Bacterial diet affects the age-dependent decline of associative learning in Caenorhabditis elegans
Figures
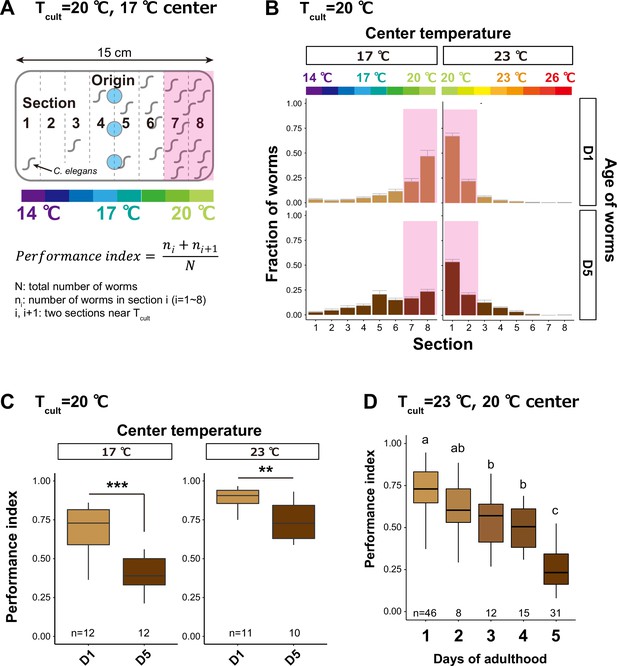
Thermotaxis performance declines with age.
(A) Schematic of thermotaxis assay. Animals were placed at light blue circles on a thermal gradient without food. The pink rectangle indicates the sections around the Tcult. After 1 hr, the number of animals in each section was counted to calculate the thermotaxis performance index using the indicated formula. (B, C) Age-dependent changes in thermotaxis behavior. D1 and D5 animals were cultivated with E. coli at 20°C and placed at the center of a 14–20 or 20–26°C gradient. (B) Distributions of animals (pink rectangle: the sections around the Tcult) on the thermotaxis plates. (C) Box plots of thermotaxis performance indices. The number of experiments is shown. Statistics: Student’s t-test compared to D1 adults. **p < 0.01, ***p < 0.001. (D) Box plots summarizing thermotaxis performance indices of animals at different ages. Animals were cultivated with E. coli at 23°C and placed at the center of a 17–23°C gradient. The number of experiments is shown. Statistics: The mean indices marked with distinct alphabets are significantly different (p < 0.05) according to one-way analysis of variance (ANOVA) followed by Tukey–Kramer test.
-
Figure 1—source data 1
Thermotaxis assays with 17 or 23°C center.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig1-data1-v1.xlsx
-
Figure 1—source data 2
Thermotaxis assays at different ages.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig1-data2-v1.xlsx
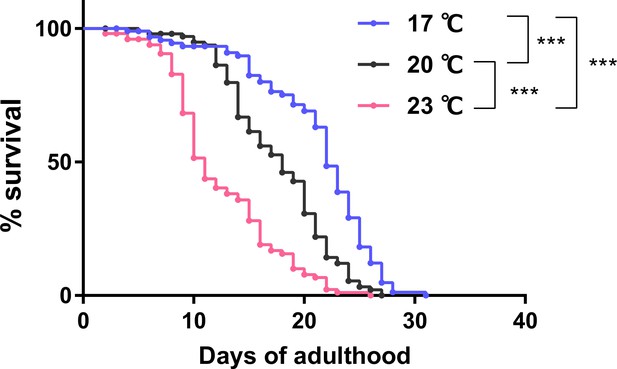
Survival curve of animals cultivated at different temperatures.
Survival curves of wild-type animals cultivated with E. coli from eggs at the indicated temperatures. N = 4 experiments with 25 animals/experiment (100 animals in total). Statistics: Log-rank test, ***p < 0.001.
-
Figure 1—figure supplement 1—source data 1
Lifespan at different temperatures.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig1-figsupp1-data1-v1.xlsx
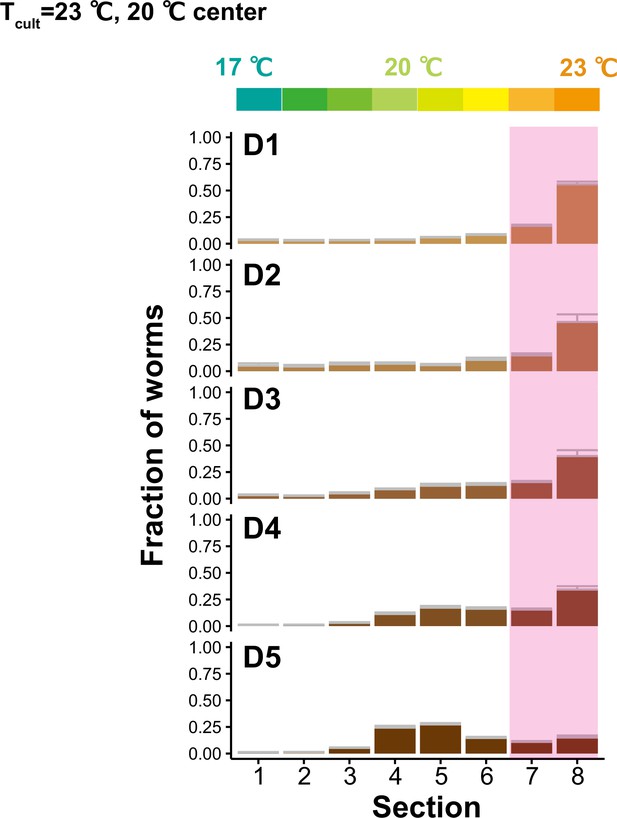
Distributions of animals at different ages on the temperature gradient.
The distributions of animals at D1–D5 on the temperature gradient after thermotaxis assays. Animals were cultivated at 23°C and placed at the center of the 17–23°C gradient. Pink rectangles indicate the sections around the Tcult.
-
Figure 1—figure supplement 2—source data 1
Distribution of animals on thermotaxis plates at different ages.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig1-figsupp2-data1-v1.xlsx

HT115-fed animals showed thermotaxis decline.
Box plots summarizing thermotaxis performance indices. Animals were cultivated with OP50 until D1 and then with OP50 or HT115 until D5. The number of experiments is shown. Statistics: The mean indices marked with distinct alphabets are significantly different (p < 0.05) according to one-way analysis of variance (ANOVA) followed by Tukey–Kramer test.
-
Figure 1—figure supplement 3—source data 1
Thermotaxis decline of HT115-fed animals.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig1-figsupp3-data1-v1.xlsx
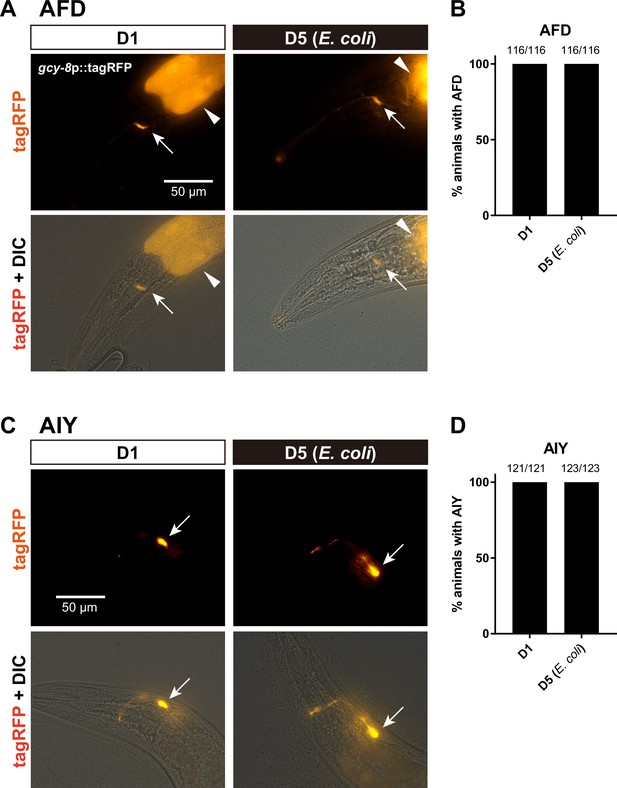
AFD and AIY are alive at D5.
TagRFP of NUJ296 knjIs15[gcy-8Mp::GCaMP6m+gcy-8Mp::tagRFP+ges-1p::tagRFP] and IK1144 njIs26[AIYp::GCaMP3+AIYp::tagRFP+ges-1p::tagRFP] were imaged to visualize AFD and AIY, respectively. (A, C) Representative images of the head of C. elegans for tagRFP (top panels) or tagRFP + differential interference contrast (DIC) (bottom panels). Scale bar: 50 µm. Arrows indicate the cell bodies of the neurons. Arrowheads in (A) indicate tagRFP expression in the intestine used as a co-injection marker. Bar graphs show the percentage of animals with AFD (B) and AIY (D) in D1 and D5, based on tagRFP signals in the cell body. The number indicates the number of animals with visible neurons over the total number of animals examined.

E. coli-fed aged animals sense food.
Food recognition assays of D1 in (A) and D5 adults in (B). Animals were pre-conditioned with (well-fed) or without (food-deprived) E. coli and assayed on plates with or without E. coli. Animals’ locomotion was evaluated by body bends in 20 s. The presence of food on the assay plate slows down the locomotion of well-fed animals (basal slowing response). Pre-conditioning animals without food enhanced the basal slowing response (enhanced slowing response). The number of animals examined is shown. Error bars: Standard error of the mean (SEM). Statistics: Two-way analysis of variance (ANOVA) with Tukey’s multiple comparison test, ***p < 0.001; **p < 0.01; ns, p > 0.05.
-
Figure 1—figure supplement 5—source data 1
Food recognition assays of young and aged animals.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig1-figsupp5-data1-v1.xlsx
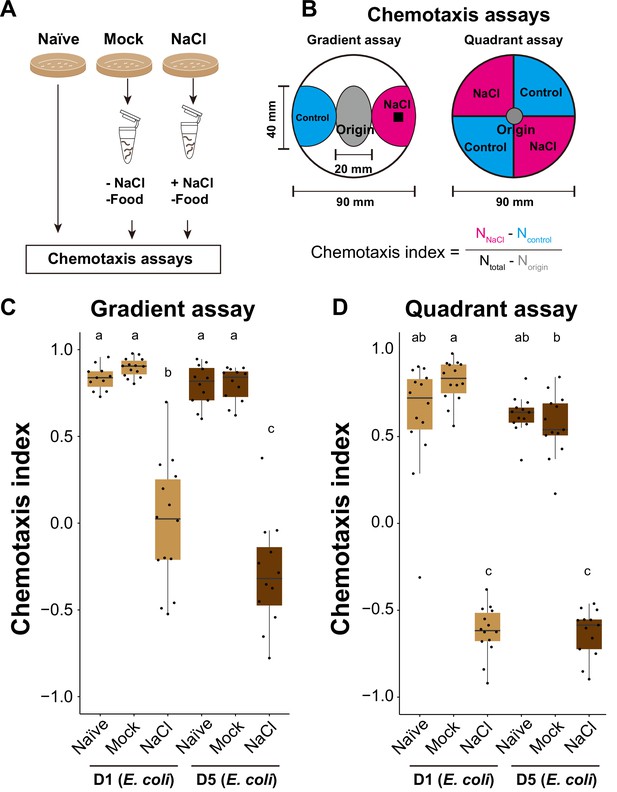
Aged E. coli-fed animals perform salt-avoidance behavior irrespective of diet.
(A) Schematic of conditioning of animals. Animals were conditioned in the absence of food with (NaCl) or without (Mock) NaCl. (B) Schematic of salt-avoidance assays. Gradient and quadrant assays were used to evaluate salt-avoidance behavior. The chemotaxis index was calculated using the indicated formula based on the number of animals in each area after the assay. (C, D) Box plots summarizing chemotaxis indices of salt-avoidance assays. The number of experiments is shown. Statistics: The mean indices marked with distinct alphabets are significantly different (p < 0.05) according to one-way analysis of variance (ANOVA) followed by Tukey–Kramer test.
-
Figure 1—figure supplement 6—source data 1
Salt-taxis assays of young and aged animals.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig1-figsupp6-data1-v1.xlsx
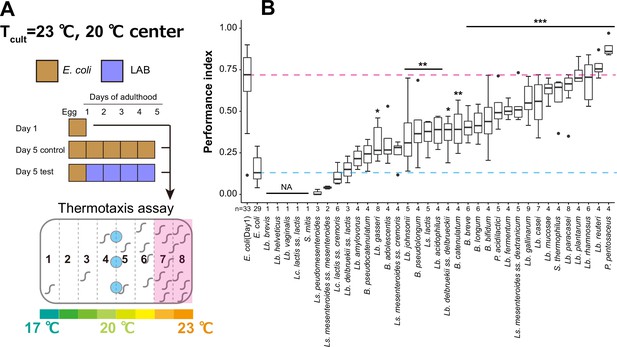
Lactic acid bacteria (LAB) screen for thermotaxis in aged animals.
(A) Schematic of the screening procedure. Animals were cultivated at 23°C with E. coli until D1 and transferred to E. coli or LAB plates every day until D5. At D5, animals were subjected to thermotaxis assays with a thermal gradient of 17–23°C. (B) Box plots comparing thermotaxis performance indices of D5 animals fed LAB to those of D1 (pink dashed line) and D5 animals (light blue dashed line) fed E. coli. ‘Not applicable’ (NA) indicates that animals fed those LAB were not subjected to the assay because they were sick or dead. Abbreviations: B, Bifidobacterium; Lb, Lactobacillus; Lc, Lactococcus; Ls, Leuconostoc; P, Pediococcus; S, Streptococcus. The number of experiments is shown. Statistics: One-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test compared to D5 adults fed E. coli, ***p < 0.001; **p < 0.01; *p < 0.05.
-
Figure 2—source data 1
Thermotaxis assays of aged animals fed with different lactic acid bacteria.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig2-data1-v1.xlsx
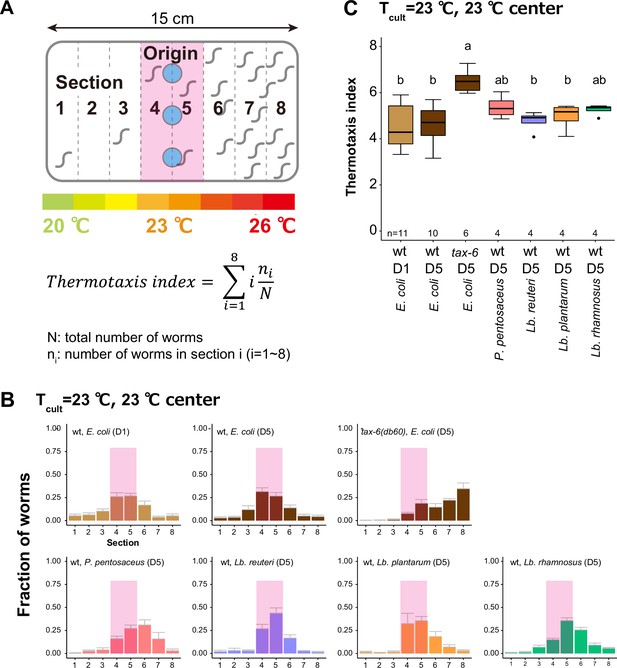
Animals fed heterofermentative lactic acid bacteria (LAB) are not thermophilic.
Animals were cultivated at 23°C and placed at the center of a 20–26°C gradient to examine the thermophilicity. D5 animals with indicated genotypes were cultivated with E. coli or Lb. reuteri from D1. tax-6(db60) was used as a thermophilic mutant. (A) Schematic of thermotaxis assay and formula to calculate thermotaxis index. (B) Distribution of animals on the temperature gradient after thermotaxis assays. Pink rectangles indicate the sections around the Tcult. (C) Box plots summarizing thermotaxis indices of animals under indicated conditions. The number of experiments is shown. Statistics: The mean indices marked with distinct alphabets are significantly different (p < 0.05) according to one-way analysis of variance (ANOVA) followed by Tukey–Kramer test.
-
Figure 2—figure supplement 1—source data 1
Thermophilicity of aged animals fed with different lactic acid bacteria.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig2-figsupp1-data1-v1.xlsx
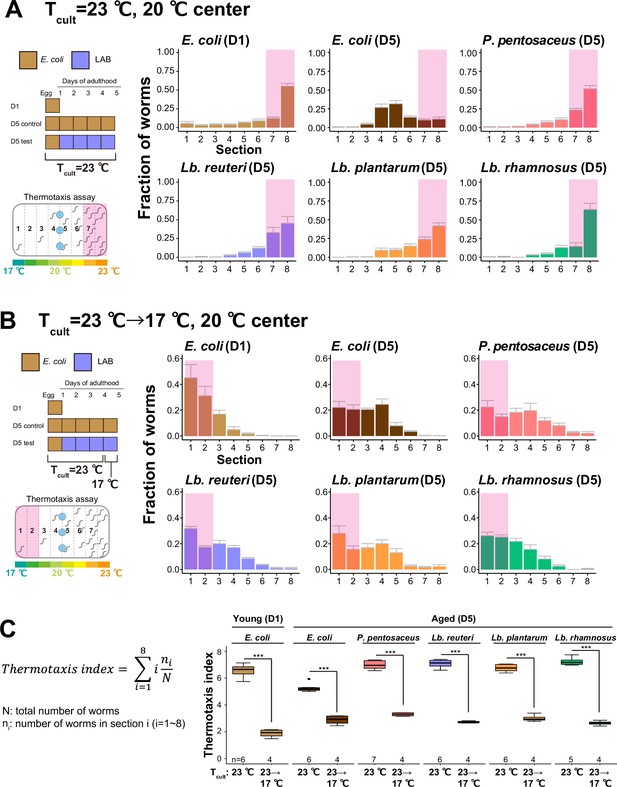
Lactic acid bacteria (LAB)-fed aged animals learn a new Tcult.
(A, B) The distribution of animals on thermotaxis plates. Pink rectangles indicate the sections around the Tcult. (A) D1 or D5 animals fed indicated bacteria were cultivated at 23°C and placed at the center of a 17–23°C gradient. (B) Temperature shift assay. Tcult was shifted from 23 to 17°C 1 day before the assay. Animals were placed at the center of the 17–23°C gradient. (C) Box plots summarizing thermotaxis indices corresponding to (A) and (B). Thermotaxis indices were calculated to examine the mean distribution of animals on thermotaxis plates using the indicated formula. The number of experiments is shown. Statistics: Student’s t-test for comparison between Tcult = 23°C and Tcult = 23°C → 17°C, ***p < 0.001.
-
Figure 3—source data 1
Thermotaxis assays of aged animals fed with the select lactic acid bacteria.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig3-data1-v1.xlsx
-
Figure 3—source data 2
Temperature shift experiment of aged animals fed with the select lactic acid bacteria.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig3-data2-v1.xlsx
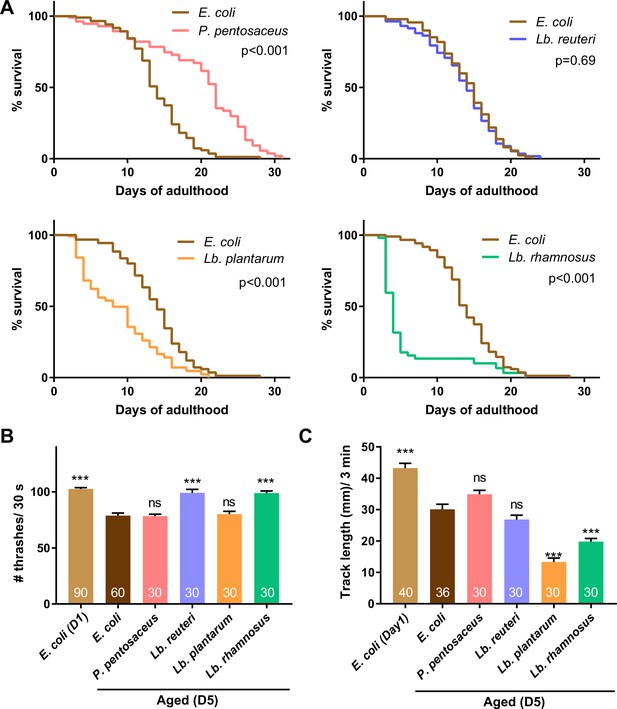
Lactic acid bacteria (LAB) show various effects on lifespan and locomotion.
Animals were fed E. coli until D1 and indicated bacteria after D1. (A) Survival curves of animals fed indicated LAB are shown with control animals fed E. coli. Nematode Growth Medium (NGM) plates without peptone were used to avoid the undesired growth of E. coli on LAB plates. N = 4 experiments with 25 animals/experiment (100 animals in total). Statistics: Log-rank test. p values are shown. The number of thrashes in liquid (B) and distance of migration in 3 min on plates with E. coli (C) were measured to examine the locomotion of aged animals. The number of animals is shown in bars. Error bars: Standard error of the mean (SEM). Statistics: One-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test compared to D5 fed E. coli, ***p < 0.001; ns, p > 0.05.
-
Figure 4—source data 1
Lifespan of animals fed with the select lactic acid bacteria.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig4-data1-v1.xlsx
-
Figure 4—source data 2
Thrashing assays of aged animals fed with the select lactic acid bacteria.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig4-data2-v1.xlsx
-
Figure 4—source data 3
Locomotion of aged animals fed with different lactic acid bacteria.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig4-data3-v1.xlsx
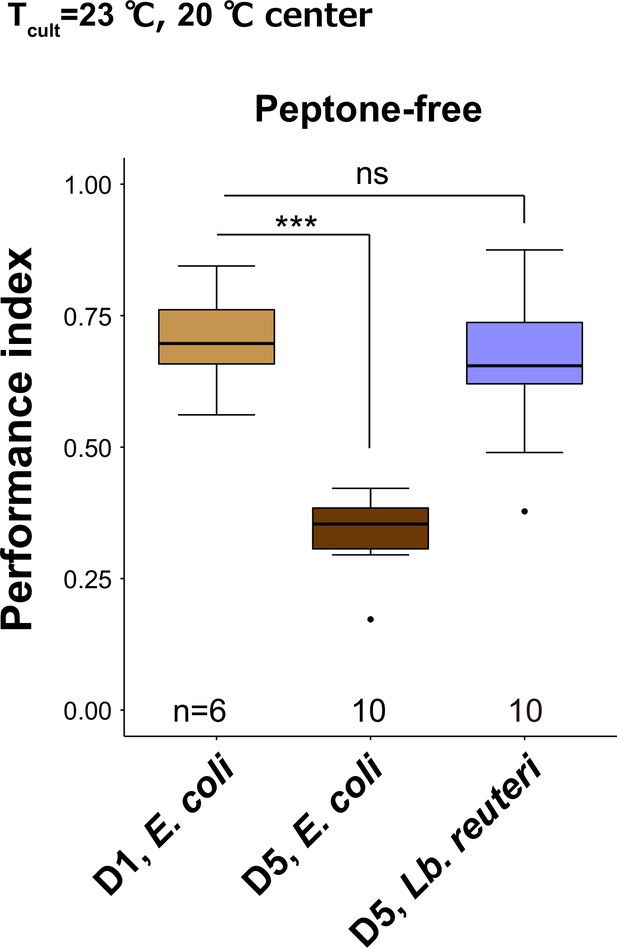
Diet affects thermotaxis of aged animals on peptone-free plates.
Box plots summarizing performance indices of animals cultivated on peptone-free Nematode Growth Medium (NGM) plates. D5 animals were cultivated at 23°C with E. coli or Lb. reuteri from D1. The number of experiments is shown. Statistics: One-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test compared to the D1 control, ***p < 0.001; ns, p > 0.05.
-
Figure 4—figure supplement 1—source data 1
Thermotaxis assays using peptone-free plates.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig4-figsupp1-data1-v1.xlsx
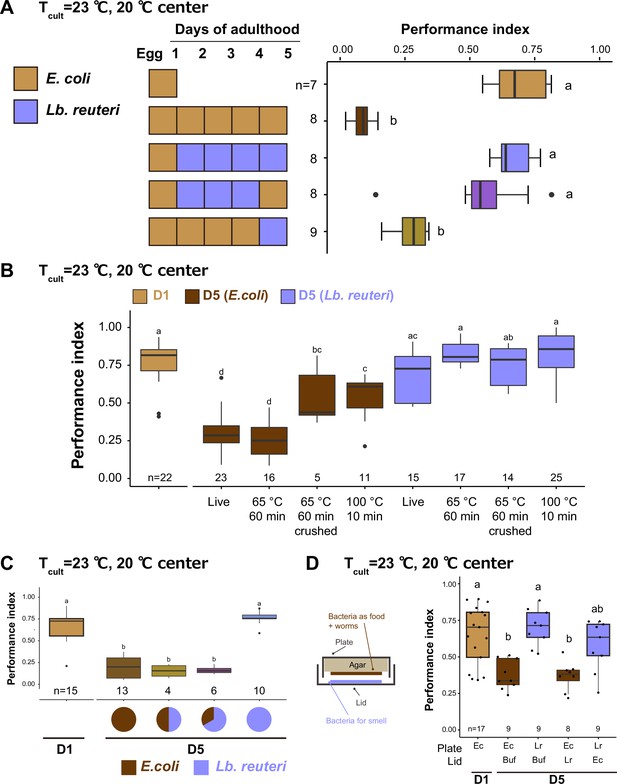
Bacteria affect thermotaxis of aged animals as nutrition.
Box plots show thermotaxis performance indices of animals fed indicated bacteria and cultivated at 23°C. Aged animals were transferred every day to new plates from D1. The number of experiments is shown. Statistics: The mean indices marked with distinct alphabets are significantly different (p < 0.05) according to one-way analysis of variance (ANOVA) followed by Tukey–Kramer test. (A) The short-term effects of diet. The diet was switched 1 day before the thermotaxis assay, as indicated in the schematic. (B) The effect of heat treatment and crushing of bacteria. The bacteria were killed by incubating at 65°C for 1 hr or 100°C for 10 min. After heat treatment, bacteria were crushed using a bead-based homogenizer for the crushed condition. (C) The mixture of bacteria. Live E. coli and Lb. reuteri were mixed at a 1:1 or 1:2 ratio with the final concentration of 0.1 g/ml and used as a diet. (D) The effect of bacterial odor. Animals were exposed to the bacterial odor by putting the bacterial solution on the lid and cultivated, as shown in the schematic. Ec: E. coli, Lr: Lb. reuteri.
-
Figure 5—source data 1
The effect of switching bacteria on the thermotaxis behavior of aged animals.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig5-data1-v1.xlsx
-
Figure 5—source data 2
The effect of heat-killed bacteria on the thermotaxis behavior of aged animals.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig5-data2-v1.xlsx
-
Figure 5—source data 3
The effect of mixed bacteria on the thermotaxis behavior of aged animals.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig5-data3-v1.xlsx
-
Figure 5—source data 4
The effect of bacterial smell on the thermotaxis behavior of aged animals.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig5-data4-v1.xlsx

Animals ingest E.coli and Lb. reuteri.
Representative images show fluorescently labeled bacteria in the intestine of C. elegans. Animals were cultivated with FICT-labeled E. coli, Lb. reuteri, or the mixture of fluorescently labeled and unlabeled bacteria. Both fluorescence and transmission light were simultaneously imaged to show that C. elegans ingested bacteria.
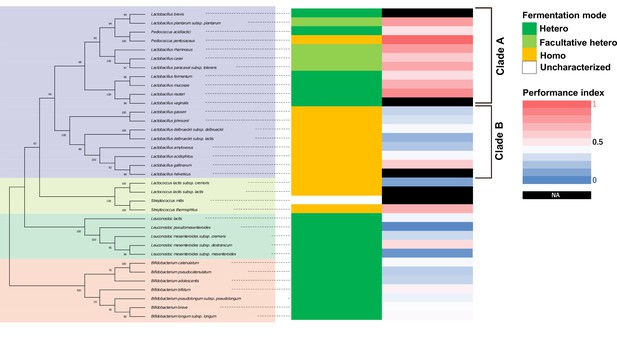
Lactobacilli in a clade are associated with high thermotaxis performance of aged animals.
Phylogenetic tree of lactic acid bacteria (LAB) based on 16S rRNA is shown with fermentation mode, and heatmap of performance indices of aged animals fed indicated LAB from D1. Bootstrap values are indicated at each node on the phylogenetic tree. Fermentation modes were categorized based on previous studies (see Table S1Supplementary file 2). The same data as Figure 2B were used for thermotaxis performance indices. NA in the performance indices heatmap indicates that animals fed those LAB were not subjected to the thermotaxis assay because they were sick or dead. Fermentation mode indicates obligatory hetero- (green), facultatively hetero- (light green), and obligatory homofermentative (orange) LAB.

Images of Gram-stained bacteria.
Images of Gram-stained E. coli and select lactic acid bacteria (LAB). E. coli and LAB are Gram-negative and -positive, respectively. Scale bar: 10 µm.
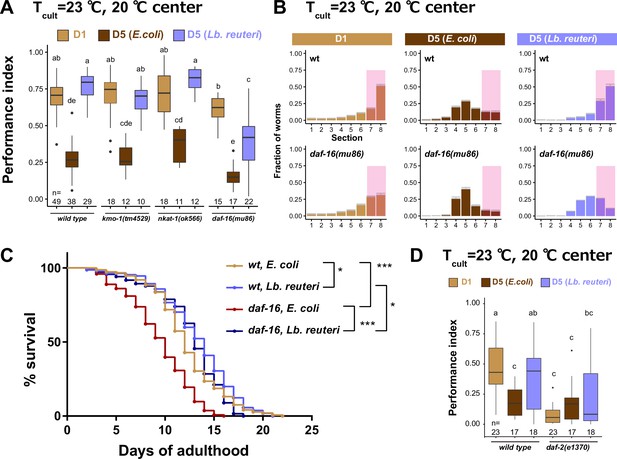
daf-16 is involved in the effect of Lb. reuteri on thermotaxis in aged animals.
(A) Box plots summarizing thermotaxis performance indices of animals with indicated genotypes in the different diet and age conditions. Animals were cultivated at 23°C with E. coli or Lb. reuteri from D1. The number of experiments is shown. Statistics: The mean indices marked with distinct alphabets are significantly different (p < 0.05) according to two-way analysis of variance (ANOVA) followed by Tukey–Kramer test. (B) Distribution of animals of indicated conditions on thermotaxis plates. Pink rectangles indicate the sections around the Tcult. (C) Survival curves of animals with indicated genotypes fed E. coli or Lb. reuteri from D1 and cultivated at 23°C. Nematode Growth Medium (NGM) plates without peptone were used. N = 6 experiments with 25 animals/experiment (150 animals in total). Statistics: Log-rank test, ***p < 0.001; *p < 0.05. (D) Box plots summarizing thermotaxis performance indices of animals with indicated genotypes. Animals were cultivated at 15°C for 96 hr to avoid dauer formation of daf-2(e1370) and then incubated at 23°C until D1 or D5 with E. coli or Lb. reuteri. The number of experiments is shown. Statistics: The mean indices marked with distinct alphabets are significantly different (p < 0.05) according to two-way ANOVA followed by Tukey–Kramer test.
-
Figure 7—source data 1
Thermotaxis of kmo-1, nkat-1, and daf-16 mutants.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig7-data1-v1.xlsx
-
Figure 7—source data 2
Distributions of daf-16 mutants on thermotaxis plates.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig7-data2-v1.xlsx
-
Figure 7—source data 3
Lifespan of daf-16 mutants fed with E. coli or Lb. reuteri.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig7-data3-v1.xlsx
-
Figure 7—source data 4
Thermotaix of daf-2 mutants.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig7-data4-v1.xlsx
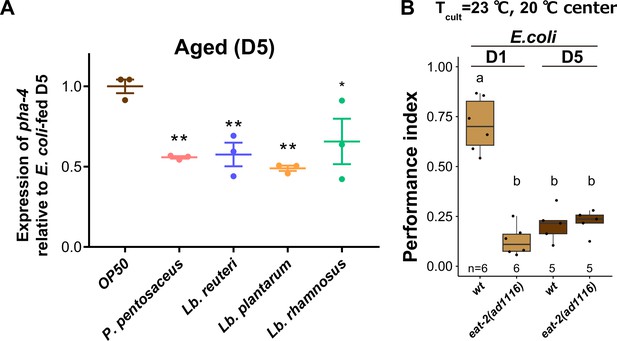
Lactic acid bacteria (LAB)-fed animals are not dietary restricted.
(A) Expression of pha-4 transcripts in aged animals fed indicated LAB relative to aged animals fed E. coli. (B) Box plots summarizing thermotaxis indices of wild type and eat-2 mutant animals cultivated at 23°C with E. coli. The number of experiments is shown. Statistics: Student’s t-test, ns, p > 0.05. Error bars: Standard error of the mean (SEM). Statistics: One-way analysis of variance (ANOVA) with Dunnett’s multiple comparison test, **p < 0.01; *p < 0.05.
-
Figure 7—figure supplement 1—source data 1
pha-4 expression of aged animals fed with the select lactic acid bacteria.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig7-figsupp1-data1-v1.xlsx
-
Figure 7—figure supplement 1—source data 2
Thermotaxis assays of eat-2 mutants.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig7-figsupp1-data2-v1.xlsx
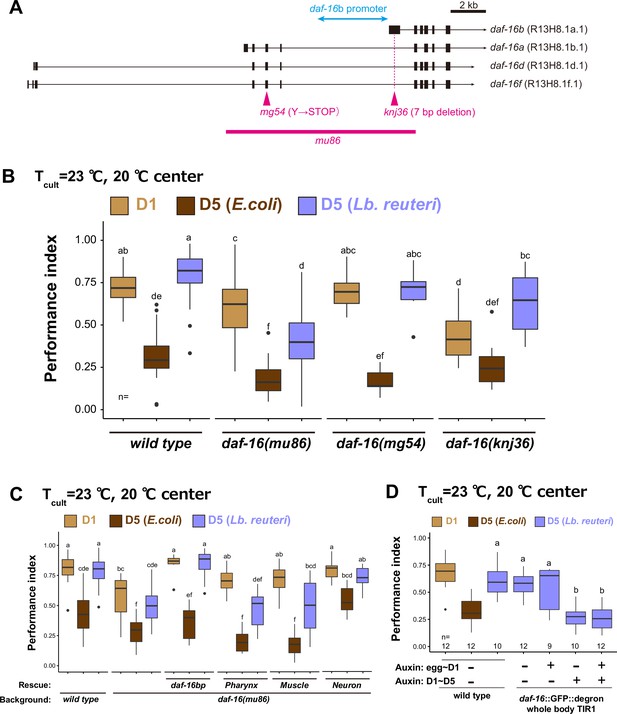
daf-16 b isoform functions in neurons to maintain high thermotaxis ability in aged animals fed Lb. reuteri.
(A) Schematic of daf-16 locus with representative isoforms based on WormBase (https://wormbase.org). Black boxes and black lines indicate exons and introns, respectively. Arrows indicate 3′UTR. Alleles used in this study are shown in magenta. The promoter of the daf-16 b isoform (4.9 kbp) is indicated in light blue. (B, C) Box plots summarizing thermotaxis performance indices of animals with indicated genotypes in the different age and diet conditions. Animals were cultivated at 23°C with E. coli or Lb. reuteri from D1. The number of experiments is shown. Statistics: The mean indices marked with distinct alphabets are significantly different (p < 0.05) according to two-way analysis of variance (ANOVA) followed by Tukey–Kramer test. (B) Analysis of different alleles of daf-16 indicated in (A). (C) Tissue-specific rescue of daf-16. The single-copy insertions of daf-16b fragment with introns under tissue-specific promoters were used to examine if it rescues daf-16(mu86): myo-2p, pharynx; myo-3p, body-wall muscle; rgef-1p, pan-neuron. (D) Time-specific knock down of daf-16. daf-16::degron animals carrying TIR1 expressed in the whole body were treated with auxin during development and/or aging.
-
Figure 8—source data 1
The effect of different daf-16 alleles on the thermotaxis behavior.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig8-data1-v1.xlsx
-
Figure 8—source data 2
Tissue-specific daf-16 rescue of the thermotaxis behavior.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig8-data2-v1.xlsx
-
Figure 8—source data 3
The effect of timing-specific knockdown of daf-16 on the thermotaxis behavior.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig8-data3-v1.xlsx
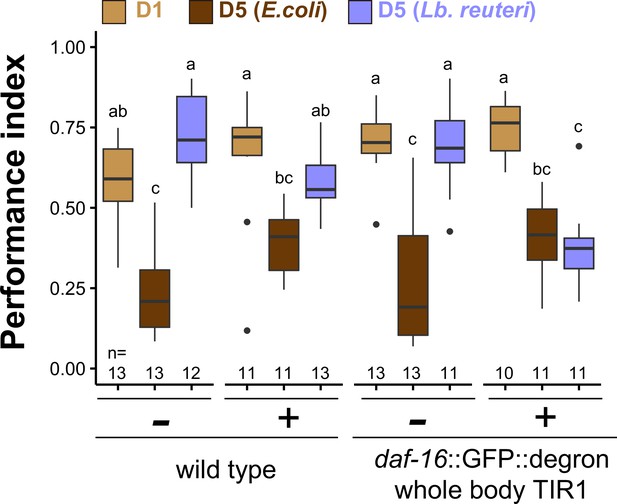
Auxin and Auxin-Inducible Degron (AID) tag do not affect the thermotaxis.
Box plots summarizing performance indices of the control conditions for AID system. D5 animals were cultivated at 23°C with E. coli or Lb. reuteri from D1. Auxin, TIR1, and AID degron tag by themselves did not affect the thermotaxis of D1, E. coli-fed D5, and Lb. reuteri-fed D5 animals. The number of experiments is shown. Statistics: The mean indices marked with distinct alphabets are significantly different (p < 0.05) according to two-way analysis of variance (ANOVA) followed by Tukey–Kramer test.
-
Figure 8—figure supplement 1—source data 1
The effect of daf-16 knockdown using AID on the thermotaxis behavior.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig8-figsupp1-data1-v1.xlsx
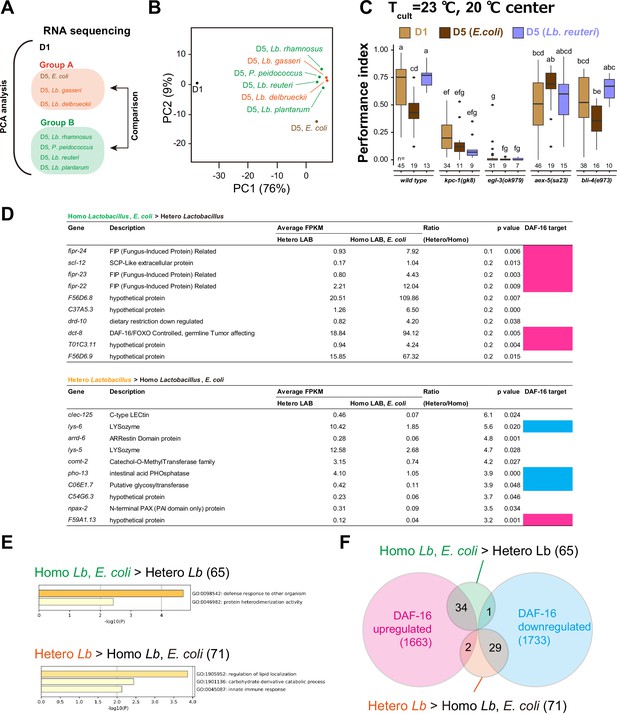
Transcriptome analysis on the effect of aging and diet.
(A) Schematic of transcriptome analysis. We carried out RNA sequencing of indicated samples (eight in total). Principal component analysis was carried out using all samples. Differentially expressed genes were analyzed using D5 samples to examine the difference between Groups A and B. (B) A scatter plots of the two variables projected on the first and second principal components. The percentage of variance explained by each principal component is indicated in brackets. (C) Mutants of the genes encoding proprotein convertases. Box plots summarizing thermotaxis performance indices of animals with indicated genotypes in the different age and diet conditions. Animals were cultivated at 23°C with E. coli or Lb. reuteri from D1. The number of experiments is shown. Statistics: The mean indices marked with distinct alphabets are significantly different (p < 0.05) according to two-way analysis of variance (ANOVA) followed by Tukey–Kramer test. (D) Differentially expressed genes (DEG) between Groups A and B in (A). DEG were ranked by the ratio between Group A’s and B’s average expression, and the top 10 genes are shown. Magenta and light blue indicate genes that are up- and downregulated by DAF-16 (Tepper et al., 2013), respectively. (E) Gene ontology analysis of differentially expressed genes between Groups A and B. x-Axis indicates log10[p-value of enrichment]. The number of genes is indicated in brackets. (F) Venn diagram showing the overlap between differentially expressed genes of our samples and DAF-16 targets (Tepper et al., 2013). The number of genes is indicated in brackets.
-
Figure 9—source data 1
Thermotaxis assays of proprotein convertase mutants.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig9-data1-v1.xlsx
-
Figure 9—source data 2
List of genes enriched in aged animals fed with different bacteria.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig9-data2-v1.xlsx
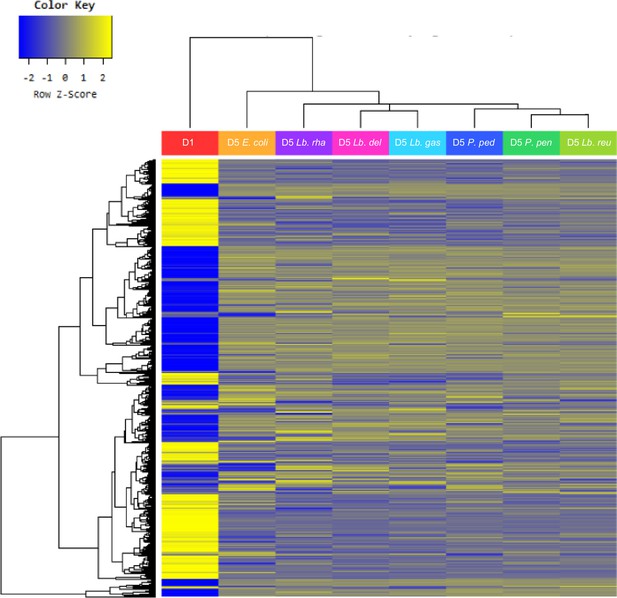
Heatmap for differentially expressed genes.
Heatmap of the one-way hierarchical clustering using Z-score for normalized value based on log2. 4426 genes satisfying fc2 are shown.
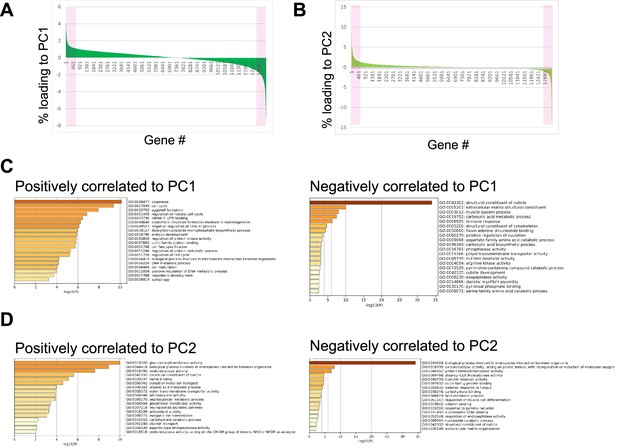
Principal component analysis of the transcriptome data of the animals of different ages and diet.
Principal component analysis was carried out for the transcriptome data of D1, and D5 animals fed E. coli or lactic acid bacteria (LAB); (Lb. gasseri, Lb. delbrueckii, P. pentosaceus, Lb. reuteri, Lb. rhamnosus, and Lb. plantarum). Loadings of genes were ranked. (A, B) Loadings of each gene to PC1 (A) and PC2. Magenta indicates the top 5% of genes most correlated and anti-correlated to PC1 or PC2. the x-axis indicates genes, but the orders are different between (A) and (B). Gene ontology analysis of the top 5% of genes correlated or anti-correlated with PC1 (C) or PC2 (D).
-
Figure 9—figure supplement 2—source data 1
Contribution of each gene on PC1.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig9-figsupp2-data1-v1.xlsx
-
Figure 9—figure supplement 2—source data 2
Contribution of each gene on PC2.
- https://cdn.elifesciences.org/articles/81418/elife-81418-fig9-figsupp2-data2-v1.xlsx
Additional files
-
Supplementary file 1
Lifespan statistics.
(a) Lifespan statistics for Figure 1—figure supplement 1. (b) Lifespan statistics for Figure 4A. (c) Lifespan statistics for Figure 7C.
- https://cdn.elifesciences.org/articles/81418/elife-81418-supp1-v1.xlsx
-
Supplementary file 2
List of LAB strains.
- https://cdn.elifesciences.org/articles/81418/elife-81418-supp2-v1.xlsx
-
Supplementary file 3
RNAseq analyses of aged animals fed with different bacteria.
(a) RNAseq data. (b) Top 5% genes positively contributing to PC1. (c) Top 5% genes negaitively contributing to PC1. (d) Gene ontology analysis of top 5% genes positively contributing to PC1. (e) Gene ontology analysis of top 5% genes negatively contributing to PC1. (f) Top 5% genes positively contributing to PC2. (g) Top 5% genes negatively contributing to PC2. (h) Gene ontology analysis of top 5% genes positively contributing to PC2. (i) Gene ontology analysis of top 5% genes negatively contributing to PC2. (j) Genes enriched in E. coli or Homo lactic acid bacteria (LAB)-fed D5. (k) Gene ontology analysis of E. coli or Homo LAB-enriched genes. (l) Genes enriched in Hetero LAB-fed D5. (m) Gene ontology analysis of Hetero LAB-enriched genes.
- https://cdn.elifesciences.org/articles/81418/elife-81418-supp3-v1.xlsx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/81418/elife-81418-transrepform1-v1.docx





