Intermittent fasting induces rapid hepatocyte proliferation to restore the hepatostat in the mouse liver
Figures
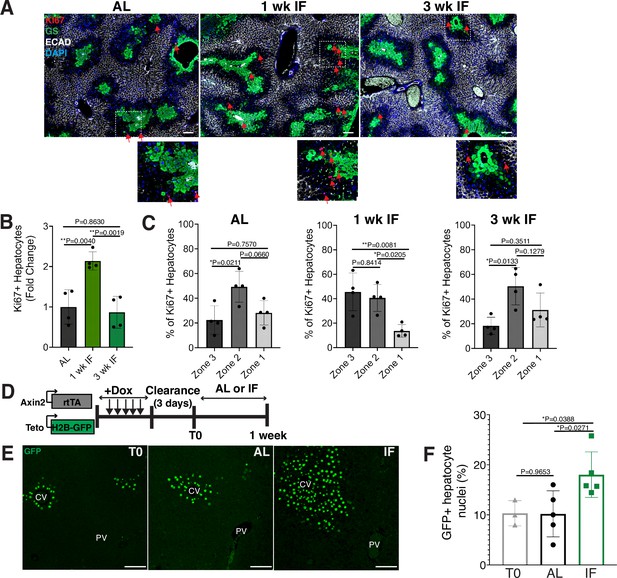
Intermittent fasting (IF) induces rapid hepatocyte proliferation.
(A) Ki67 immunofluorescence for the detection of proliferating cells in ad libitum (AL), 1- and 3-week IF-treated livers. IF-treated livers were analyzed 30 min after re-feeding cycle. (B, C) Quantification of spatial distribution and percentage of Ki67+ hepatocytes in AL- and IF-treated livers. One-way analysis of variance (ANOVA), N = 4.(D) Dox inducible Axin2-rtTA; Teto-H2BGFP system to label Axin2+ pericentral hepatocytes and trace cell proliferation. Mice were pulsed with dox for 7 days, cleared of dox for 3 days and AL-fed or intermittently fasted for 6 days. (E) GFP immunofluorescent images showing increased hepatocyte expansion in AL and IF compared to T0. (F) Percentage of GFP+ hepatocyte nuclei in AL, IF livers from A. One-way ANOVA, N = 3 (T0), 5 (AL), 5 (IF). **p < 0.01; *p < 0.05. Error bars indicate standard deviation. Scale bar, 100 µm. wk, weeks.
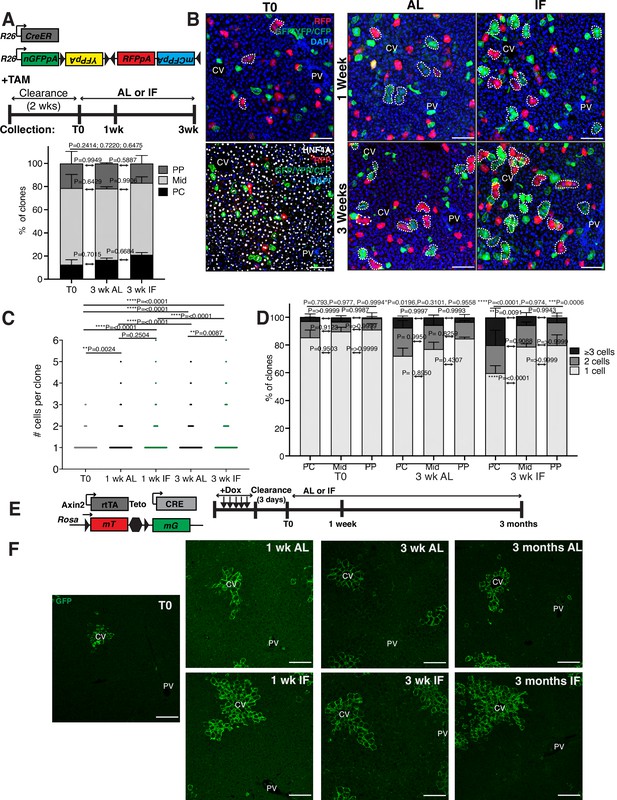
Hepatocyte proliferation kinetics in ad libitum (AL) fed and intermittent fasted animals.
(A) Schematic of unbiased system to trace cell proliferation during AL feeding and intermittent fasting (IF). R26-CreER mice were crossed to R26-Confetti mice enabling permanent cell labeling and lineage tracing by four fluorescent reporters after tamoxifen administration. (B) At T0, mostly single, HNF4A+ hepatocytes were labeled throughout the lobule. At 1 and 3 weeks, multicellular hepatocytes clones (dotted circles) grew in AL and IF livers, with increased pericentral growth in IF. (C) The number of hepatocytes per 3D clone at each collection in A. One-way analysis of variance (ANOVA), 439–602 clones analyzed at T0, 430–1085 clones at 1 wk AL, 625–904 clones at 1 wk IF, 523–615 clones at 3 wk AL, and 442–615 at 3 wk IF. N = 3. (D) Percentage of 3D clones consisting of different cell sizes, from C, in different liver lobule locations. PC, pericentral; Mid, midlobular; PP, periportal. Differences between 1-, 2-, and >3-cell clones in periportal and pericentral zones are indicated by p values above bars. Two-way ANOVA. N = 3. (E) Schematic of system to trace hepatocyte proliferation over 3 months of IF or AL treatment. Axin2-rtTA; Teto-Cre; Rosa26-mTmG mice were induced with tamoxifen enabling permanent cell labeling and lineage tracing by reporter GFP. (F) GFP immunofluorescent images highlighting Axin2+ hepatocyte tracing from T0, 1 week, 3 weeks, and 3 months after AL or IF treatment. ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05. Error bars indicate standard deviation. Scale bar, 100 µm.
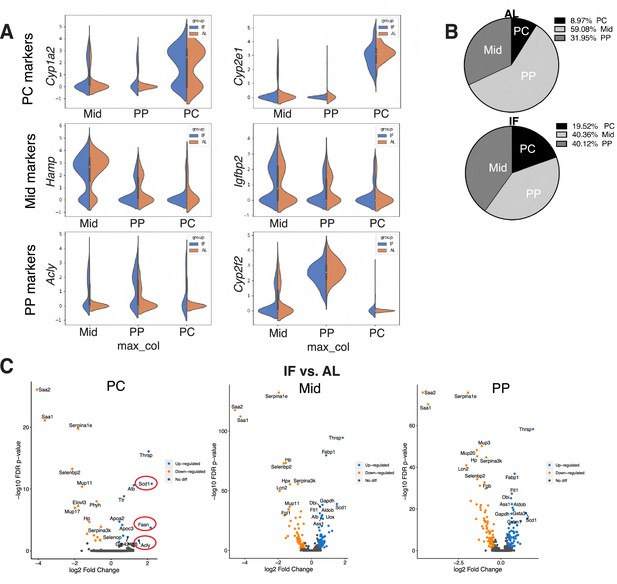
Single-cell RNA-seq comparing hepatocytes in ad libitum (AL) fed and intermittent fasted livers.
(A) Violin plots, from scRNA-seq, demonstrating zonal marker gene expression used to classify single hepatocytes from AL and intermittent fasting (IF) livers as pericentral (PC), midlobular (Mid), and periportal (PP) hepatocytes. (B) Pie charts of hepatocyte zonal populations identified in scRNA-seq highlighting increase in PC hepatocytes in IF compared to AL livers. (C) Volcano plots highlighting differentially expressed transcripts in IF versus AL livers in PC, Mid, and PP hepatocytes. Red circles highlighting transcripts involved in de novo lipogenesis.
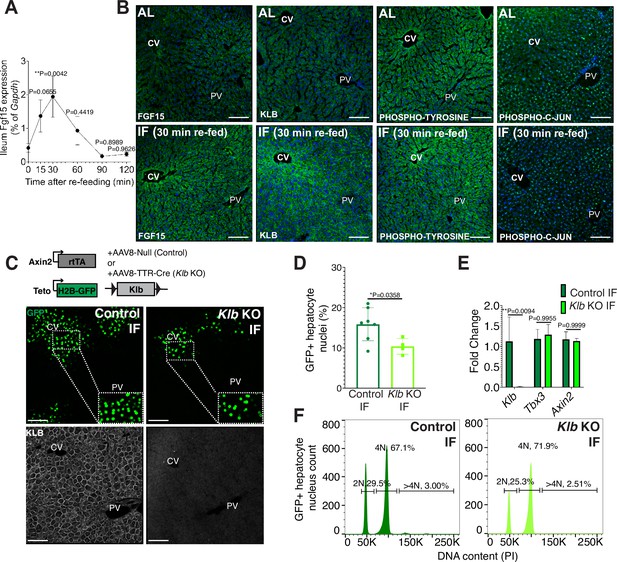
Endocrine FGF15-β-KLOTHO (KLB) signaling is required for hepatocyte proliferation during intermittent fasting (IF).
(A) Quantitative real-time PCR analysis highlighting rapid increase in Fgf15 expression in ileum 30 min after re-feeding in 1-week IF-treated livers. One-way analysis of variance (ANOVA), comparison with time 0, N = 3. (B) Immunofluorescence for endocrine FGF pathway components highlighting pathway activation in 1-week IF-treated livers 30 min after re-feeding (ZT12). (C) Schematic of method to deplete hepatocytes of Klb. Axin2-rtTA; Teto-H2BGFP; Klb flox/flox mice were injected with AAV8-TTR-Cre (Klb KO). GFP and KLOTHO immunofluorescent images showing decrease in hepatocyte expansion and loss of KLOTHO in Klb KO compared to control livers. (D) Percentage of GFP+ hepatocyte nuclei in Klb KO and control livers. (E) Quantitative real-time PCR analysis confirming loss of Klb but not WNT target genes, Tbx3 and Axin2, in Klb KO livers. Two-way ANOVA, N = 3. (F) Ploidy distribution of GFP+ hepatocyte nuclei incontrol IF and Klb KO IF livers. Unpaired t-test. N = 7 (control), 4 (Klb KO). **p < 0.01, *p < 0.05. Error bars indicate standard deviation. Scale bar, 100 µm.
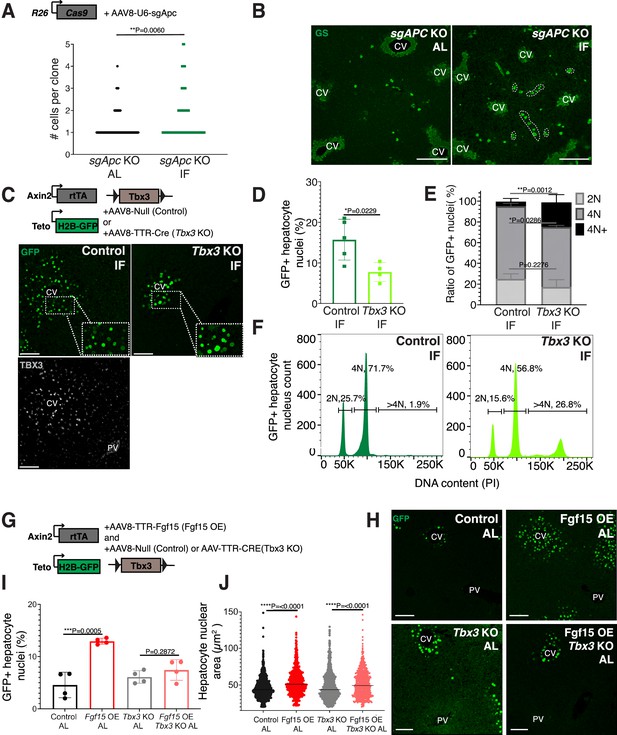
Paracrine WNT and WNT target gene Tbx3 promote hepatocyte proliferation during intermittent fasting (IF).
(A) Method to constitutively activate WNT signaling in midlobular, periportal cells. AAV8-U6-sgAPC was injected into the tail vein of Rosa26-Cas9 mice. Animals were IF treated for 1 week before analysis. GS immunofluorescent images for detection of Apc mutant clones in ad libitum (AL) and IF livers. (B) The number of Apc mutant hepatocytes per 3D clone expand in IF compared to AL livers. Mann–Whitney test. 130 clones analyzed in AL. 74 clones analyzed in IF. N = 3. White dashed lines demarcate multicellular non-pericentral GS+ clones. (C) Schematic of method to deplete hepatocytes of the WNT target, Tbx3. Axin2-rtTA; Teto-H2BGFP; Tbx3flox/flox mice were intraperitoneally injected with AAV8-TTR-Cre (Tbx3 KO). GFP and TBX3 immunofluorescent images to show IF-induced proliferation and Tbx3 depletion, respectively, in control and Tbx3 KO livers. (D) Percentage of GFP + hepatocyte nuclei decreased in Tbx3 KO IF compared to control IF livers. Unpaired t-test, (N = 7 control IF), N = 4 (Tbx3 KO IF). (E, F) Nuclear ploidy distribution of GFP+ hepatocytes highlighting hyper-polyploidization in Tbx3 KO IF compared to control IF livers. Two-way analysis of variance (ANOVA), N = 3. (G) Schematic for Fgf15 overexpression. Axin2-rtTA; Teto-H2BGFP; Tbx3 flox/flox mice were injected with AAV-TTR-FGF15 (Fgf15 OE) and AAV8-Null (control) or AAV-TTR-CRE (Tbx3 KO). (H) GFP immunofluorescent images from c AL, Fgf15 OE AL, Tbx3 KO AL, Fgf15 OE; Tbx3 KO AL livers. (I) Percentage of GFP+ hepatocyte nuclei highlighting lack of hepatocyte proliferation in Tbx3 KO livers. Unpaired t-test, N = 4. (J) Dot plot highlighting increase in nuclear area with Fgf15 overexpression both with and without Tbx3. Unpaired t-test. ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05. Error bars indicate standard deviation. Scale bar, 100 µm.
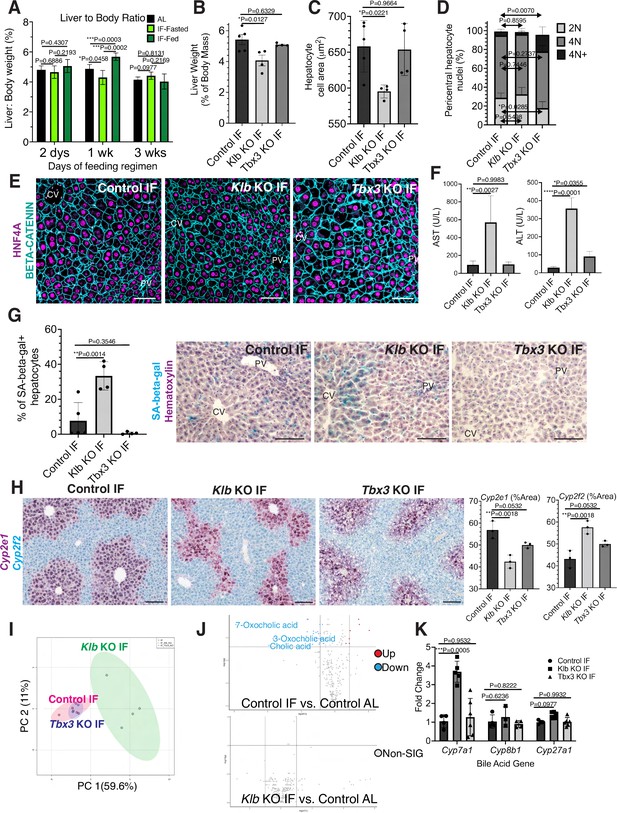
Hepatocyte proliferation or compensatory polyploidization maintains the hepatostat during intermittent fasting (IF).
(A) Liver-to-body weight ratio in wild-type livers during 2 days, 1 week, and 3 weeks of IF and ad libitum (AL) feeding. (B–K) Liver analyses after 3 weeks of IF treatment in control, Klb KO, and Tbx3 KO livers. (B) Liver-to-body weight ratio. (C) Hepatocyte nuclear area. (D) Nuclear ploidy distribution of pericentral hepatocytes with hyper-polyploidization in Tbx3 KO IF livers. (E) Immunofluorescence images for β-CATENIN and HNF4A highlighting hepatocyte cell and nuclear area during IF. (F) AST and ALT liver injury marker presence in serum. (G) Quantification and representative images of senescence-associated β-galactosidase stains. (H) RNAscope images and quantification for pericentral marker Cyp2e1 and periportal marker Cyp2f2. (I) Metabolomics PCA plot comparing control IF, Klb KO IF, and Tbx3 KO IF livers. (J) Volcano plots comparing metabolites between control AL and control IF livers and control AL and Klb KO IF livers. The top 3 most significantly changed bile metabolites are labeled in blue. (K) Expression of bile acid pathway enzymes genes in livers. Quantitative PCR for genes for critical enzymes in bile acid pathway. All statistics were performed on N=3-5 animals using one-way analysis of variance (ANOVA). ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05. Error bars indicate standard deviation. Scale bar, 100 µm.
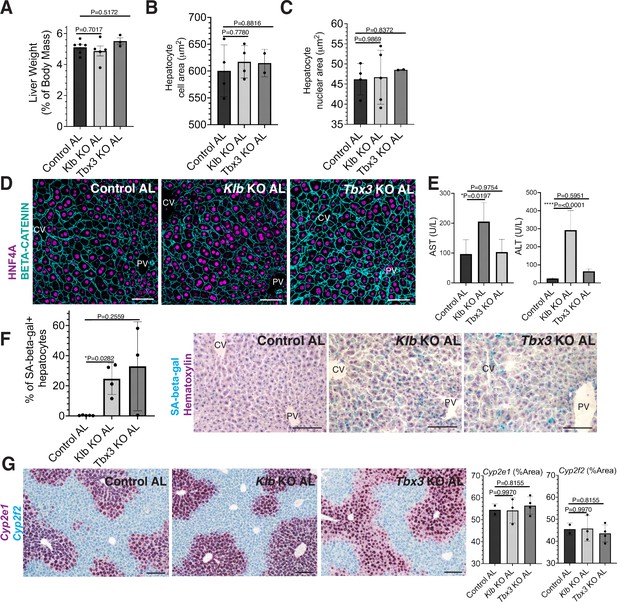
Short-term loss of Tbx3 or Klb does not disrupt the hepatostat during ad libitum (AL) feeding.
A-H AL Klb KO, AL Tbx3 KO, and control AL livers were assessed at the same time point in Figure 4 (3 weeks after AL feeding). (A) Liver-to-body weight ratio. (B, C) Hepatocyte cell and nuclear area. (D) Immunofluorescence images forβ-CATENIN and HNF4A highlighting hepatocyte cell and nuclear area during AL livers. (E) AST and ALT liver injury marker presence in serum. (F) Quantification and representative images of senescence-associated β-galactosidase stains. (G) RNAscope for pericentral marker Cyp2e1 and periportal marker Cyp2f2. All statistics were performed on N=3-5 animals using one-way analysis of variance (ANOVA). ****p < 0.0001, *p < 0.05. Error bars indicate standard deviation. Scale bar, 100 µm.
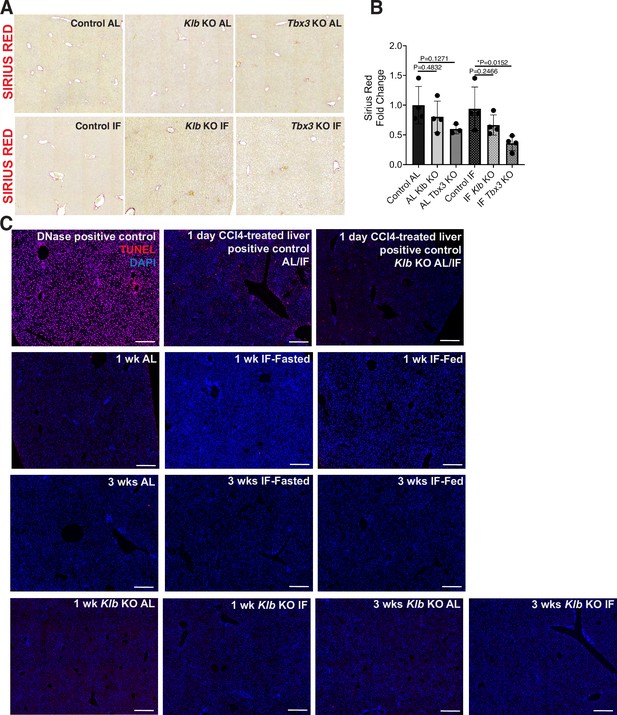
Fibrosis and cell death assessment of Control, Klb KO, and Tbx3 KO IF- and ad libitum (AL)-treated livers.
(A, B) Sirius red staining and quantification to assess for liver fibrosis. (C) TUNEL stains on livers. All statistics were performed on N=3-5 animals using one-way analysis of variance (ANOVA). *p < 0.05. Error bars indicate standard deviation. Scale bar, 100 µm.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| gene (Mus musculus) | Fgf15 | GenBank | BC021328 cloneID 5066286 | Male |
| Strain, strain background (Mus musculus, male) | C57BL/6 J | The Jackson Laboratory | Cat# 000664 RRID:IMSR_JAX:000664 | |
| Strain, strain background (Mus musculus, male) | Rosa26-CreERT2 | The Jackson Laboratory | Cat# 008463 RRID:IMSR_JAX:00846 | |
| Strain, strain background (Mus musculus, male) | Rosa26-Confetti | The Jackson Laboratory | Cat# 017492 RRID:IMSR_JAX:008463 | |
| Strain, strain background (Mus musculus, male) | Axin2-rtTA | The Jackson Laboratory | Cat# 016997 RRID:IMSR_JAX:016997 | |
| Strain, strain background (Mus musculus, male) | TetO-H2B-GFP | The Jackson Laboratory | Cat# 005104 RRID:IMSR_JAX: 005104 | |
| Strain, strain background (Mus musculus, male) | TetO-Cre | The Jackson Laboratory | Cat#006234 RRID:IMSR_JAX:006234 | |
| Strain, strain background (Mus musculus, male) | Rosa26-mTmG | The Jackson Laboratory | Cat# 037456 RRID:IMSR_JAX: 037456 | |
| Strain, strain background (Mus musculus, male) | Rosa26-Cas9 | The Jackson Laboratory | Cat# 026179 RRID:IMSR_JAX: 026179 | |
| Strain, strain background (Mus musculus, male) | Tbx3 flox | Dr. Anne Moon | N/A | |
| Strain, strain background (Mus musculus, male) | Klb flox | Dr. David Mangelsdorf | N/A | |
| antibody | anti-GFP (Chicken polyclonal) | Abcam | Cat# ab13970 RRID:AB_300798 | 1:500 IF |
| antibody | Anti-RFP (Rabbit polyclonal) | Rockland | Cat# 600-401-379 RRID:AB_2209751 | 1:500 IF |
| antibody | Anti-Hnf4 (Mouse monoclonal) | Abcam | Cat# ab41898 RRID:AB_732976 | 1:500 IF; 50 IHC |
| antibody | Anti-Klotho (Rat monoclonal) | DSHB | Klotho KL-115 RRID:AB_2618099 | 1:50 IF |
| antibody | Anti-FGF15 (Mouse monoclonal, IgG2a) | Santa Cruz | sc-514647 RRID NA | 1:50 IF |
| antibody | Anti-Phospho-Tryosine (mouse monoclonal) | Cell Signaling Technology | Cat# 9411 RRID:AB_331228 | 1:50 IF |
| antibody | Anti-Phospho-c-Jun (Ser73) (rabbit monoclonal) | Cell Signaling Technology | Cat# 3270, RRID:AB_2129575 | 1:50 IF |
| antibody | Anti-Tbx3 (Goat polyclonal) | Santa Cruz Biotechnology | Cat# sc-17871 RRID:AB_661666 | 1:50 IHC |
| antibody | Anti-Goat IgG (Donkey polyclonal) | Jackson Immuno Research Labs | Cat# 705-065-147 RRID:AB_2340397 | 1:200 IHC |
| antibody | Anti-Glutamine Synthetase (Mouse monoclonal) | Millipore | Cat# MAB302 RRID:AB_2110656 | 1:500 IHC |
| antibody | Anti-Catenin, beta (mouse monoclonal) | BD Biosciences | Cat# 610154 RRID:AB_397555 | 1:50 IHC |
| antibody | Anti-Hnf4 (Rabbit polyclonal) | Santa Cruz Biotechnology | Cat# sc-8987 RRID:AB_2116913 | 1:50 IHC |
| antibody | KI67(SolA15) (Rat, monoclonal) | Thermo Fisher Scientific | Cat# 14-5698-82 RRID:AB_10854564 | 1:50 IHC |
| recombinant DNA reagent | pAAV-Guide-it-Down | Clontech Laboratories Inc. | Cat# 041315 | |
| recombinant DNA reagent | pscAAV-TTR-mFgf15 | This paper and Addgene | Currently Deposit 81516 | |
| sequence-based reagent | sgAPC_F | This paper | Assembly primers for pAAV-Guide-it-Down targeting | CCGGAGGCTGCATGAGAGCACTTG3 |
| sequence-based reagent | sgAPC_F | This paper | Assembly primers for pAAV-Guide-it-Down targeting | AAACCAAGTGCTCTCATGCAGCCT3 |
| sequence-based reagent | sgRNA: targeting Apc | This paper | Targeting sequence | AGGCTGCATGAGAGCACTTG |
| commercial assay or kit | In-Fusion HD Cloning | Clontech | Cat# 639647 | |
| commercial assay or kit | RNAscope probe-Mm-Cyp2f2 | Advanced Cell Diagnostics | Cat# 451851 | target region: 555–169 |
| commercial assay or kit | RNAscope probe-Mm-Cyp2e1-C2 | Advanced Cell Diagnostics | Cat# 402781 C2 | target region: 458–1530 |
| commercial assay or kit | RNeasy Mini Isolation Kit | Qiagen | Cat# 74004 | |
| commercial assay or kit | High Capacity cDNA Reverse Transcription Kit | Life Technologies | Cat# 4368814 | |
| commercial assay or kit | Taqman Gene Expression Assay (Gapdh) | ThermoFisher Scientific | Cat# 4331182; Mm99999915_g1 | |
| commercial assay or kit | Expression Assay (Klb) | ThermoFisher Scientific | Cat# 4331182; Mm00473122_m1 | |
| commercial assay or kit | Expression Assay (Fgf15) | ThermoFisher Scientific | Cat# 4331182; Mm00433278_m1 | |
| commercial assay or kit | Expression Assay (Tbx3) | ThermoFisher Scientific | Cat# 4331182; Mm01195719_m1 | |
| commercial assay or kit | Chromium Single Cell 3” Reagents Kit V3 | 10 x Genomics | Discontinued | |
| commercial assay or kit | NovaSeq S2 v.1.5 Reagent Kits | Illumnina | NA | Discontinued |
| commercial assay or kit | Filter Microplates | Agilent Technologies | Cat#203980–100 | |
| chemical compound, drug | Tamoxifen | Sigma Aldrich | Cat# T5648-1G | |
| chemical compound, drug | Doxycycline hyclate | Sigma-Aldrich | Cat# D9891 | |
| chemical compound, drug | HistoClear | Natural Diagnostics | Cat# HS2001GLL | |
| chemical compound, drug | Antigen Unmasking Solution, Tris-Based | Vector Labs | Cat# H-3301 | |
| chemical compound, drug | Avidin/Biotin Blocking Kit | Vector Labs | Cat# SP-2001 | |
| chemical compound, drug | Click-iT Plus TUNEL Assay Kits for In Situ Apoptosis Detection | ThermoFisher Scientific | Cat# C10619 | |
| chemical compound, drug | FxCycle PI/RNase | ThermoFisher Scientific | Cat# F10797 | |
| chemical compound, drug | TRIzol Reagent | Invitrogen | Cat# 15596026 | |
| software, algorithm | ImageJ | NIH https://imagej.net/ | RRID:SCR_003070 | |
| software, algorithm | GraphPad Prism 5.0 software | GraphPad Software; http://www.graphpad.com | RRID:SCR_002798 | |
| software, algorithm | Cell Ranger Software (v3.1.0, mm10 ref genome) | 10 x Genomics Software; https://support.10xgenomics.com/single-cell-gene-expression/software/pipelines/latest/what-is-cell-ranger | RRID:SCR_017344 | |
| software, algorithm | Seraut Software (v3.0, R package) | Seurat Software; https://satijalab.org/seurat/get_started.htm | RRID:SCR_016341 | |
| software, algorithm | BD FACS Diva 8.0 software (BD) | BD FACS Diva software; http://www.bdbiosciences.com/instruments/software/facsdiva/index.jsp | RRID:SCR_001456 | |
| software, algorithm | MS-DIAL v4.60 software | MS-DIAL software; (Tsugawa et al., 2020) | NA | |
| software, algorithm | MetaboAnalyst 5.0 software | MetaboAnalyst software; https://www.metaboanalyst.ca/ | RRID:SCR_015539 | |
| Other | AAV/DJ8-Ttr-Cre | Vector Bio Labs | 7102 | AAV-DJ8 virus that expresses an improved Cre under a liver-specific Ttr promoter |
| Other | AAV8-Null | Vector Bio Labs | 7077 | AAV serotype 8 virus that has a CMV promoter with no transgene. It's used as control AAV in the paper. |
Additional files
-
Supplementary file 1
Metabolomics intermittent fasting (IF) versus ad libitum (AL significantly changed metabolites between IF and AL samples from metabolomic studies).
- https://cdn.elifesciences.org/articles/82311/elife-82311-supp1-v1.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/82311/elife-82311-mdarchecklist1-v1.docx