hz, 10–30 Hz, and 60–100 Hz, the percentages of dorsal medial prefrontal cortex (dmPFC) (A) and basolateral amygdala (BLA) (B) Local field potential (LFP) power averaged over an entire period of a session in the target session relative to those in the no target session (n = 14 mice). Data are presented as the mean ± SEM. Each gray dot represents an individual data points. No significant differences were found in these comparisons (p>0.05, paired t-test followed by Bonferroni correction, target vs no target). (C, D) Supplementary datasets for Figure 5F. For the frequency bands of 1–4 Hz, 7–10 Hz, 10–30 Hz, and 60–100 Hz, LFP power in the dmPFC (C) and BLA (D) was compared power between approach and leaving behavior (n = 14 and 6 mice respectively). Data are presented as the mean ± SEM. No significant differences were found in these comparisons (p>0.05, paired t-test followed by Bonferroni correction, approach vs leaving).
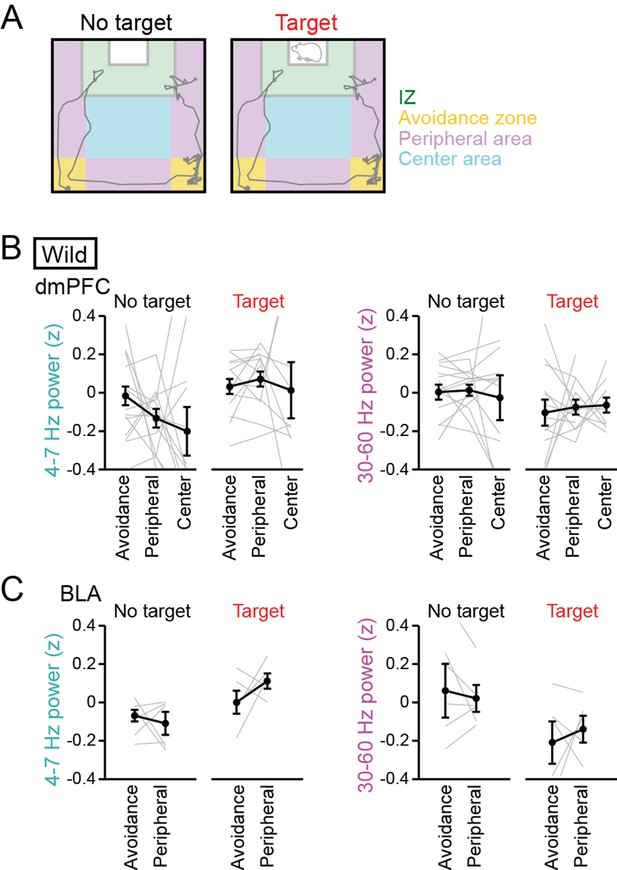
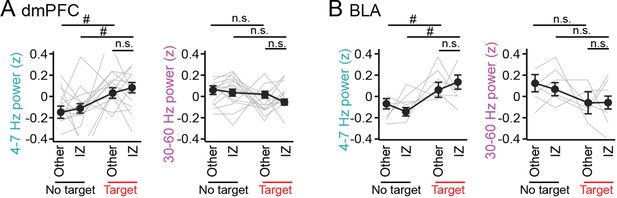
No pronounced changes in 4–7 Hz and 30–60 Hz power in the dorsal medial prefrontal cortex (dmPFC) and basolateral amygdala (BLA) in areas outside the interaction zone (IZ).
(A) Schematic illustration showing social avoidance zones, peripheral areas, and a center area in the SI test. (B) Comparisons of dmPFC 4–7 Hz and 30–60 Hz power across avoidance zones, peripheral areas, and a center area (n = 14 mice). Data are presented as the mean ± SEM. Each line represents each mouse. p>0.05, Mann-Whitney U test followed by Bonferroni correction. (C) Same as B but for the BLA (n = 6 mice). The center area was removed from this analysis because of the limited number of samples.
-
Figure 2—source data 1
Individual data for Figure 2.
- https://cdn.elifesciences.org/articles/78428/elife-78428-fig2-data1-v1.xlsx
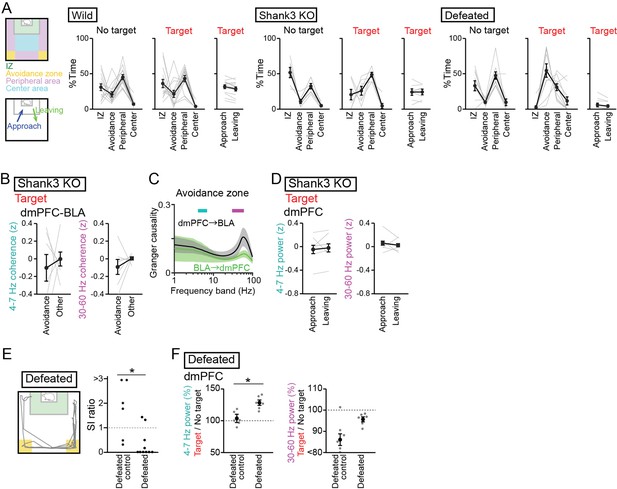
Supplementary datasets for analyses of socially deficient mice.
(A) Summary of the percentages of stay areas in the two sessions (left) and the percentages of approach/leaving behavior in the target session (right) in the three mouse groups. Data are presented as the mean ± SEM. Each line represents each mouse. (B) Supplementary datasets for Figure 4B. Comparison of dorsal medial prefrontal cortex (dmPFC)-basolateral amygdala (BLA) 4–7 Hz and 30–60 Hz coherence between the avoidance zones and the other areas in Shank3 knockout (KO) mice (n = 7 mice). p>0.05, paired t-test followed by Bonferroni correction. (C) Supplementary datasets for Figure 4B. Spectral Granger causality in the avoidance zones in the target session averaged over dmPFC-BLA electrode pairs in Shank3 KO mice (n = 7 mice). p>0.05, Mann-Whitney U test. (D) Supplementary datasets for Figure 5F. Comparison of 4–7 Hz and 30–60 Hz power in the dmPFC between approach and leaving behavior in Shank3 KO mice (n = 7 mice). Data are presented as the mean ± SEM. Each gray line represents each mouse. p>0.05, paired t-test followed by Bonferroni correction. (E) Supplementary datasets for Figures 1B and 3A. SI ratios in defeated control and defeated mice (n = 6 defeated control mice and 10 defeated mice). Each dot represents an individual mouse. The data from defeated mice similar to those shown in Figure 3A are presented for comparison. *p<0.05, Mann-Whitney U test. (F) Supplementary datasets for Figure 3A and B. The percentages of dmPFC 4–7 Hz (left) and 30–60 Hz (right) local field potential power in the target session relative to those in the no target session in defeated mice (n = 6 defeated control mice and 6 defeated mice). Data are presented as the mean ± SEM. Each gray dot represents each mouse. The data from defeated mice similar to those shown in Figure 3B are presented for comparison. *p<0.05, Mann-Whitney U test followed by Bonferroni correction.
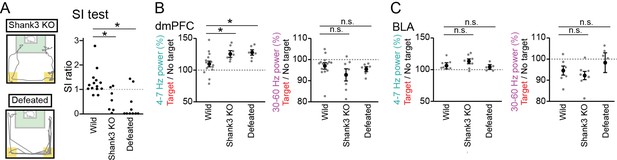
Further increases in dorsal medial prefrontal cortex (dmPFC) 4–7 Hz power in the target session in Shank3 knockout (KO) mice and defeated mice.
(A) (Left) Movement trajectory of a Shank3 KO mouse and a defeated mouse in a target session. The orange areas represent the avoidance zones. (Right) SI ratios for Shank3 KO and defeated mice (n = 14 wild, 7 Shank3 KO, and 10 defeated mice). Each dot represents an individual animal. The data from wild-type mice similar to those shown in Figure 1B are presented for comparison. *p<0.05 versus wild, Mann-Whitney U test followed by Bonferroni correction. (B) The percentages of dmPFC 4–7 Hz (left) and 30–60 Hz (right) local field potential (LFP) power in the target session relative to those in the no target session (n = 14 wild, 7 Shank3 KO, and 6 defeated mice). Data are presented as the mean ± SEM. Each gray dot represents an individual data points. The data from wild-type mice similar to those shown in Figure 1F are presented for comparison. *p<0.05, versus wild, Mann-Whitney U test followed by Bonferroni correction. (C) Same as B but for the basolateral amygdala (BLA) (n = 6, 7, and 5 mice).
-
Figure 3—source data 1
Individual data for Figure 3.
- https://cdn.elifesciences.org/articles/78428/elife-78428-fig3-data1-v1.xlsx
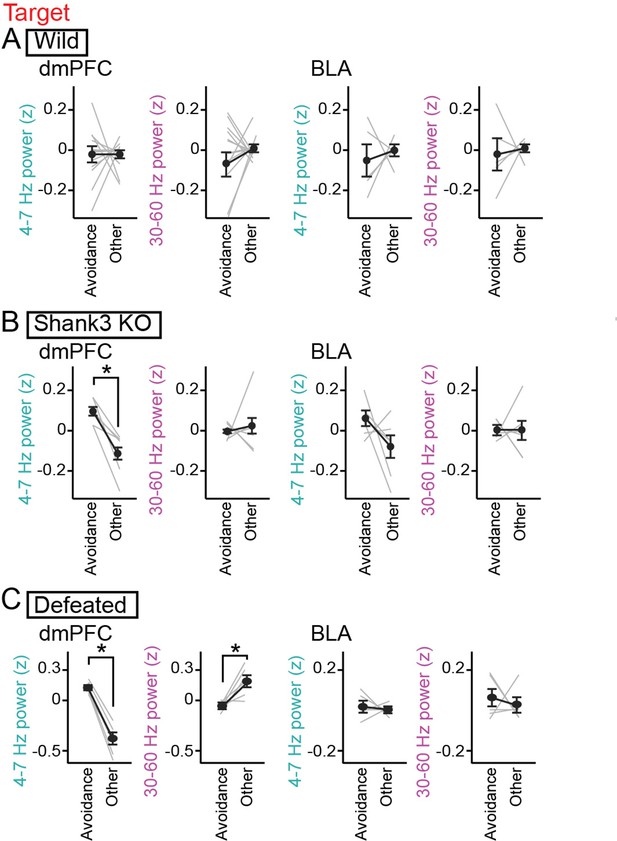
Increases in dorsal medial prefrontal cortex (dmPFC) 4–7 Hz power during social avoidance in Shank3 knockout (KO) mice and defeated mice.
(A) Comparisons of 4–7 Hz and 30–60 Hz power in the dmPFC and basolateral amygdala (BLA) between the avoidance zones and the other areas in the target session in wild-type mice (n = 14 and 6 mice). Data are presented as the mean ± SEM. Each gray line represents each mouse. p>0.05, paired t-test followed by Bonferroni correction. (B) Same as A but for Shank3 KO mice (n = 7 and 7 mice). *p<0.05, paired t-test followed by Bonferroni correction. (C) Same as A but for defeated mice (n = 6 and 5 mice).
-
Figure 4—source data 1
Individual data for Figure 4.
- https://cdn.elifesciences.org/articles/78428/elife-78428-fig4-data1-v1.xlsx
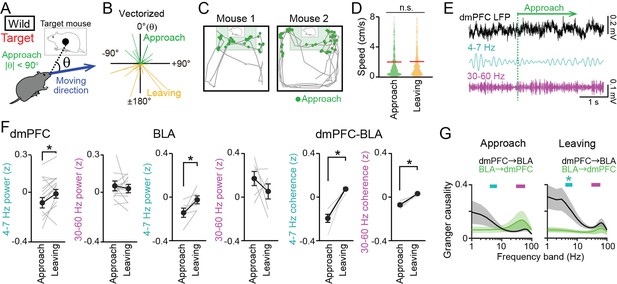
Decreases in dorsal medial prefrontal cortex (dmPFC) 4–7 Hz local field potential (LFP) power during social approach behavior in the target session.
(A) Social approach and leaving behavior in wild-type mice was defined when absolute cage-oriented moving directions (|θ|) were less than and more than 90°, respectively, in the half of the box containing the interaction zone (IZ). (B) A polar plot of vectorized instantaneous animal trajectories (bin = 1 s) as a function of cage-oriented moving direction. (C) Trajectories from two representative mice (gray). Green dots represent social approach behavior. (D) Distributions of moving speed during approach and leaving behavior (n = 644 and 577). The red lines show the average. p>0.05, Mann-Whitney U test. (E) Unfiltered and bandpass (4–7 Hz and 30–60 Hz)-filtered dmPFC LFP traces in the target session. The green line indicates the onset of a social approach behavior. (F) Comparison of 4–7 Hz and 30–60 Hz power in the dmPFC (left, n = 14 mice) and basolateral amygdala (BLA) (middle, n = 6 mice) and dmPFC-BLA 4–7 Hz and 30–60 Hz coherence (right, n = 6 mice) between approach and leaving behavior. Data are presented as the mean ± SEM. Each gray line represents each mouse. *p<0.05, paired t-test. (G) Spectral Granger causality during approach (top) and leaving (bottom) behavior in the target session averaged over dmPFC-BLA electrode pairs. *p<0.05, Mann-Whitney U test followed by Bonferroni correction.
-
Figure 5—source data 1
Individual data for Figure 5.
- https://cdn.elifesciences.org/articles/78428/elife-78428-fig5-data1-v1.xlsx
local field potential (LFP) power changes in the interaction zone in the target session.
(A, B) Supplementary datasets for Figure 5F. No significant changes in dorsal medial prefrontal cortex (dmPFC) and basolateral amygdala (BLA) LFP power between the interaction zone (IZ) and the other areas in the target session. In the dmPFC and BLA, 4–7 Hz (left) and 30–60 Hz (right) power was compared between the IZ and the other areas (A; dmPFC, n = 14 mice; 4–7 Hz: t13 = 0.76, p=0.46; 30–60 Hz: t13 = 1.38, p=0.19; B; BLA, n = 6 mice; 4–7 Hz: t5 = 0.76, p=0.48; 30–60 Hz: t5 = 0.02, p=0.98). Each gray line shows each mouse. Data are presented as the mean ± SEM. #p<0.05, paired t-test followed by Bonferroni correction.
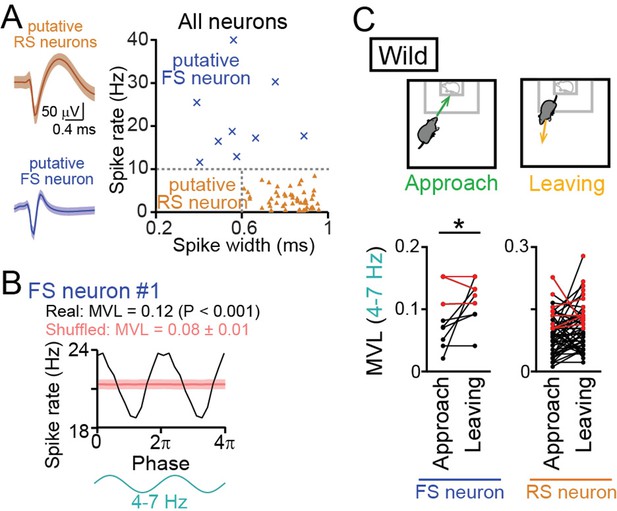
Entrainment of oscillatory spike patterns of dorsal medial prefrontal cortex (mPFC) neurons.
(A) (Left) Typical spike waveforms of a putative regular-spiking (RS) neuron (top) and a putative fast-spiking (FS) neuron (bottom). Data are presented as the mean ± SD. (Right) For individual neurons in wild-type mice, baseline spike rates and spike width are plotted. Each dot represents an individual neuron. Neurons plotted in orange and cyan regions are classified as putative excitatory RS pyramidal neurons (triangle, n = 48 neurons) and inhibitory FS interneurons (cross-mark, n = 9 neurons), respectively. (B) A representative putative FS neuron that fired time-locked to the 4–7-Hz local field potential (LFP) oscillations. Instantaneous spike rates of this neuron are plotted against the phase of 4–7-Hz oscillatory cycles. From a phase-spike distribution, a mean vector length (MVL) was computed as 0.12. The red line and the shaded area represent the mean and SD computed from the corresponding 1000 shuffled datasets. (C) Comparisons of MVL for the 4–7-Hz LFP oscillations between approach and leaving behavior. RS and FS neurons were separately analyzed. Each dot and line represents each neuron. The red dots indicate significant MVL, computed from shuffled datasets. The red lines indicate neurons showing significant MVL in both of the periods, which were considered as behavior-irrelevant phase-locked neurons and excluded from the statistical analyses. p<0.05, paired t-test for the datasets shown in black.
-
Figure 6—source data 1
Individual data for Figure 6.
- https://cdn.elifesciences.org/articles/78428/elife-78428-fig6-data1-v1.xlsx
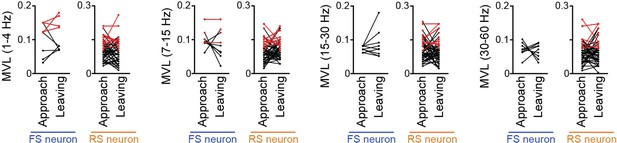
Comparisons of mean vector length (MVL) for the oscillations (at 1–4 Hz, 4–7 Hz, 7–15 Hz, 15–30 Hz, and 30–60 Hz) other than the 4–7-Hz local field potential (LFP) oscillations between approach and leaving behavior.
Supplementary datasets for Figure 6C. RS and FS neurons were separately analyzed. Each dot and line represent each neuron. The red dots indicate significant MVL, computed from shuffled datasets. The red lines indicate neurons showing significant MVL in both periods, which were considered as behavior-irrelevant phase-locked neurons and excluded from statistical analyses. In all comparisons, no statistical differences were observed (P > 0.05) (1–4 Hz: FS: n = 5 neurons; t4 = 0.33, P > 0.99; RS: n = 39 neurons; t38 = 0.28, P > 0.99; 7–15 Hz: FS: n = 7 neurons; t6 = 0.86, P > 0.99; RS: n = 39 neurons; t38 = 1.16, P > 0.99; 15–30 Hz: FS: n = 9 neurons; t8 = 1.06, P > 0.99; RS: n = 41 neurons; t40 = 1.69, P > 0.99; 30–60 Hz: FS: n = 9 neurons; t8 = 0.42, P > 0.99; RS: n = 44 neurons; t43 = 0.64, P > 0.99; paired t-test followed by Bonferroni correction).
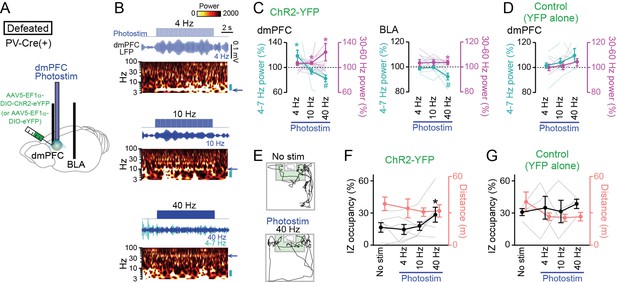
Restoration of social approach behavior by optogenetic photostimulation with a decrease in 4–7 Hz power and an increase in 30–60 Hz power in the dorsal medial prefrontal cortex (dmPFC).
(A) Schematic illustration. PV-positive interneurons expressing ChR2-YFP (or YFP alone) in the dmPFC were optogenetically stimulated, while local field potential (LFP) signals were recorded from the dmPFC and basolateral amygdala (BLA) in PV-Cre mice subjected to social defeat stress. (B) (From top to bottom) Photostimulation at 4, 10, and 40 Hz for 10 s in defeated mice injected with AAV5-EF1a-DIO-ChR2-eYFP. Each panel shows photostimulation patterns (upper), representative dmPFC LFP traces filtered at the frequency band similar to that of photostimulation (middle) and its wavelet spectrum (lower). At 40-Hz photostimulation, the LFP trace filtered at 4–7 Hz is superimposed as the cyan trace. The blue arrows beside the wavelet spectrum show the corresponding band and the cyan bars represents the 4–7 Hz band. (C) Average 4–7 Hz (cyan) and 30–60 Hz (magenta) power changes by dmPFC photostimulation in the dmPFC (left) and BLA (right) in defeated mice injected with AAV5-EF1a-DIO-ChR2-eYFP. (n = 7 mice). Each thin line represents each mouse. * and # represent a significant increase and decrease, respectively (p<0.05, paired t-test versus baseline). (D) Same as C but for control mice injected with AAV5-EF1a-DIO-eYFP (n = 4 mice). (E) Movement trajectories of a defeated mouse in a target session with no stimulation and 40-Hz photostimulation. (F) The percentage of time spent in the IZ (left axis, black) and total travel distance (right axis, thin red) in defeated mice injected with AAV5-EF1a-DIO-ChR2-eYFP (n = 7 mice). Data are presented as the mean ± SEM. Each gray line represents each mouse. *p<0.05, paired t-test followed by Bonferroni correction. (G) Same as F but for defeated mice injected with AAV5-EF1a-DIO-eYFP (n = 5 mice).
-
Figure 7—source data 1
Individual data for Figure 7.
- https://cdn.elifesciences.org/articles/78428/elife-78428-fig7-data1-v1.xlsx
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Mus musculus, male) | C57BL/6 J | SLC | Jax:000664 | |
| Strain, strain background (Mus musculus, male) | ICR | SLC | Jax:009122 | |
| Genetic reagent (M. musculus) | PV-Cre | The Jackson laboratory | Jax:008069 | |
| Genetic reagent (M. musculus) | Shank3KO | PMID:21423165 | ||
| Chemical compound, drug | Isoflurane | Pfizer Inc. | RRID: AB_2734716 | |
| Chemical compound, drug | Phosphate-buffered saline | FUJIFILM Wako Pure Chemical Corporation | Cat# 166‐23,555 | |
| Chemical compound, drug | Paraformaldehyde | Sigma-Aldrich | CAS No. 30525-89-4 | |
| Chemical compound, drug | Cresyl violet | Sigma-Aldrich | CAS No. 10510-54-0 | |
| Software, algorithm | Fiji | Fiji is just ImageJ, NIH (https://imagej.net/Fiji) | Fiji, RRID:SCR_002285 | |
| Software, algorithm | Matlab | Mathworks | RRID:SCR_001622 | Version R2020 |
| Other | AAV5-EF1a-DIO-eYFP | UNC Vector Core | In-Stock AAV Vectors –Dr. Karl Deisseroth, 100 ul Aliquots | 1.0×1,013 vg/ml |
| Other | AAV5-EF1a-DIO-ChR2-eYFP | UNC Vector Core | In-Stock AAV Vectors –Dr. Karl Deisseroth, 100 ul Aliquots | 1.0×1,013 vg/ml |





