Involvement of ILC1-like innate lymphocytes in human autoimmunity, lessons from alopecia areata
Figures
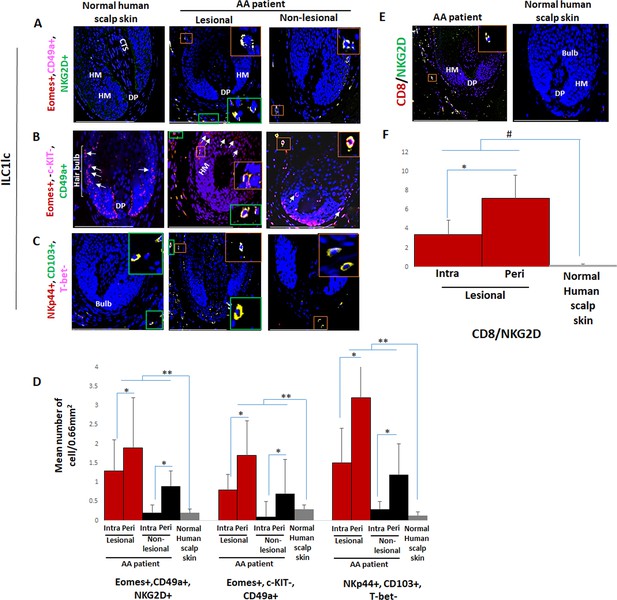
Immunofluorescence microscopy analyses of ILC1lc and CD8+/NKG2D+ cells in alopecia areata (AA) scalp skin.
(A) ILC1lc (EOMES+, CD49a+, and NKG2D+) around HF in normal scalp skin, intrafollicular and perifollicular ILC1lc infiltrates in lesional and in non-lesional AA scalp patient. (B) EOMES+, c-KIT-,CD49a+, and (C) NKp44+, CD103+, T-bet- ILC1lc. For each panel, yellow staining indicates double staining A-EOMES+, NKG2D+; B- EOMES+, CD49a+; C- NKp44+,CD103+ (D) Quantitative immunohistomorphometry (qIHM) shows an increased number of ILC1lc in AA patients as compared to normal volunteers and increased number of the cells in lesional versus non-lesional areas of the patients. There is a significant increased perifollicular than intrafollicular ILC1lc in the lesional and non lesional areas. (E) CD8+/NKG2D+ cells around HF in AA scalp patient and absence of these cells in normal scalp skin of normal scalp skin. (F) There is an increased number of CD8+/NKG2D+ cells in HFs of AA patients compared to normal scalp skin and a significant lower number of ILC1lc versus CD8+/NKG2D+ cells in AA scalp skin. N=6 biopsies /AA patients and six biopsies /healthy donors from six independent donors, three areas were evaluated per section, and three sections per biopsy. Following Shapiro-Wilk test, Student’s t-test: *p<0.05, **p<0.01 or Mann Whitney U test: #p<0.05. Scale bars, 50 µm. CTS- connective tissue sheath, DP - dermal papilla, HM - hair matrix, White arrow- c-KIT stained melanocyte.
-
Figure 1—source data 1
Quantitative data for immunofluorescence microscopy analyses of ILC1lc and CD8+/NKG2D+ cells in AA scalp skin.
- https://cdn.elifesciences.org/articles/80768/elife-80768-fig1-data1-v1.xlsx
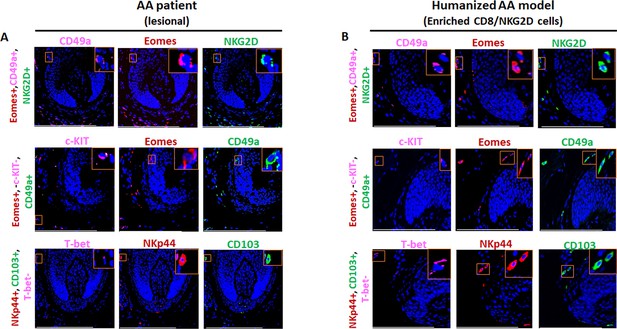
Single channels immunofluorescence microscopy analyses of various markers in AA scalp skin and in AA-induced xenotransplants.
(A) Single channels of EOMES, T-bet, CD49a+, NKG2D+, c-kit, CD103, and NKp44 expressing cells in alopecia areata (AA) scalp patient and (B) in the humanized AA model. N=4 biopsies from AA patients and five biopsies from the humanized AA mouse model. Scale bars, 50 µm.
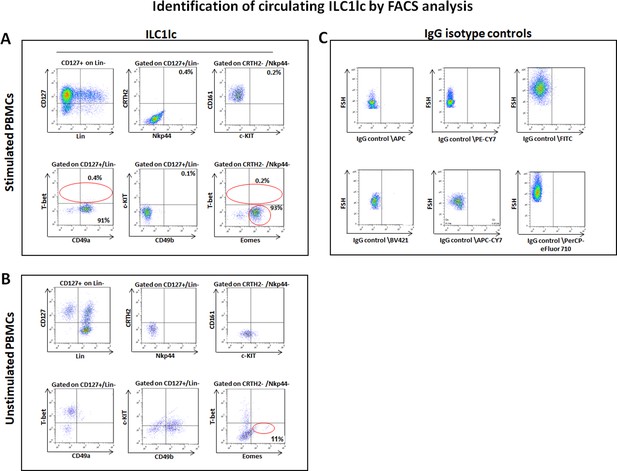
Circulating ILC1lc expanded and characterized by FACS analysis.
(A) PBMCs activated by IL-18, IL-33 and IL-12 were sorted by FACS Aria and characterized by FACS analysis. ILC1lc markers were identified by the expression of CD127+, CD161+, c-KIT-, and CRTH2-, high levels of integrin α1 (CD49a) expression, combined with the absence of integrin α2 (CD49b) and transcription factors Eomeshi and T-betlo (B) unstimulated PBMCs (C) isotype controls. N=10 blood donors, 1.5 × 106 cells/blood donor, analysis was performed in triplicates from each of the blood donors. Following Shapiro-Wilk test, Student’s t-test, p<0.05.
-
Figure 2—source data 1
Quantitative data for circulating ILC1lc expanded and characterized by FACS analysis.
- https://cdn.elifesciences.org/articles/80768/elife-80768-fig2-data1-v1.xlsx
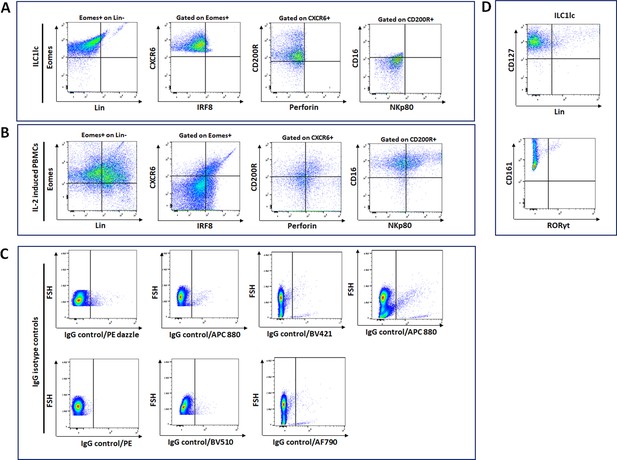
Circulating ILC1lc expanded and characterized by FACS analysis.
(A) PBMCs activated by IL-18, IL-33, and IL-12 were sorted by FACS Aria and characterized by FACS analysis. ILC1-like cell markers were identified by the expression of Eomes+, CXCR6+, CD200R+, IRF8-, Perforin-, CD16-, and NKP80 (B) IL-2 induced PBMCs (C) isotype controls. (D) expression of CD127+, CD161 +, and RORγt-. N=4 blood donors, 1.5 × 106 cells/blood donor, analysis was performed in triplicates from each of the blood donors. Following Shapiro-Wilk test, Student’s t-test, p<0.05.
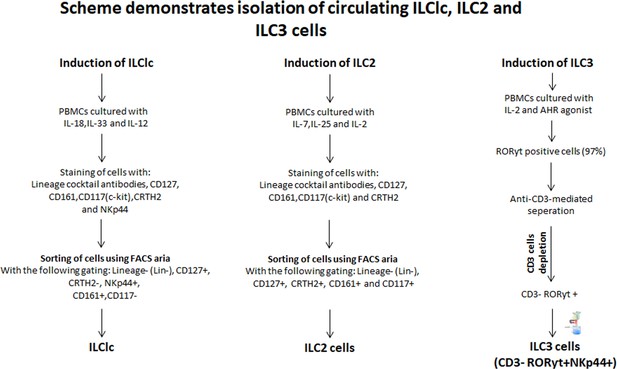
Scheme demonstrating the isolation of ILC1lc, ILC2s, and ILC3s cells from PBMCs of healthy human volunteers.
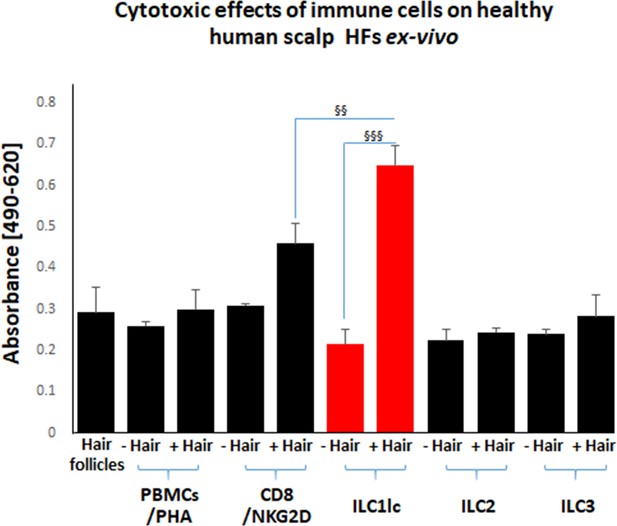
Cytotoxic effects of CD8+/NKG2D+and ILC1lc on normal human scalp HF ex vivo.
These cell populations were placed separately into wells with (+Hair) dissected HFs and without (-Hair). Cytotoxic effects of these cell populations on normal human scalp HF ex vivo were studied by measuring the spontaneous release of lactate dehydrogenase (LDH) from the microdissected HFs. Increased cytotoxicity of ILC1lc co-cultured with HFs compared to CD8+/NKG2D+, as well as to ILC2s and ILC3s, and PBMCs/PHA cells. N=20–24 HFs/group derived from three independent donors analyzed in three independent HF organ culture experiments. Following Shapiro-Wilk test and Dunn’s test §p<0.05, §§p<0.01, §§§p<0.001.
-
Figure 3—source data 1
Quantitative data for cytotoxic effects of CD8+/NKG2D+ and ILC1lc on normal human scalp HF ex vivo.
- https://cdn.elifesciences.org/articles/80768/elife-80768-fig3-data1-v1.xlsx
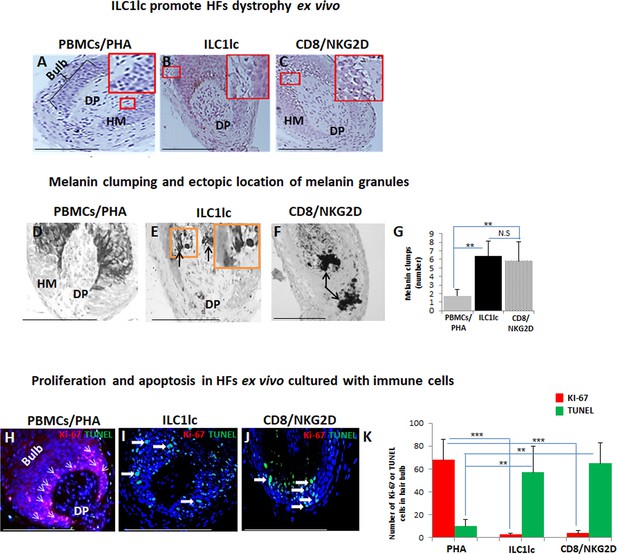
Hair follicles (HFs) dystrophy, melanin clumping, and apoptosis in normal human scalp HF ex vivo co-cultured with ILC1lc and CD8+/NKG2D+ cells.
(A–C) H&E staining revealed undifferentiated and prominent matrix cells, condensed dermal papilla, and the appearance of apoptotic cells, N=15–19 HFs/group from three independent donors. (D–G) Masson-Fontana histochemistry revealed melanin clumping and ectopic location of melanin granules only in HFs co-cultured with CD8+/NKG2D+and ILC1lc, but not in HFs cultured with PBMCs/PHA. N=7–11, HFs/group from three independent donors. Following Shapiro-Wilk test,Student’s t-test: *p<0.05, **p<0.01, ***p<0.001. (H–K) HFs co-cultured with ILC1lc or CD8+/NKG2D+ cells showed a significantly decreased proliferation (pink, arrowhead) and increased apoptosis (green, wide arrows). N=6 HFs/group from two independent donors, three areas were evaluated per section. Following Shapiro-Wilk test, Student’s t-test: *p<0.05, **p<0.01, ***p<0.001 in the anagen hair bulb compared to HFs cultured with PBMCs/PHA. Scale bars, 50 µm. DP - dermal papilla, HM - hair matrix.
-
Figure 4—source data 1
Quantitative data for HFs dystrophy, melanin clumping and apoptosis in normal human scalp HF ex vivo co-cultured with ILC1lc and CD8+/NKG2D+ cells.
- https://cdn.elifesciences.org/articles/80768/elife-80768-fig4-data1-v1.xlsx
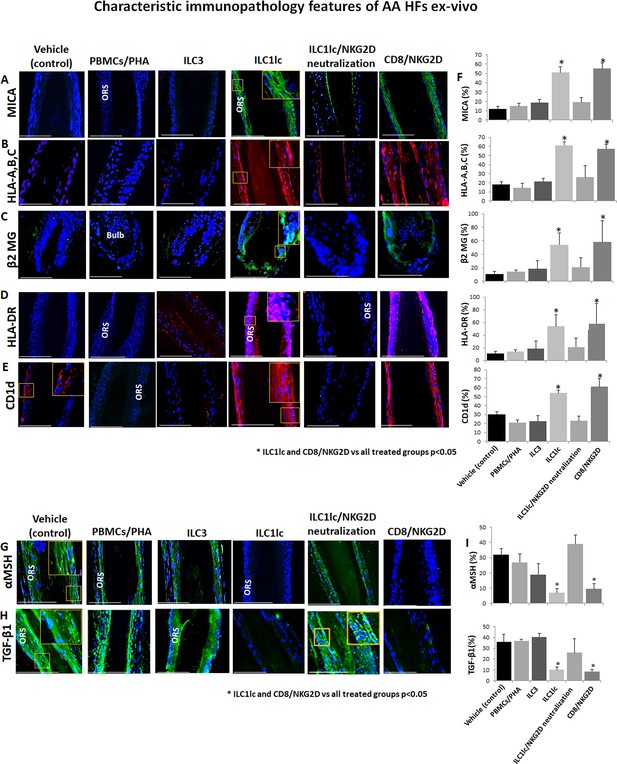
Characteristic immunopathology features of alopecia areata (AA) hair follicles (HFs).
(A) MICA, (B) HLA-A,B,C, (C) β2 MG, (D) HLA-DR, and (E) CD1d, expression by HFs epithelium, which had been co-cultured with either ILC1lc or CD8+/NKG2D+ cells but not in the control HFs, which had been co-cultured with either ILC3s, PBMCs/PHA, ILC1lc /NKG2D neutralization or in the untreated HFs. (F) quantitation. (G) The immune inhibitory HF immune privilege guardians, α-MSH and (H) TGF-β1 almost disappeared in HFs/ ILC1lc and HFs/NKG2D but were prominently present in ILC1lc /NKG2D neutralization and control HFs, N=9–12 HFs/group from three independent donors, three areas were evaluated per section. Following Shapiro-Wilk test,Student’s t-test, *p<0.05. Scale bar, 100 µm. ORS - outer root sheet.
-
Figure 5—source data 1
Quantitative data for characteristic immunopathology features of AA HFs.
- https://cdn.elifesciences.org/articles/80768/elife-80768-fig5-data1-v1.xlsx
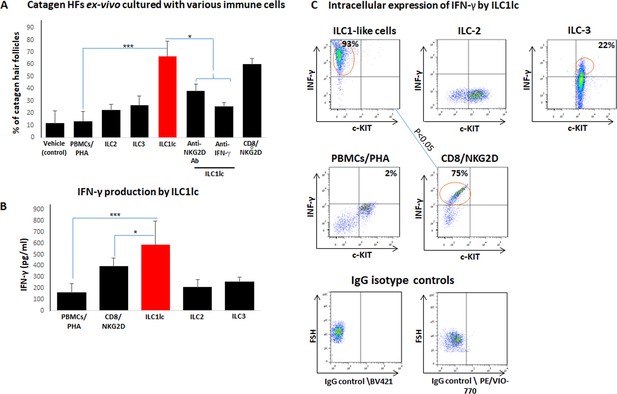
Transition of anagen to catagen hair follicles (HFs) following culture with ILC1lc or CD8+/NKG2D+ cells in human scalp HF ex vivo.
(A) These immune cells significantly accelerated the transformation of anagen HFs into catagen HFs ex vivo compared to ILC2, ILC3, PBMCs/PHA, and neutralizing anti- IFN-γ, anti-NKG2D antibodies. N=28–34 HFs/group taken from six independent donors, Student’s t-test: *p<0.05, **p<0.01, ***p<0.001. (B) ELISA analysis revealed increased IFN-γproduction by ILC1lc /HFs compared to production by CD8+/NKG2D+ cells, ILC2s, ILC3s, and PBMCs/PHA. N=6 healthy donors, 6 × 106 cells from each donor. Following Shapiro-Wilk test, Student’s t-test: *p<0.05, **p<0.01, ***p<0.001. (C) FACS analysis revealed a significant increased intracellular IFN-γexpression in ILC1lc co-cultured with HFs compared to the effector CD8+/NKG2D+and to ILC2s and ILC3s, N=6 blood donors, 1.5 × 106 cells/blood donor. Student’s t-test, p<0.05.
-
Figure 6—source data 1
Quantitative data for transition of anagen to catagen HFs following culture with ILC1lc or CD8+/NKG2D+ cells in human scalp HF ex vivo.
- https://cdn.elifesciences.org/articles/80768/elife-80768-fig6-data1-v1.xlsx
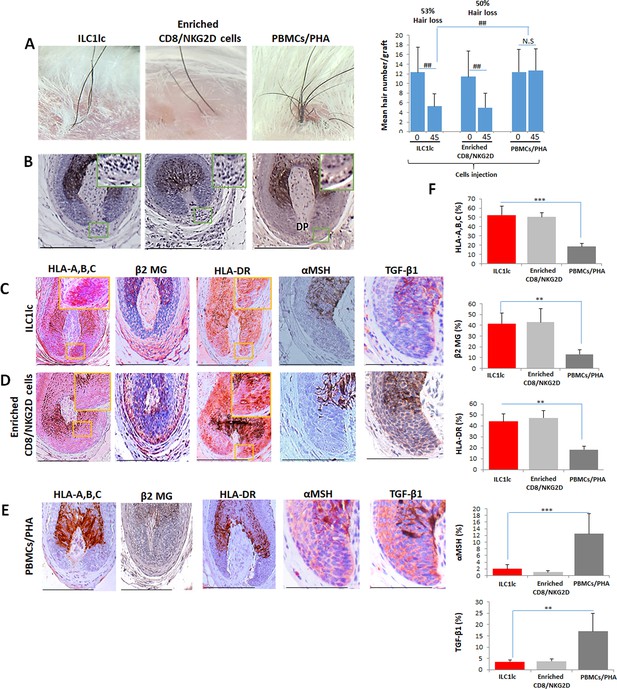
Development of alopecia areata (AA) in the humanized mouse model treated with ILC1lc.
(A) Significant hair loss is observed following the injection of ILC1lc and enriched CD8/NKG2D cells, while in the PBMCs/PHA treated group, hair number remains almost constant. N=7–9 xenotransplants/group from three independent donors, Following Shapiro-Wilk test, Mann-Whitney U test: #p<0.05, ##p<0.01. (B) HF dystrophy and perifollicular lymphocytic infiltrates around anagen hair follicles (HFs) (H&E staining) combined with strong expression of (C) HLA-A,B,C, β2 MG, HLA-DR, and downregulation of α-MSH and TGF-β1 in the ILC1lc and in (D) enriched CD8/NKG2D cells versus xenotransplants treated with (E) PBMCs/PHA (IHC staining) (F) quantitative data. N=5–9 xenotransplats/group from three independent donors. 4–5 defined reference areas were evaluated per section, and three sections per xenotransplants. Following Shapiro-Wilk test, Student’s t-test: *p<0.05, **p<0.01, ***p<0.001. Scale bar, 50 µm. DP - dermal papilla, HM - hair matrix.
-
Figure 7—source data 1
Quantitative data for development of AA in the humanized mouse model treated with ILC1lc.
- https://cdn.elifesciences.org/articles/80768/elife-80768-fig7-data1-v1.xlsx
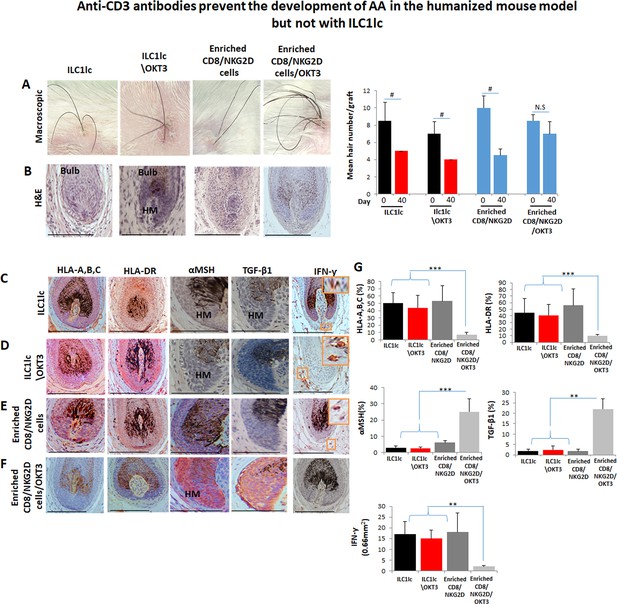
Anti-CD3 antibodies prevent the development of alopecia areata (AA) in scalp skin xenotransplants treated with enriched CD8/NKG2D but not with ILC1lc.
(A) Significant hair loss was observed following the injection of ILC1lc, ILC1lc /OKT3, or enriched CD8/NKG2D cells into normal scalp skin on SCID/beige mice, but not in those treated with enriched CD8+/NKG2D+/anti-CD3 (OKT3), N=5 xenotransplants/group from two independent donors. Following Shapiro-Wilk test, Mann-Whitney U test: #p<0.05, ##p<0.01. Hair follicle (HF) dystrophy combined with perifollicular lymphocytic infiltrate around anagen HFs (H&E staining) in xenotransplants treated with (B) ILC1lc, ILC1lc /OKT3, or enriched CD8/NKG2D. Normal HF in anagen in xenotransplant treated with enriched CD8/NKG2D/OKT3. Induction of HLA-A,B,C and of HLA-DR, downregulation of α-MSH and TGF-β1 by the follicular epithelium and increased dermal IFN-γ cells (IHC staining) in xenotransplants treated with (C) ILC1lc and similarly in (D) ILC1lc /OKT3 and (E) enriched CD8/NKG2D treated groups. Reduced expression of HLA-A,B,C, HLA-DR, and upregulation of α-MSH and TGF-β1 combined with decreased number of dermal IFN-γ cells were observed in xenotransplants treated with (F) enriched CD8/NKG2D/OKT3 cells. (G) Quantitative data, N=5–6 xenotransplants/group from two independent donors. 3–4 areas were evaluated per section, and three sections per xenotransplant. Following Shapiro-Wilk test, Student’s t-test: *p<0.05, **p<0.01, ***p<0.001. Scale bar, 50 µm. HM - hair matrix.
-
Figure 7—figure supplement 1—source data 1
Quantitative data for anti-CD3 antibodies prevent the development of AA in scalp skin xenotransplants treated with enriched CD8/NKG2D but not with ILC1lc.
- https://cdn.elifesciences.org/articles/80768/elife-80768-fig7-figsupp1-data1-v1.xlsx
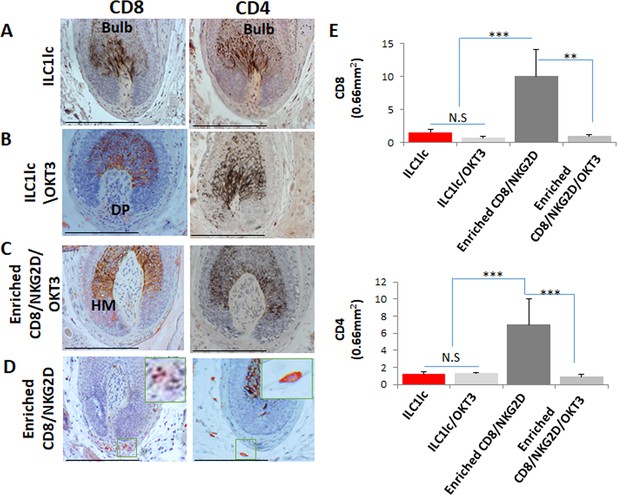
Dermal infiltrates of the various treated xenotransplants.
Several CD8 and CD4 in xenotransplants treated with (A) ILC1lc and similarly in xenotransplants treated with (B) ILC1lc /OKT3, and (C) enriched CD8/NKG2D/OKT3 cells versus increased CD8 and CD4 in xenotransplants treated with (D) enriched CD8/NKG2D cells. (E) Quantitative data,N=5–6 xenotransplants/group from two independent donors, 3–four areas were evaluated per section, and three sections per xenotransplant. Following Shapiro-Wilk test, Student’s t-test: *p<0.05, **p<0.01, ***p<0.001. Scale bar, 50 µm. DP - dermal papilla,HM - hair matrix.
-
Figure 7—figure supplement 2—source data 1
Quantitative data for dermal infiltrates of the various treated xenotransplants.
- https://cdn.elifesciences.org/articles/80768/elife-80768-fig7-figsupp2-data1-v1.xlsx
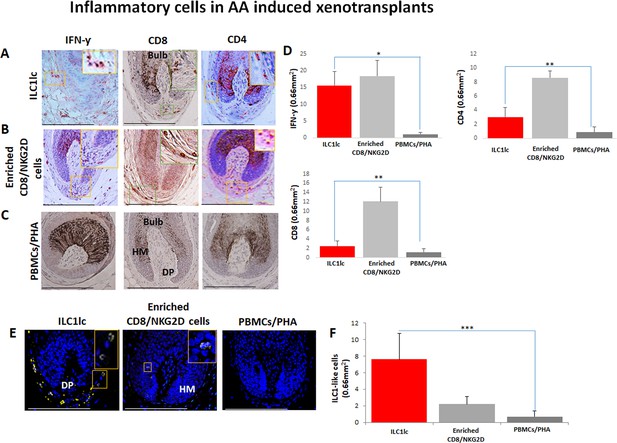
Development of alopecia areata (AA) in normal human scalp skin xenotransplants treated with (A) ILC1lc.
Infiltrates of IFN-γ +, CD8, and CD4 cells in group treated with (B) enriched CD8/NKG2D cells. Decreased number of IFN-γ +, CD8, and CD4 cells in the (C) PBMCs/PHA treated one. (D) Quantitative data (E) presence of ILC1lc in transplants injected with ILC1lc compared to the virtual absence of these cells in enriched CD8/NKG2D and PBMCs/PHA treated mice.(F) Quantitative data N=5–9 xenotransplats/group from three donors. 4–5 areas were evaluated per section, and three sections per xenotransplant. Following Shapiro-Wilk test, Student’s t-test: *p<0.05, **p<0.01, ***p<0.001. Scale bar, 50 µm. DP - dermal papilla,HM - hair matrix.
-
Figure 7—figure supplement 3—source data 1
Quantitative data for development of AA in normal human scalp skin xenotransplants treated with ILC1lc, enriched CD8/NKG2D and PBMCs/PHA.
- https://cdn.elifesciences.org/articles/80768/elife-80768-fig7-figsupp3-data1-v1.xlsx
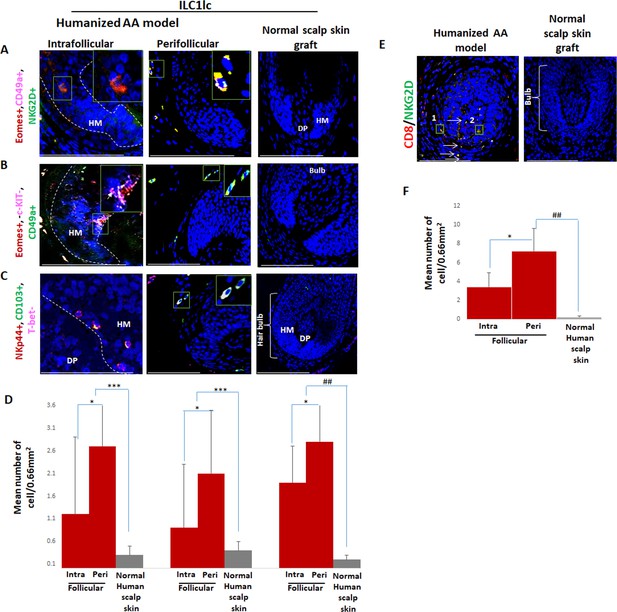
Immunofluorescence microscopy analysis of ILC1lc and enriched CD8/NKG2D cells in AA-induced xenotransplant.
(A) EOMES+, CD49a+, and NKG2D+ around hair follicle (HF) in normal scalp skin, intrafollicular and perifollicular ILC1lc infiltrates in AA-induced xenotransplants (B) EOMES+,c-KIT-,CD49a+, and (C) NKp44+, CD103+, T-bet- ILC1lc. Absence of these cells in normal scalp xenotransplant. For each panel, yellow staining indicates double staining A-EOMES+, NKG2D+; B- EOMES+,CD49a+; C- NKp44+, CD103+. (D) Quantitation. (E) CD8+/NKG2D+ cells around HF in AA-induced xenotransplant versus absence of the cells in normal xenotransplant. (F) The quantitative data demonstrate the significant increased CD8+/NKG2D+ cells in HFs of alopecia areata (AA) humanized mice compared to normal scalp xenotransplants. N=6 xenotransplants/ group from three independent donors, three areas were evaluated per section. Following Shapiro-Wilk test, Student’s t-test: *p<0.05, **p<0.01, ***p<0.001. Mann Whitney U test: #p<0.05, ##p<0.01.Scale bar, 50 µm. DP - dermal papilla, HM - hair matrix, White arrow- c-KIT stained melanocyte.
-
Figure 8—source data 1
Quantitative data for immunofluorescence microscopy analysis of ILC1lc and enriched CD8/NKG2D cells in AA-induced xenotransplant.
- https://cdn.elifesciences.org/articles/80768/elife-80768-fig8-data1-v1.xlsx
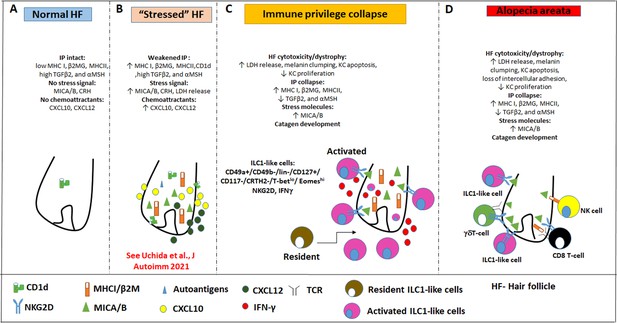
Pathobiology scenario: How ILC1lc can induce alopecia areata (AA).
(A) ILC1lc are rarely detected around the bulb of healthy human scalp hair follicles (HFs), which exhibit relative immune privilege and low or absent expression of MICA and MHC class I, and CD1d. (B) Various tissue stressors (in the current study: hair follicle microdissection and organ culture), can transiently weaken the hair follicle’s physiological immune privilege by upregulating the expression of MHC class I, MICA (a key activating NKG2D ligand), and of CD1d, along with the secretion of chemoattractants such as CXCL12. (C) This recruits and activates ILC1lc, which migrates towards the ‘stressed’ hair follicle and secretes IFN-γ, thus ultimately inducing HF-IP collapse. (D) Either alone or in conjunction with other recognized AA-inducing immune cells (i.e. CD8 +T cells which recognize hair follicle autoantigens now exposed by ectopically expressed MHC class I; NK cells, and γδTCs), ILC1lc can then induce the full AA phenotype, characterized by HF-IP collapse, premature hair follicle regression (catagen), and hair follicle dystrophy.
Additional files
-
Supplementary file 1
Microdissected, organ-cultured HFs are ‘stressed’ on day 1, but become equilibrated on day 3, as assessed by the listed objective read-out parameters that indicate a temporarily weakened HF immune privilege and HF damage as well as MICA overexpression on day 1.
Instead, the expression of HF immune privilege guardians (αMSH, TGFß2) (Bertolini et al., 2020) is preserved. This makes freshly microdissected healthy human scalp HFs one day after initiation of HF organ culture optimally suited as ‘stressed’ human (mini-) organs that strongly express the NKG2D-activating ‘danger’ ‘signal,’ MICA, which also is overexpressed by human AA HFs (Li et al., 2016) (these data are repeated from Uchida et al., 2021 to illustrate the HF distress/partial IP collapse of microdissected human scalp HFs one day 1 after initiation of organ culture).
- https://cdn.elifesciences.org/articles/80768/elife-80768-supp1-v1.docx
-
Supplementary file 2
Route of injections, volume, and the number of immune cells injected into the normal healthy human xenotransplants.
- https://cdn.elifesciences.org/articles/80768/elife-80768-supp2-v1.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/80768/elife-80768-mdarchecklist1-v1.docx





