Altered basal ganglia output during self-restraint
Figures
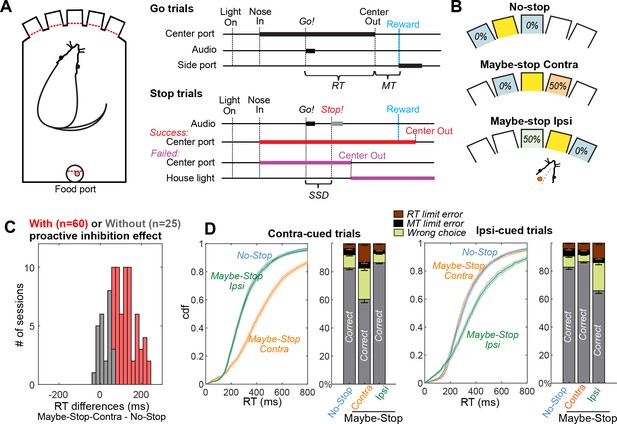
Selective proactive inhibition.
(A) Left, operant box configuration, with dashed red lines indicating photobeams for nose detection; right, event sequence for Go and Stop trials. RT, reaction time; MT, movement time; SSD, stop-signal delay; Reward, delivery of a sugar pellet to the food port. (B) Trial start location indicates stop probabilities. In this example configuration with left SNr recording, illumination of the middle hole indicates that Go! cues instructing rightward movement may be followed by a Stop! cue, but Go! cues instructing leftward movements will not (‘Maybe-Stop-Contra’). (C) Overall, the sessions in which SNr units were successfully recorded showed strong proactive inhibition effect (n=85, Wilcoxon signed rank tests on median RT differences between Maybe-Stop-Contra and No-Stop-Contra conditions, p=8.2 × 10-15). Among them, the individual sessions are considered to show proactive inhibition (red) if the reaction time difference between Maybe-Stop-Contra and No-Stop trials is statistically significant (one-tail Wilcoxon rank sum test, p<0.05). (D) Cumulative distribution functions (cdf) of RTs of Maybe-Stop-Contra condition show selective slowing for the contra-cued trials, but not for the ipsi-cued trials. Response ratios also show selective increase of wrong choice and RT limit errors for the Maybe-Stop direction. Shaded band and error bars, SEM across n=60 sessions with proactive inhibition effect. RT limit error = nose remained in Center port for >800 ms after Go! cue onset; MT limit error = movement time between Center Out and Side port entry >500 ms.
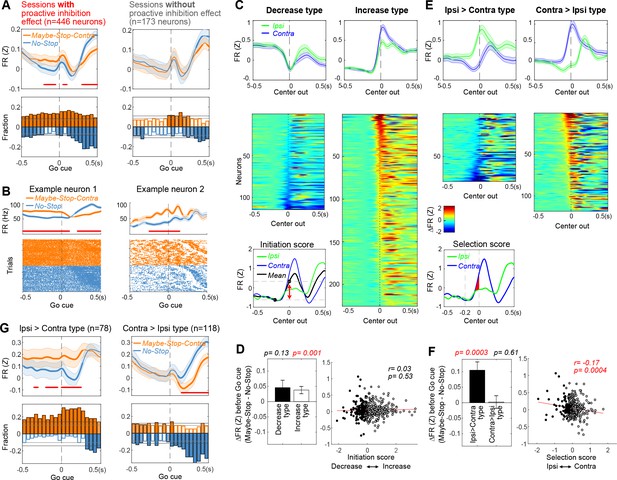
Elevated firing rates of specific substantia nigra pars reticulata (SNr) subpopulations with selective proactive inhibition.
(A) Top left: before the Go! cue SNr firing is elevated in Maybe-Stop-Contra compared to No-Stop conditions. This occurs selectively in sessions with behavioral evidence of proactive inhibition (left). Each neuron’s firing rate is Z-scored and averaged (over all trials in which a Go! cue was presented, regardless of Stop! cues). Shaded band, ± SEM across n=446 (left) or n=173 neurons (right). Thicker lines indicate significant differences between conditions (p<0.05; Wilcoxon signed rank tests at each time point) and red lines at the bottom indicate times with significant difference remaining after Bonferroni correction (by the number of 50 ms time bins; p<0.05). Bottom left: fraction of SNr neurons whose firing rate significantly differs between conditions, across time (p<0.05; Wilcoxon rank sum tests in each 50 ms bin). Higher firing with Maybe-Stop-Contra, No-Stop conditions are shown as positive (orange) or negative (blue), respectively. Horizontal gray lines indicate thresholds for a significant proportion of neurons (binomial test, p<0.05 without or with multiple-comparisons correction, light and dark gray lines, respectively). Light and dark color-filled bars are those for which the threshold was crossed without or with multiple-comparisons correction. Right, sessions without significant behavioral evidence of proactive inhibition do not show this firing rate difference between conditions (same format as left panels; n=173 neurons). (B) Two individual example neurons demonstrating the proactive elevation of firing rate before the Go! cue. Top: averaged firing rates in each condition. Shaded band, ± SEM across trials. Bottom: raster plots of individual trials. Trials are sorted by reaction times (RTs). (C) Neurons were categorized as decrease-type or increase-type, based on an ‘Initiation Score’. We defined the ‘Initiation Score’ for each neuron as the change in (Z-scored) firing rate in the 0.2 s before Center Out (inset shows example neuron). Plots show average firing of each subpopulation (top; ± SEM) and individual cells (normalized average firing, subtracting ‘baseline’ firing at 0.2 s before Center Out) sorted by Initiation Score (bottom). (D) No relation between Initiation Score and proactive inhibition (assessed as the difference between Maybe-Stop-Contra and No-Stop trials, in the 200 ms before Go! cue). Bar graph (inset) shows that on average, both increase- and decrease-type neurons modestly increase firing with proactive inhibition (Wilcoxon signed rank tests in each group). Error bar is ± SEM across neurons. (E) We defined the ‘Selection Score’ for each neuron as the integral of the difference in (Z-scored) firing rate between Contra and Ipsi actions during the 0.2 s epoch before Center Out. Remainder of panel is as C, but for Selection Score (individual cell plots show normalized firing rate for Contra minus Ipsi actions). (F) Significant negative correlation between Selection Score and proactive inhibition. Bar graph (inset) shows that Ipsi>Contra neurons preferentially increase activity on Maybe-Stop-Contra trials. (G) Same result as F, using the format of panel A to illustrate time course.
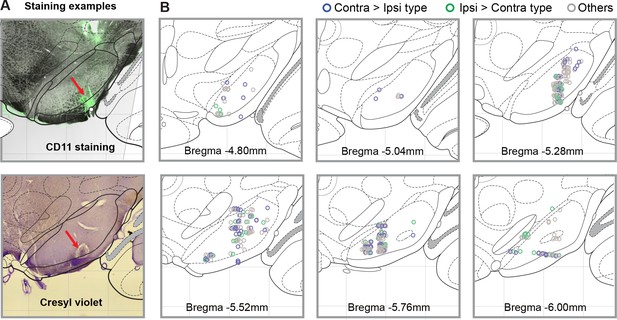
Locations of recorded cells.
(A) Brain slice examples of using CD11 antibody or Cresyl violet to mark electrode tip after making lesion. The grid represents 1mm. (B) Estimated locations of recorded cells, within coronal atlas sections (Paxinos and Watson, 2006).
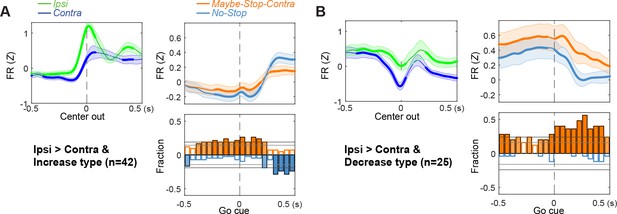
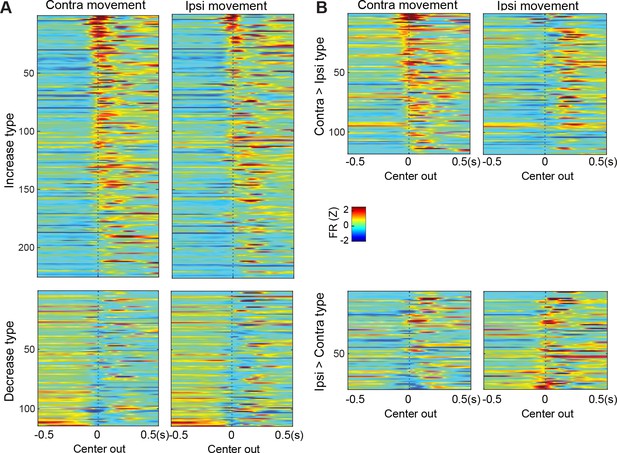
Ipsi>Contra type cells.
(A) (B) Ipsi>Contra type cells are further divided into increase (A) and decrease (B) type cells.Both group of cells show significantly increase firing rates before Go! cues. Same formats as Figure 2.
Normalized firing rates.
(A) The normalized firing rates of categorized increase and decrease type neurons are shown separately for Contra and Ipsi movements. The cells are sorted by Initiation score (same as in Figure 2C). (B) Same as in (A) for Ipsi>Contra and Contra >Ipsi type cells and sorted by Selection score (same as Figure 2D).
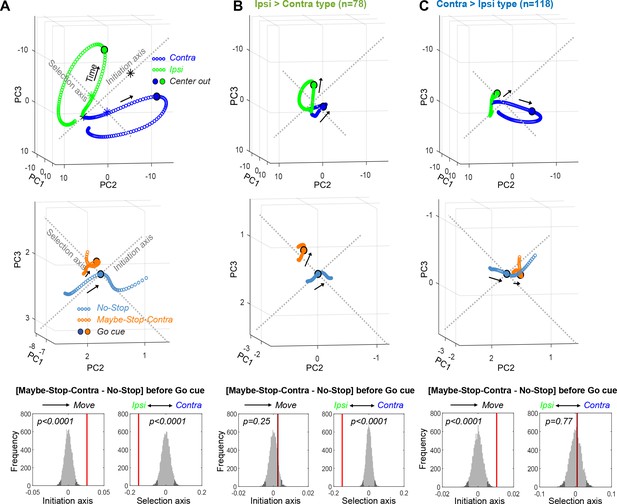
Biased substantia nigra pars reticulata (SNr) population dynamics during proactive inhibition.
(A) Top, Overall SNr state-space trajectories before and during Contra (blue) and Ipsi movements (green), in the state space of the first three principal components (PCs). Trajectories show ±250 ms around detected movement onset (Center Out, larger circles), with each small circle separated by 4 ms. ‘Initiation Axis’ joins the positions (black asterisks) 200 ms before and at action initiation (averaging Contra and Ipsi actions). ‘Selection Axis’ joins the means of each trajectory in the same epoch (colored asterisks). Middle, Comparing Maybe-Stop-Contra (orange) and No-Stop (blue) trials (±100 ms around Go! cue) in the same state space as above. Overall population state is visibly biased toward Ipsi along the Selection Axis. Bottom, Permutation tests of bias along each axis (average during –200 to 0 ms relative to Go! cue), using all 10 PCs. Red bars, observed results; gray, distributions of surrogate data from 10,000 random shuffles of trial-type labels. Dark gray indicates 5% of distributions at each tail. (B) As A, but for Ipsi>Contra cells only. The proactive bias toward Ipsi along the Selection Axis is more clearly visible. For comparisons, the PC dimension scale and Initiation/Selection axis are matched to the graph in A. (C) As A–B, but for Contra>Ipsi cells. These do not show a proactive bias on the Selection Axis, but on the Initiation Axis instead.
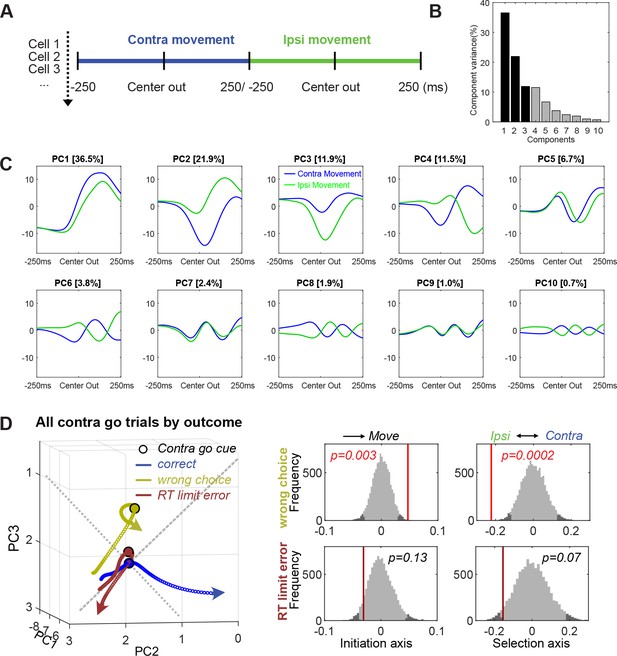
Principal component analysis (PCA).
(A) PCA was performed using 500 ms epoch around Center Out for Contra and Ipsi movements (averaged, normalized firing rates were concatenated). (B) Variance explained by each of the first 10 principal components (PCs). (C) The first 10 PCs. (D) Population dynamics of different trial outcomes (200 ms around Go! cue) show different neural trajectory patterns. Permutation test shows positioning differences at Go! cue compared to correct contra go trials. The trials with wrong choice are biased toward Ipsi action initiation at the time of Go! cue. Formats of permutation histograms are same as in Figure 3.
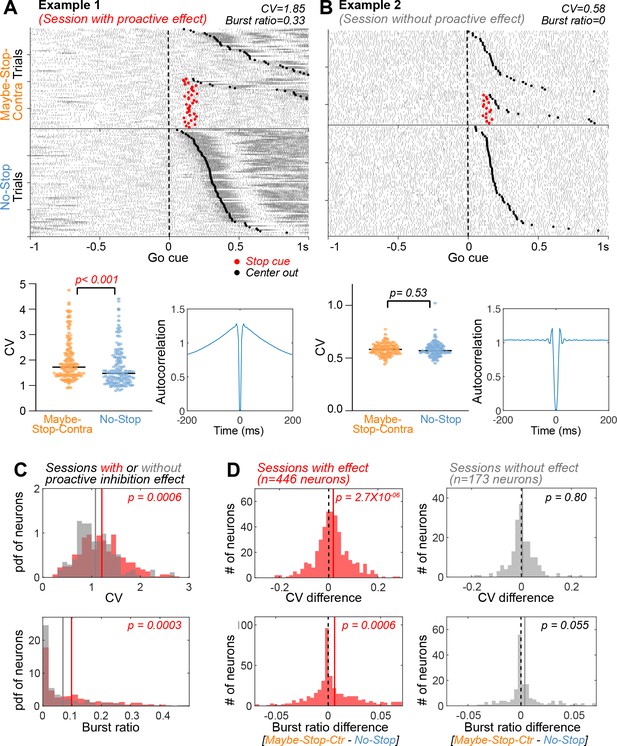
Substantia nigra pars reticulata (SNr) firing is more irregular and bursty with proactive inhibition.
(A) An example neuron from a session with proactive inhibition effect, showing spike rasters (top; aligned on contra Go! cues, sorted by reaction times [RTs]), CVs of individual trials (bottom left, for the 3 s preceding Go! cues, Wilcoxon rank sum test) and autocorrelograms (bottom right; for the 3 s preceding Go! cues). CV: coefficient of variation of inter-spike intervals. (B) An example neuron from a session without a proactive inhibition effect. Same format as in (A). (C) Elevated CV and burst ratio of neurons recorded in sessions with behavioral evidence of proactive inhibition, compared to sessions without (Wilcoxon rank sum tests). This effect was also seen at the level of individual rats (Figure 4—figure supplement 1C). Gray and red lines indicate mean of sessions with and without proactive inhibition effect, respectively. (D) Within-session comparison of Maybe-Stop-Contra and No-Stop trials shows increased CV and burst ratio when proactive inhibition is engaged (left). This effect is not present on sessions without significant proactive slowing of reaction times (right). Comparisons within each functional cell type and within each individual are shown in Figure 4—figure supplements 2 and 3. Wilcoxon signed rank tests. Dotted black lines indicates zero and colored lines indicate mean of the neurons.
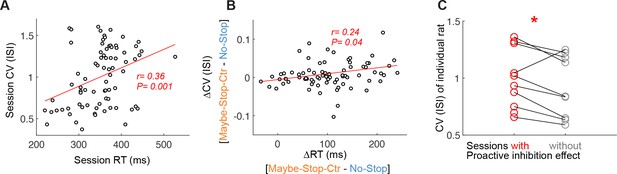
Coefficient of variation (CV) in individual sessions and rats.
(A) Sessions with high CV (averaged across units) shows longer reaction times (mean reaction times of all trials). Each data point shows the value of each session. (B) Sessions with bigger increase of CV during proactive inhibition shows bigger proactive inhibition effects. (C) Sessions with proactive inhibition effect show bigger CV compared to the sessions without proactive inhibition effect in individual rats (one-tailed Wilcoxon signed rank test, p<0.05).
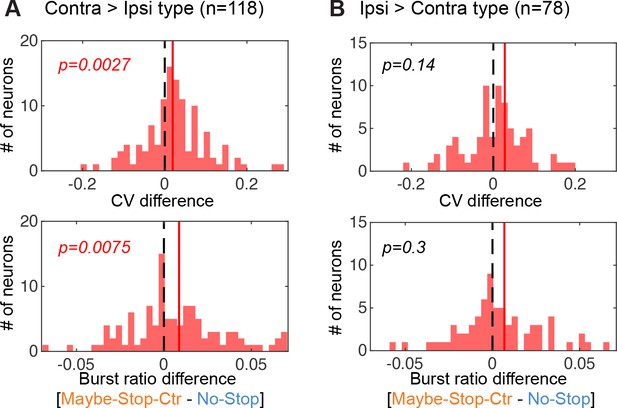
Spiking varibilities of response selective cells.
Both Contra>Ipsi and Ipsi>Contra type of cells show increased coefficient of variation(CV) and burst ratios with selective proactive inhibition, however only Contra>Ipsi cells shows a statistically significant effect (Wilcoxon signed rank test). Same format as in Figure 4D.
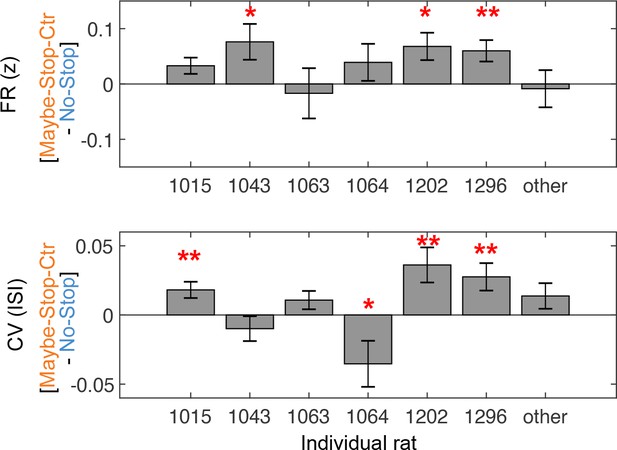
Individual rat data.
Individual rat data shows firing rates (during 200 ms before Go! cue) and coefficient of variation (CV) (3 s before Go! cue) differences between two conditions. *p<0.05 without correction, **p<0.05 with Bonferroni correction (Wilcoxon signed rank tests in each subject). Error bar indicate ± SEM.
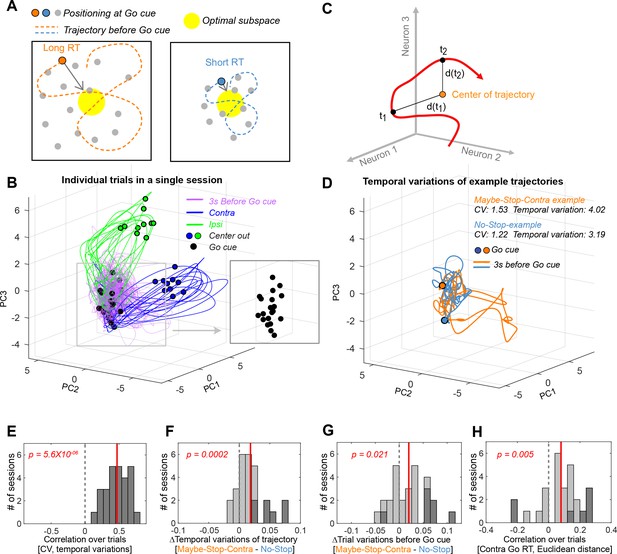
Altered variability of state trajectories with proactive inhibition.
(A) Conceptual illustration for the relationship between trajectory variability and reaction times (RTs). Larger fluctuations in trajectory will tend to result in a position further away from the ‘optimal subspace’ when the Go! cue arrives. (B) Individual trial trajectories (10 trials each for Contra and Ipsi movements) for one example session (n=46 neurons). Trajectories are shown after principal component analysis (PCA) for visualization. (C) Trajectory variability was defined as the mean of the Euclidean distances at each time point to the mean position over the trajectory (in the full neural state space, without PCA). (D) Example trajectories for Maybe-Stop-Contra (orange) and No-Stop (blue) trials, before the Go! cue. Trajectories are shown after PCA for visualization. (E) Correlations between coefficient of variation (CV) and trajectory variability for each session. (F) Trajectory variability increased on Maybe-Stop-Contra, compared to No-Stop trials. (G) Across trials, the state-space position at Go! cue (–200 to 0 ms) was more variable for Maybe-Stop-Contra, compared to No-Stop trials. (H) Variability across trials of the state-space position at Go! cue (–200 to 0 ms) was positively correlated with RT. Trials with less than 100 ms reaction times were excluded because they would have already initiated the movement trajectory. For (E–H), sessions with more than five neurons (# of session = 27) were used for analysis (Wilcoxon signed rank test across session values). Dotted gray line indicates zero and red line indicates mean of the session values. Dark gray bars indicate sessions showing significant correlation (p<0.05 for (E), (H)), or conditional differences (Wilcoxon rank sum test, p<0.05 for (F), (G)).
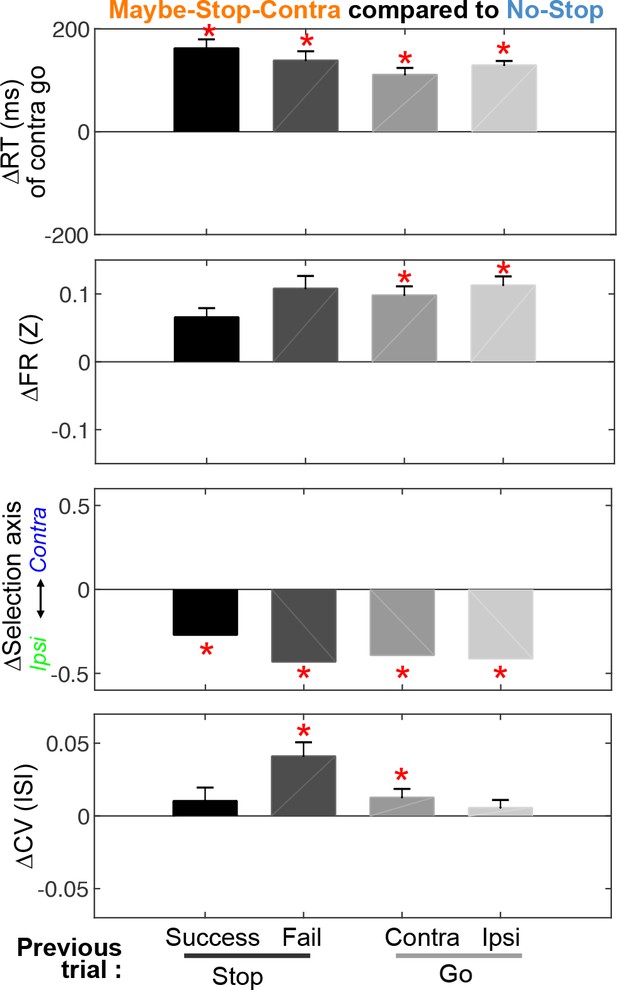
Feedback effect by previous trials and relation to trial outcomes.
Differences in reaction time, firing rates, selection axis positions, and coefficients of variation (CVs) of Maybe-Stop-Contra trials, compared to the No-stop condition (zero), and separated by previous trial type. The effect of previous trial type reached significance for the difference in CV (Friedman’s test, X2(3)=13.38, p=0.004), but not for contra go RT (X2(3)=2.84, p=0.42) nor in firing rates (X2(3)=5.09, p=0.17). Firing rates and selection axis measures use Ipsi>Contra cells (during 200 ms before Go! cue); all cells are included in the CV calculation. *p<0.05 with Bonferroni multiple comparison correction (Compared to No-Stop trials, permutation test as in Figure 3 for Selection axis, and Wilcoxon signed rank test for others). Error bars are ± SEM across sessions (n=60) for reaction times and across neurons (n=446) for firing rates and CV.
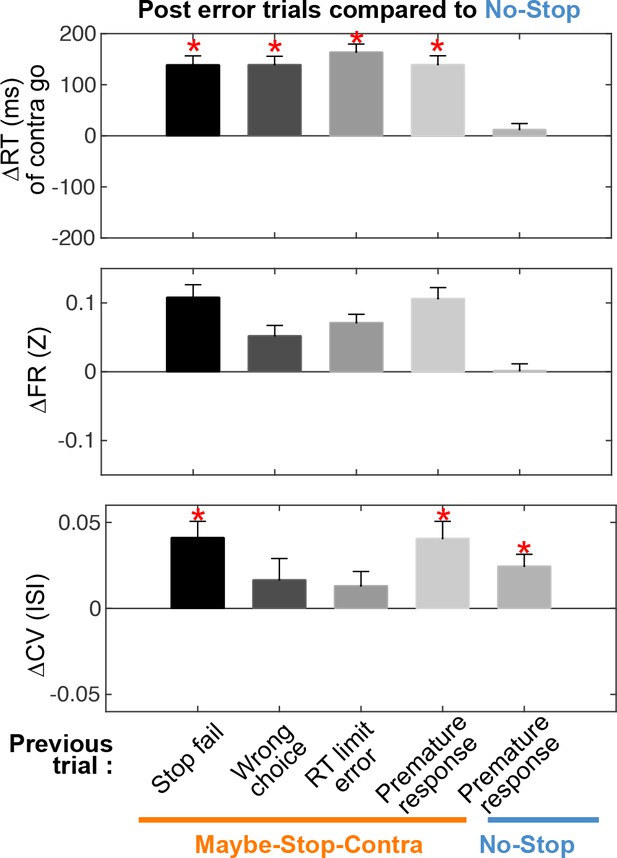
Post error trials.
Differences in reaction time, firing rates, and coefficients of variation (CVs) of post-error trials, compared to the No-stop condition (zero, including all trials), and separated by previous error type (same format as Figure 6).
Premature response refers to action initiation before the Go! cue (Center Out during foreperiod; these premature responses are excluded in all other analyses). A significant CV increase was specifically observed after Stop-failures and premature responses, but not after other error types. The CV increase after premature responses is seen for both Maybe-Stop and No-stop conditions.
Tables
Information on individual rats.
| RAT | Port location with stop probability(port1, port2 port3) | # of sessions (with/ without proactive inhibition effect) | # of cells (from sessions with/ without proactive inhibition effect) | Sessions with contralateral proactive inhibition effect (Mean ± SD) | |||
|---|---|---|---|---|---|---|---|
| # of trials per session (Maybe-Stop-Contra/No-Stop conditions)* | Contralateral RTs (Maybe-Stop-Contra/No-Stop conditions, ms) | Ipsilateral RTs (Maybe-Stop-Contra/No-Stop conditions, ms) | Stop success rates (%) | ||||
| 1015 | 0, L50, R50% | 7/1 | 184/20 | 104±27/103±12 | 563±39/363±35 | 428±45/415±42 | 55.3±10.3 |
| 1019 | L50, 0, R50% | 5/2 | 6/4 | 110±33/122±25 | 369±47/275±30 | 271±30/321±11 | 47.8±10.9 |
| 1042 | L50, 0, R50% | 2/0 | 3/0 | 94±5/88±10 | 399±27/293±10 | 277±4/377±37 | 81.1±5.5 |
| 1043 | L50, R50, 0% | 8/1 | 30/5 | 124±25/131±35 | 474±49/353±48 | 384±51/38747 | 70.5±5.6 |
| 1063 | L50, R50, 0% | 8/4 | 21/15 | 124±31/110±25 | 347±57/226±29 | 266±49/191±18 | 26.2±5.6 |
| 1064 | L50, 0, R50% | 6/4 | 23/26 | 121±20/123±18 | 446±34/338±35 | 303±13/309±20 | 57.3±4.8 |
| 1098 | 0, L50, R50% | 5/2 | 5/2 | 102±18/109±27 | 456±17/330±38 | 324±21/327±54 | 41.7±3.1 |
| 1202 | 0, R50, L50% | 10/1 | 114/12 | 107±23/118±32 | 494±49/328±36 | 359±38/356±28 | 56.3±10.6 |
| 1296 | R50, 0, L50% | 7/2 | 53/11 | 101±17/92±11 | 435±39/330±38 | 344±41/345±28 | 61.5±13.0 |
| 1328 | R50, L50, 0% | 2/8 | 7/78 | 127±2/147±19 | 370±13/298±36 | 275±18/368±65 | 51.8±2.5 |
-
*
Number of trials includes only those trials in which the Go! cue was presented (i.e. trials with premature center out are excluded).





