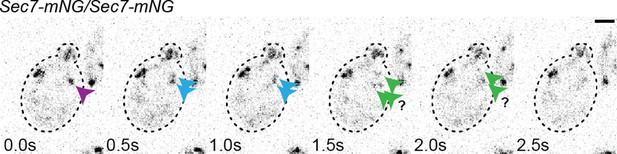
High-resolution secretory timeline from vesicle formation at the Golgi to fusion at the plasma membrane in S. cerevisiae
Figures
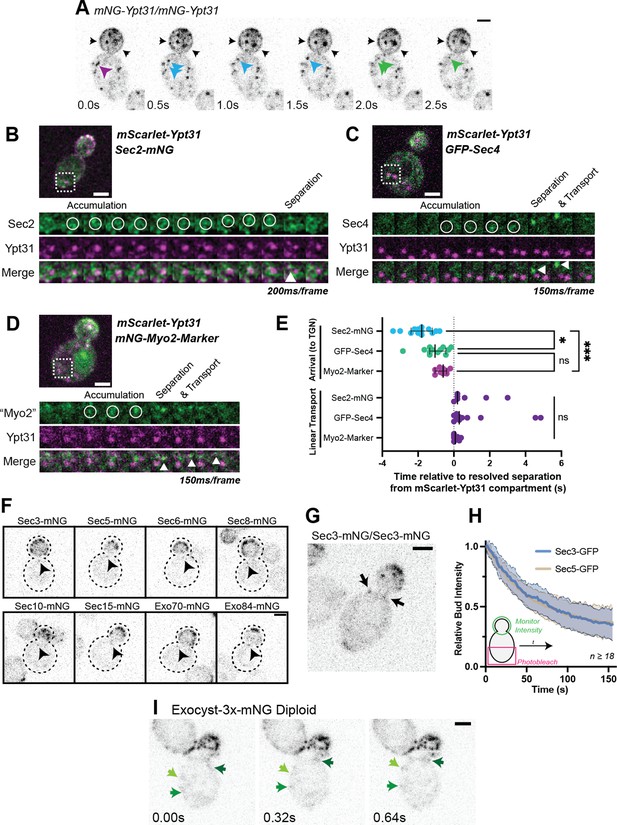
Recruitment of Sec2, Sec4, and Myo2 precede secretory vesicle budding from the TGN and all exocyst components are on secretory vesicles before arrival at the plasma membrane.
(A) An example of mNG-Ypt31 TGN vesiculation in a diploid cell. Colored arrowheads follow successive fragmentation of an initial compartment. Black arrows highlight aberrant mNG-Ypt31 on the plasma membrane. (B) Timeseries example of Sec2-mNG recruitment to and budding from an mScarlet-Ypt31-marked compartment in a diploid cell. Single plane video. (C) Timeseries example of GFP-Sec4 recruitment to and budding from an mScarlet-Ypt31-marked compartment in a diploid cell. Single plane video. (D) Timeseries example of an mNG- Myo2-marker being recruited to and budding from an mScarlet-Ypt31-marked compartment in a diploid cell. Single plane video. (E) Order of recruitment to Ypt31 TGN compartments aligned by apparent separation of signal from the compartment. Budded vesicles were generally transported linearly towards the bud in under a second. Median ±95% CI. n≥10. *, p≤0.05; ***, p≤0.001. (F) Localization of mNG-tagged exocyst components in haploid cells. Sum projection of a 1.5 μm vertical volume surrounding the bud neck. Arrowheads indicate a single vesicle approaching the bud neck in each. All scaled equally; Bar on Exo84, 2 μm. (G) Additional example of mNG-Sec3 localizing to puncta approaching the bud neck in homozygously-tagged diploid cells. (H) Fluorescence Loss in Photobleaching (FLIP) experiment comparing the recycling of Sec3 or Sec5 from the bud. Mean curve ± SD, n≥18. (I) Exocyst-3x-mNG faintly localizes to vesicles earlier in the mother. Compare the moving signals identified by green arrows to any punctum within in the bud. All bars, 2 μm.
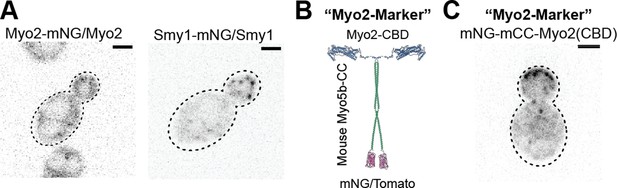
Rationale for and construction of the Myo2 marker.
(A) One copy of endogenous Myo2 tagged with mNG in a diploid cell (left) shows no clear punctate localization due to the relatively high cytosolic background, however, one copy of Smy1-mNG (right) permits identification of clear vesicles. (B) mNeonGreen or one copy of Tomato were tagged to a portion of the coiled coil domain of mouse myosin 5b of similar length to the Myo2 coiled coil, to facilitate dimerization of the marker without hetero- dimerizing with endogenous Myo2. This was fused to the C-terminal cargo-binding domain of Myo2 to maintain normal Myo2 cargo recognition. (C) an example of mNG-mCC-Myo2(CBD) localization in a diploid cell.
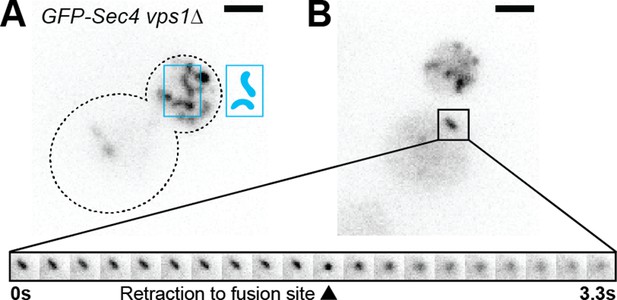
Sec4 localizes to larger fusion-competent compartments in a vps1-null strain.
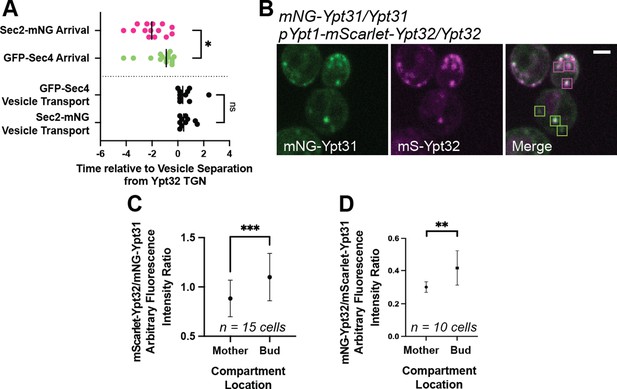
While vesiculation dynamics of the underlying compartments appear similar, Ypt31 and Ypt32 are enriched on subtly different membranes marked by localization to either the mother cell or daughter bud.
(A) Replication of Sec2/Sec4 separation from Ypt32-positive TGN compartments. n≥10. *, p≤0.05. (B) Example of Ypt31/32 localization, highlighting more Ypt31-dominant compartments in the mother and Ypt32-rich compartments in the bud. (C and D) quantification of arbitraryYpt32/Ypt31 fluorescence intensity ratio for compartments in the mother and bud for mNG-Ypt31 mScarlet-Ypt32 (C) or mScarlet-Ypt31 mNG-Ypt32 (D). Compared via paired students t-test. *, p≤0.05; ***, p≤0.001.
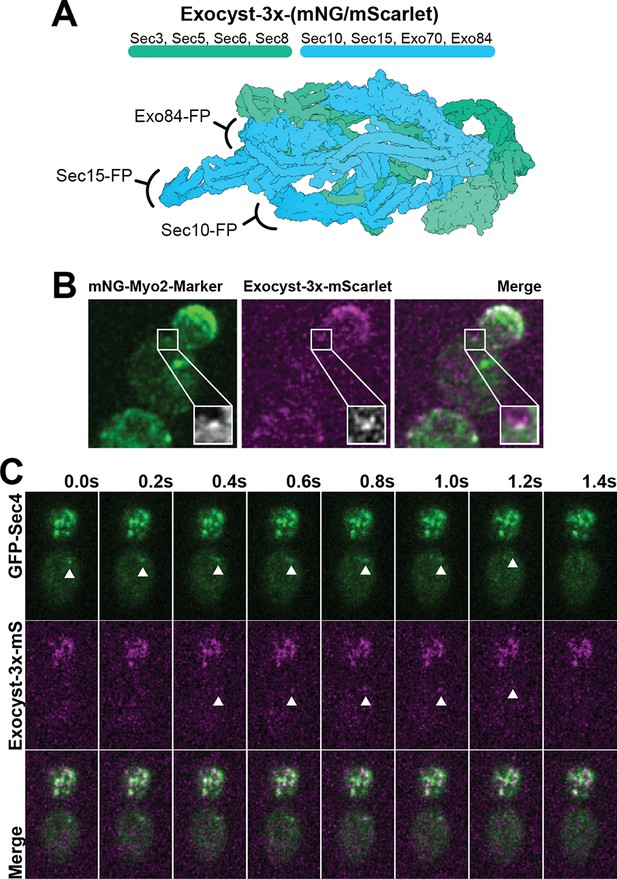
Construction of the exocyst marker and colocalization examples with other markers used in this study.
(A) Exocyst-3x-tag construction. (B) Colocalization example of Exocyst-3x-mScarlet with Myo2-Marker. Single Plane. (C) Colocalization example of Exocyst-3x-mScarlet with GFP-Sec4 on a vesicle in the mother cell. Single Plane.
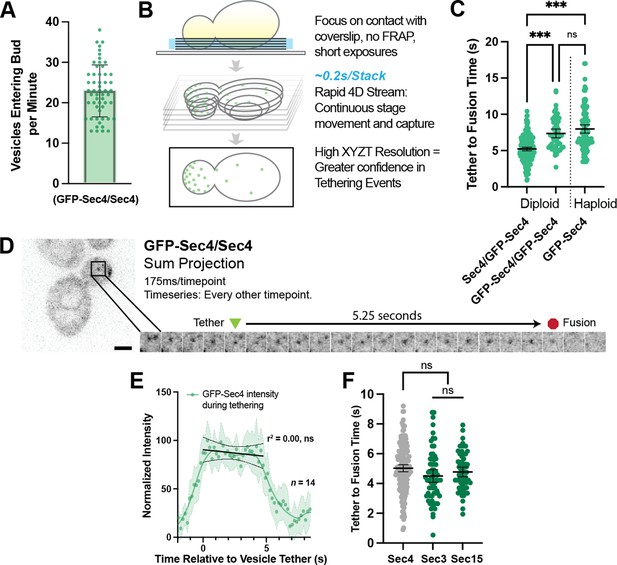
Secretory vesicles tether for about 5 s before fusion.
(A) Approximately 22 vesicles enter the bud per minute. n=55 cells. Mean ± SD. (B) Schematic diagram of improved volumetric imaging technique used in this study. See Video 1 for example. (C) Collected data of all timed GFP-Sec4 tethering events in wildtype GFP-Sec4/Sec4 cells as well as homozygously-tagged diploids and GFP-Sec4 haploids Means ±95% CI. GFP- Sec4/Sec4 Mean: 5.02 s, n=180 events, others n>50. ***, p≤0.001. (D) Example of GFP-Sec4 vesicle tethering and fusion from a GFP-Sec4 heterozygously-tagged diploid. Sum projection. See Video 2. (E) GFP-Sec4 fluorescence intensity is roughly constant through tethering. Local weighted regression (LOWESS; green) and linear regression curves (during tethering; black) added for visual interpretation. (F) Apparent tethering time of vesicles marked carrying Sec3-mNG (Mean: 4.55 s) or Sec15-mNG (Mean: 4.77 s) is similar to tethering time of vesicles marked by GFP-Sec4. Mean ±95% CI.
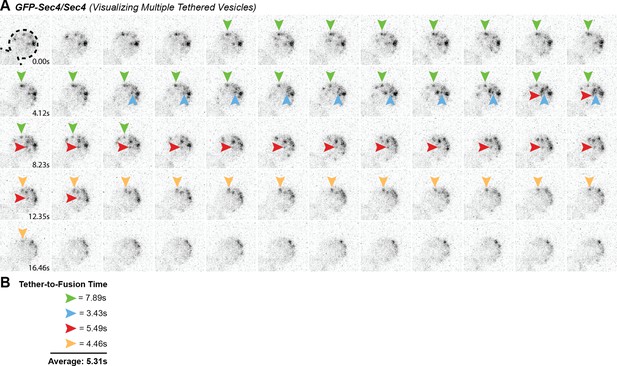
Multiple GFP-Sec4 vesicle tethering and fusion events can be visualized and timed in a single cell.
(A) Example of multiple vesicle tethering events within a single bud. Colored arrows follow single events. (B) Collected timings of events shown in A.
Tethering Hotspots Exist.
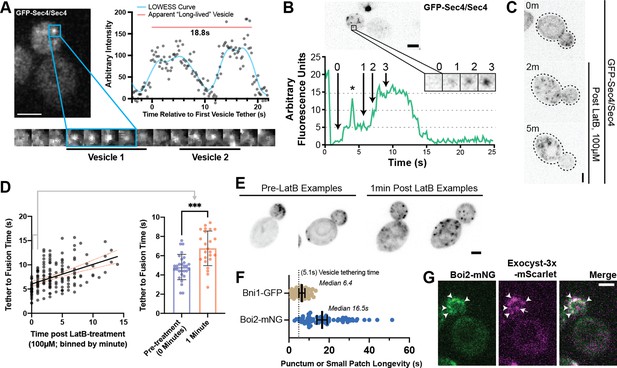
(A) Sequentially tethering vesicles may appear as long-lived events in captures with low spatiotemporal resolution, especially when considering the elongated tethering time in cells with no untagged Sec4 (as in Figure 2C). Images were captured at 176ms per frame. Inset time-lapse shown with 10 x lower time resolution. (B) Additional example of “hot-spot” tethering shows 3 vesicles arriving and tethering in rapid succession at one un-resolvable location (see Video 3). Although they tether at separate times, they appear to fuse at roughly the same time. Asterisk marks signal from a bright vesicle that passed the observed position. (C) Secretory vesicle formation continues even in the absence of actin cables. (D) Disruption of actin cables with LatrunculinB (LatB) immediately results in elongated GFP-Sec4 vesicle tether-to-fusion time. ***, p≤0.001 by t-test. (E) Several examples of clustered GFP-Sec4 secretory vesicle localization in diploid cells not treated with LatB and examples of more dispersed vesicle tethering locations in cells 1 min after LatB treatment. Sum projection of cell bottom. (F) Boi2 patches are longer lived than individual vesicles or even Bni1 patches, suggesting a potential explanation for the observation of tethering ‘hot-spots’. (G) Exocyst-3x-mScarlet frequently colocalizes with Boi2-mNG puncta on the plasma membrane. Arrowheads indicate vesicles colocalized with Boi2, arrow indicates a tethered vesicle not colocalized with Boi2.
Duration of vesicle tethering is modulated by the level of Sec4:GTP on the vesicle.
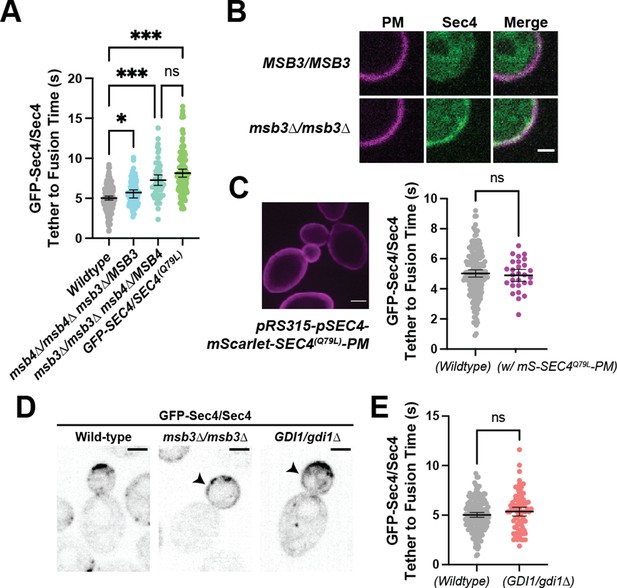
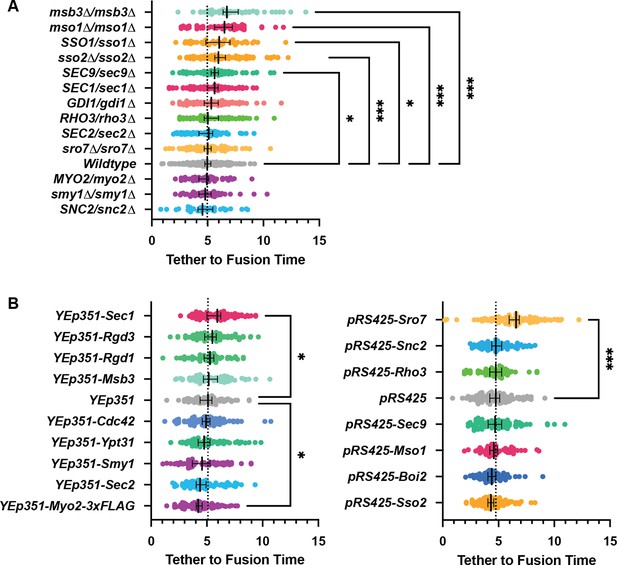
Msb3 likely acts on Sec4 twice to aid in efficient tethering and Sec4 recycling. (A) Homozygous deletion of msb3 or replacement of one copy of Sec4 with a constitutively active allele results in significantly longer tether-to-fusion time, whereas similar deletion of msb4 has a much milder effect. *, p≤0.05; ***, p≤0.001. (B) Deletion of msb3 results in GFP-Sec4 accumulation on the plasma membrane (PM). Unbudded cells shown for simplicity, though the same occurs in budded cells. PM is marked with mCherry-Ist2tail(2 X). Bar, 1 μm. (C) Expression of a constitutively active and plasma-membrane-bound Sec4 has no effect on GFP-Sec4 vesicle tethering time. Ectopic Sec4:GTP on the plasma membrane is not the cause of elongated tethering times observed in msb3∆ and Sec4(Q79L). (D) Heterozygous deletion of Gdi1 (an essential protein) also induces Sec4 accumulation on the plasma membrane. (E) Heterozygous deletion of Gdi1 has no significant effect on secretory vesicle tether-to-fusion time.
Defining the location and timing of exocytic components individually.
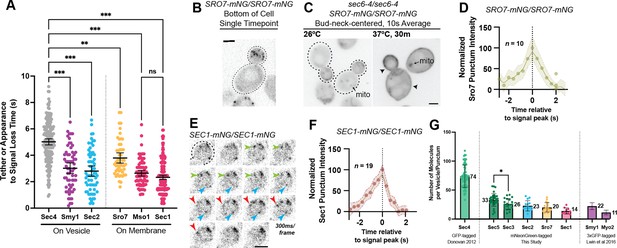
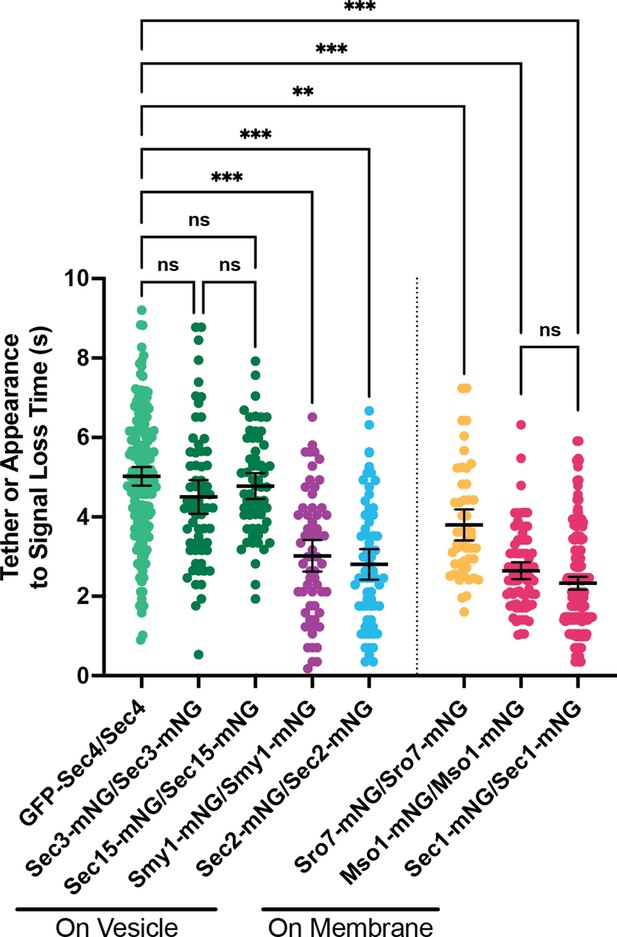
(A) Independent timing from tether to disappearance for components residing on vesicles (Smy1-mNG, Sec2-mNG) and timing of punctum appearance to disappearance for components apparently residing on the plasma membrane (Sro7- mNG, Sec1-mNG, Mso1-mNG). All imaged in homozygously-tagged diploids in a manner as in Figure 2B. **, p≤0.005; ***, p≤0.001. See Figure 5—figure supplement 2 for combined statistics. (B) Sro7, a Sec4 effector, does not localize directly to vesicles and instead appears in short-lived puncta at the plasma membrane. Sum projection. Bar, 2 μm. (C) Disruption of secretory vesicle tethering via sec6-4 does not result in Sro7 accumulation on cytosolic vesicles, but instead accumulation at the plasma membrane. Mitochondrial autofluorescence is apparent due to the low signal intensity of Sro7-mNG. Bar, 2 μm. (D) Averaging multiple events shows that the timing of arrival and departure for Sro7 is roughly symmetrical. (E) Time-lapse of several Sec1-mNG puncta within the bud. Sum Projection. Captured as in Figure 2B. ~300ms per frame. Bar, 2 μm. (F) Averaging several Sec1-mNG localization events shows faster signal dissipation than accumulation. This is different from what is observed for another PM-localized protein Sro7 in (C). (G) Collected data of number of molecules per vesicle of various components measured both in this study and other studies from our lab. *, p≤0.05.
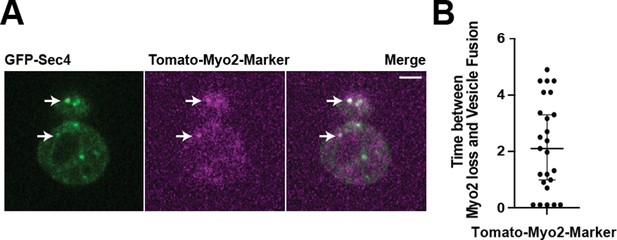
A Myo2-marker co-imaged with GFP-Sec4 indicates loss of Myo2 occurs midway through vesicle tethering.
(A) Tomato-Myo2 marker can be seen on GFP-Sec4 marked vesicles in both the mother and bud. (B) Myo2 departs secretory vesicles just over 2 s before vesicle fusion on average, but this time varies widely.
Combined data from Figures 2F and 5A.
All n≥50 events, analyzed blind. To minimize the number of comparisons performed, these data were tested for differences among the group via Kruskal-Wallis and only the indicated post-hoc tests were performed, with corrections. See methods for additional details. **, p≤0.005; ***, p≤0.001.
Relative ordering of exocytic events and the tether-to-fusion timeline.
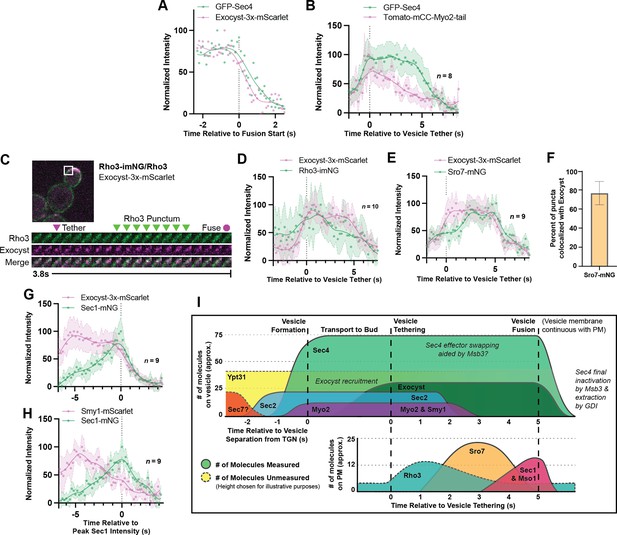
(A) Single example of vesicle fusion showing near simultaneous loss of Sec4 signal and the exocyst in GFP-Sec4/Sec4 Exocyst-3x- mScarlet. (B) Averaging of several Sec4 vesicle tethering events shows that on average Myo2 begins dissociating from the vesicle around the start of tethering. (C) Internally tagged Rho3-imNG on the plasma membrane concentrates briefly after Exocyst-3x-mScarlet vesicle tethering. (D) Averaging several vesicle tethering events as in C illustrates that Rho3-imNG membrane intensity rises with Exocyst-3x-mScarlet arrival and peaks ~1 s after vesicle tethering. Aligned by visual start of tethering. (E) Averaging several Exocyst-3x-mScarlet vesicle tethering events shows that Sro7-mNG localization to vesicles peaks 3–4 s after tethering. Aligned by visual start of tethering. (F) Not all Sro7-mNG puncta clearly colocalize with exocyst- marked secretory vesicles. The fraction of Sro7-mNG puncta visually colocalized with the exocyst in still images was manually counted for >10 cells across three biological replicates. Mean ± SD. (G) Averaging of several vesicle tethering events shows colocalization of Sec1-mNG and Exoycst-3x-mScarlet with Sec1 signal peaking around the moment of exocyst loss. Aligned by moment of peak Sec1 intensity. (H) The start of Smy1- mScarlet loss from vesicles occurs approximately 5 s before Sec1-mNG peak. Aligned by moment of peak Sec1 intensity. (I) Timeline of events from secretory vesicle formation to plasma membrane fusion. Timing of appearance and disappearance of proteins in this timeline is based on the individual component data (where available) and aligned with the dual component imaging data.

Tether-to-fusion timing of GFP-Sec4 in Exocyst-3x-mScarlet diploid cells.

Example of Rho3-imNG on its own in a haploid cell.
Arrowheads indicate transient patches/puncta of increased Rho3 intensity.
Secretion is remarkably robust.
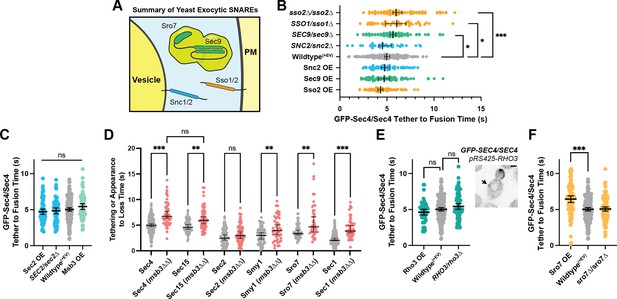
(A) Expanded summary diagram of exocytic SNARE localization. The cytosolic SNARE Sec9 is shown bound to Sro7. (B) SNARE limitation significantly elongates vesicle tether-to-fusion time, while overexpression has the minimal, but statistically insignificant effect of decreasing tether-to-fusion time. Median ±95%CI. (C) Overexpression of Sec2, Msb3 (or heterozygous deletion of Sec2) has no significant effect on secretory vesicle tether-to-fusion time. Mean ±95% CI. (D) In msb3∆∆ msb4∆ diploid cells, all components measured, except Sec2, remain significantly longer on tethered vesicles/plasma- membrane. All but Sec4 were homozygously tagged with mNG. Individual comparisons performed via Mann- Whitney test. Median ±95% CI. (E) Overexpression or heterozygous deletion of Rho3 has no significant effect on secretory vesicle tether-to-fusion time in the bud, however, aberrant, longer lived vesicle tethering events could be found in the mother cell when Rho3 was overexpressed. Mean ±95%CI. (F) Overexpression of Sro7 significantly elongates vesicle tether-to-fusion time. Mean ±95% CI. All panels: Wildype(+EV) shown for visual clarity. All overexpressions were compared to a relevant empty vector control, while deletions were compared the prior wildtype vesicle tethering data. See Figure 7—figure supplement 1 for complete data and methods for more details. *, p≤0.05; **, p≤0.005; ***, p≤0.001.
All GFP-Sec4/Sec4 vesicle tether-to-fusion times; deletions and over expression comparisons and controls.
All n>40 events (average n=72 events) analyzed blind. To minimize the number of comparisons performed, these data were tested for differences among the shown groups via Kruskal-Wallis tests and then corrected post-hoc tests were performed comparing each experimental condition to the indicated control. Only significant results are indicated. All Median ±95% CI. Dotted vertical line from each Wildtype/control shown. See Materials and methods for additional details. *, p≤0.05; **, p≤0.005; ***, p≤0.001.
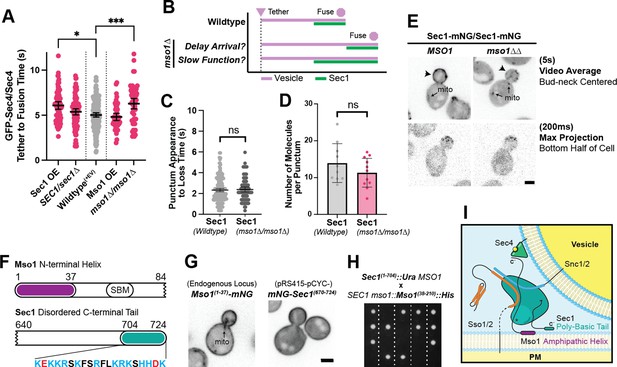
Sec1 and Mso1 both contribute to Sec1 membrane recruitment and function.
(A) Sec1 overexpression and deletion of mso1 significantly elongate vesicle tether-to-fusion time, while heterozygous deletion of Sec1 has little effect. *, p≤0.05; ***, p≤0.001. (B) Two potential models for elongation of vesicle tethering induced by loss of mso1. Mean ±95%CI. (C) Sec1-mNG puncta have similar longevity to wildtype in mso1∆ cells. Mean ± SD. (D) The number of Sec1 molecules per membrane punctum is unchanged in mso1∆ cells. One-tailed unpaired t-test, n≥10, p=0.1. (E) Broad plasma-membrane association of Sec1 is diminished in mid-sized mso1∆ cells. (F) Schematic diagram of Mso1 N-terminal amphipathic helix and Sec1-Binding Motif (SBM) and the sequence of a portion of the Sec1 C-terminal tail. (G) The amphipathic alpha-helical N- terminus of Mso1 (aa1-37) and the C-terminus of Sec1 (aa670-724) both aid in plasma membrane localization. (H) Tetrad dissections show that loss of both the Mso1 N-terminus (aa1-37) and the last 20 residues of the Sec1 C-terminus is synthetically lethal. Five representative dissections are shown. See Figure 8—figure supplement 1 for controls. (I) With initial localization aid via Mso1, Sec1 templates the assembly of trans-SNARE complexes. A theoretical, but likely, intermediate state with Sec1 simultaneously bound to Sso1/2 and Snc1/2 is shown (Baker et al., 2015). Mso1’s N-terminus binds to the plasma membrane and interacts with Sec1 through its Sec1-binding motif (*) while Sec1 also interacts directly with the plasma membrane through its poly-basic tail. Loss of both of these PM-binding motifs is lethal. Mso1 may also contribute through reported interactions with the SNAREs and its C-terminus (C’) may interact with Sec4 to aid in recruitment of the complex to tethered vesicles (Weber et al., 2010; Weber-Boyvat et al., 2011).
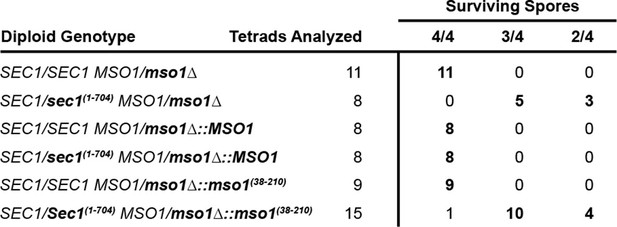
Controls and complete results for Sec1 truncation and replacement of mso1 for tetrad dissection experiments.

Alignment of many fungal Sec1 tails.
(A) Positively charged residues (blue) are highly concentrated in the Sec1-tail and (B) shown in the context of the entire alignment.
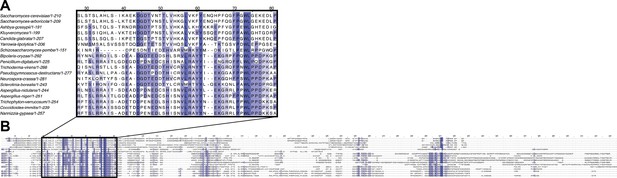
Alignment of several Mso1 protein sequences of select ascomycetes from Figure 8—figure supplement 2.
(A) The Sec1-binding region represents the primary conserved motif of Mso1 proteins while the rest of the protein (B) varies more between species.
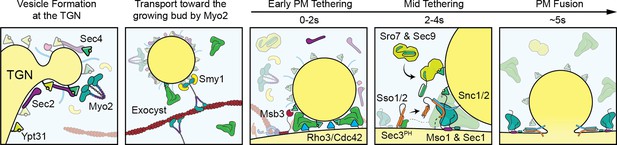
Visual timeline of secretion from Golgi to plasma-membrane fusion.
Active Ypt31/32 on Trans-Golgi Network (TGN) membranes recruits the secretory Rab-GEF Sec2, which thereby recruits Sec4 to the TGN ahead of vesicle formation. Though vesiculation of the Golgi occurs by as-yet-unknown mechanisms, newly activated Sec4 recruits the myosin-V motor protein Myo2 to the Golgi around the time of separation from TGN compartments and the Myo2-vesicle interaction is enhanced by the kinesin-related protein Smy1. During transport to the plasma membrane of the bud the complete exocyst complex, including Sec3, is recruited to vesicles through interactions with multiple proteins including Sec4, Myo2, and Sec2. Upon reaching the plasma membrane, Rho-proteins on the membrane (Rho3, but likely Cdc42 as well) concentrate slightly at the vesicle interface facilitating the activation of the exocyst, thereby aiding tethering. The initial plasma-membrane recognition and selection of tethering sites likely involves many other inputs, including but not limited to the PIP composition of the membrane, position of actin-cable termini, and scaffolding proteins like Boi1/2. Shortly after tethering, Sec2, Smy1, and Myo2 dissociate from the tethered vesicle and Msb3 begins inactivating portions of the vesicle-bound Sec4 population. Sro7 (and Sec9) are then recruited to the vesicle-PM interface through active Sec4 as well as multivalent interactions with the exocyst. SNARE assembly at the interface is subsequently aided by the exocyst—including Sec6 and Sec8, as well as Sec3, which is partially responsible for activating Sso1/2—and, finally, Mso1. Shortly before fusion, Mso1 and Sec1, which are enriched on the plasma membrane of the bud by their respective membrane-binding domains, begin accumulating at the vesicle interface and rapidly dissipates around the moment of exocyst complex disassociation and vesicle fusion. After membrane fusion, any remaining active Sec4 is returned to an inactive state by Msb3/4 to facilitate removal from the plasma membrane.
Videos
Example of a typical three-dimensional live-cell vesicle tethering video showing a single diploid cell containing one endogenously-tagged copy of GFP-Sec4 over 26 seconds.
Six planes were captured with 25ms exposures during continuous stage movement, resulting in 176ms per stack or ‘timepoint’. Several vesicle fusion events, defined as rapid loss of GFP-Sec4 signal from stationary puncta, are highlighted with green arrows. Video is played back at 2 x capture speed.
Sum projection video of the vesicle tethering and fusion event illustrated in Figure 2D.
Video is played back at 2 x capture speed.
Maximum intensity projection video of multiple vesicles tethering and apparently fusing at a single, un-resolvable location following a photobleaching event.
Green circle illustrates the region from which intensity data was extracted in Figure 3B. Video is played back at approximately 3 x capture speed.
Sum projection video of Sec1 localization events in a Sec1-mNG homozygous diploid cell.
Six planes were captured with 50ms exposure times, resulting in 350ms per timepoint. For ease of visibility, video is shown using a two-frame rolling signal average. Video is played back at 4 x capture speed.
Tables
Proteins discussed in this work.
| Protein | Brief Description | Interactions Relevant to this Work |
|---|---|---|
| Ypt31/32 | Paralogous Rab proteins which localize to late Golgi to facilitate TGN transport by Myo2 and help drive vesicle formation. | Sec2, Myo2, Exocyst (Sec15) |
| Sec4 | The secretory vesicle Rab protein which recruits effectors critical to exocytosis. | Sec2, Myo2, Msb3/4, Exocyst (Sec15), Sro7 |
| Sec2 | GEF (Guanine-nucleotide Exchange Factor) for Sec4. | Ypt31/32, Sec4, Exocyst (Sec15) |
| Msb3/4 | Redundant GAPs (GTPase Accelerating Proteins) for Sec4. | Sec4 |
| Myo2 | Class V Myosin motor protein responsible for transporting secretory vesicles (among other cargo) to the growing bud. Effector of Ypt31 and Sec4. | Sec4, Exocyst (Sec15), Ypt31/32 |
| Smy1 | Kinesin-related protein which increases Myo2 affinity for Sec4. Does not function as a typical kinesin. | Myo2 |
| Exocyst | The hetero-octameric secretory vesicle tethering complex. | See below: |
| Sec3 | Aids in plasma membrane binding, SNARE assembly. Exocyst component. | Rho1, Cdc42, Sso1/2, PI(4,5)P2 |
| Sec5 | Exocyst component. | N/a |
| Sec6 | Aids in SNARE assembly. Exocyst component. | Snc1/2, Sec1, Sec9 |
| Sec8 | Exocyst component. | N/a |
| Sec10 | Exocyst component. | N/a |
| Sec15 | Directs the exocyst to secretory vesicles. Exocyst component. | Ypt31/32, Sec2, Sec4, Myo2 |
| Exo70 | Aids in initial plasma membrane binding. Exocyst component. | Rho3, Cdc42, PI(4,5)P2 |
| Exo84 | Aids in SNARE assembly. Exocyst component. | Sro7 |
| Rho3 | Polarity regulating Rho-GTPase. Helps “activate” the exocyst through Exo70. | Exocyst (Exo70) |
| Cdc42 | Polarity regulating Rho-GTPase. | Exocyst (Sec3, Exo70) |
| Snc1/2 | Secretory vesicle resident SNARE proteins. | Sso1/2, Sec9, Sec1, Mso1 |
| Sso1/2 | Plasma membrane resident SNARE proteins. | Snc1/2, Sec9, Sec1, Mso1 |
| Sec9 | Cytosolic SNARE protein. | Snc1/2, Sso1/2, Sro7, Sec6, Sec1, Mso1 |
| Sro7/77 | Lgl/Tomosyn homologs and effectors of Sec4. Aids in vesicle tethering and Sec9 regulation. | Sec4, Exocyst (Exo84), Sec9 |
| Sec1 | Sec1-Munc18 Family protein essential for secretory vesicle fusion with the plasma membrane. | Snc1/2, Sso1/2, Sec9, Mso1 |
| Mso1 | Small fungal Sec1 accessory which is thought to aid in SNARE assembly. Reported Sec4 effector. | Sec1, Sec4 |
Additional files
-
Supplementary file 1
All supplementary files described in methods.
(1a) Yeast strains, (1b) plasmids, and (1c) oligos used in this study. (1d) Mouse Myosin-Vb sequence used for generating the fluorescent Myo2 markers used. (1e) All sample sizes for statistical tests. (1f) All raw tethering and component lifetime data.
- https://cdn.elifesciences.org/articles/78750/elife-78750-supp1-v2.xlsx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/78750/elife-78750-mdarchecklist1-v2.docx