Theta-phase-specific modulation of dentate gyrus memory neurons
Figures
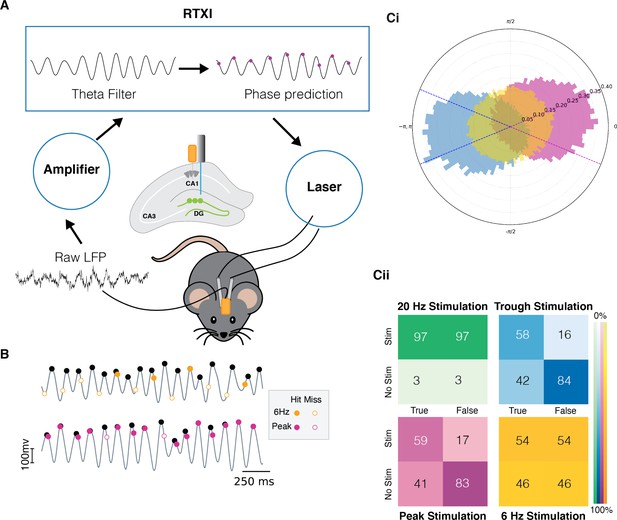
Quantification of the real-time phase detection algorithm performance.
(A) LFP signal recorded from the hippocampus is amplified and processed in Real-Time eXperimental Interface (RTXI) (https://github.com/ndlBU/phase_specific_stim). The signal is first filtered using an FIR filter in the theta range (4–12 Hz) and then a real-time phase detection algorithm predicts the next extrema. At the predicted time, a TTL pulse is sent to the laser which delivers light through fiber optics to the dentate gyrus (DG) region of the hippocampus to activate tagged neurons. (B) Sample stimulation shows the superior performance of the predictive algorithm in comparison to the 6 Hz periodic stimulation. (C) (Ci) Normalized polar histogram shows the phases of stimulation in the cases of peak, trough, and periodic 20 Hz and 6 Hz stimulation. Dotted lines indicate the accepted peak (pink) and trough (blue) stimulation phase (within quarter cycle). Note that 20 Hz stimulation overlaps completely with 6 Hz stimulation because both are fixed frequencies. (Cii) Confusion matrices indicate that peak and trough stimulation are specific to the desired phase of the stimulation. Stimulations are considered true if they take place within –π/4 to π/4 of the desired phase. The false entry for the No Stim case represents the true negative rate (TNR) or specificity. The true entry for the Stim case represents the true positive rate (TPR) or sensitivity.
Summary data for excluded animals.
For 17 animals, we observed less than 5% light-induced freezing in at least three of the four light-induced stimulation conditions (20 Hz, 6 Hz, peak, trough). Data from these animals were excluded from further analysis.
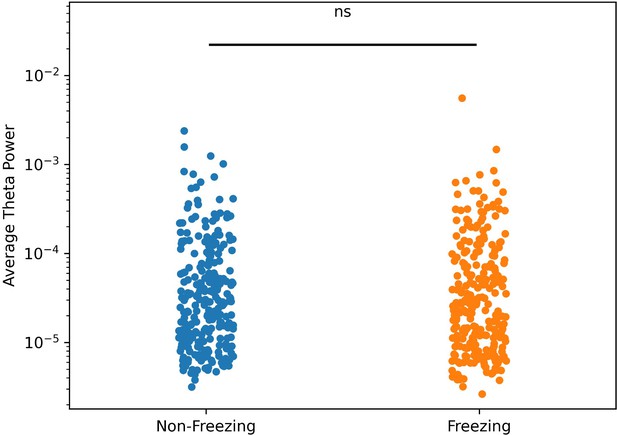
Theta power during motion and freezing epochs.
The average theta power did not change between motion and freezing epochs (p>0.05, t-test on log-normalized data).
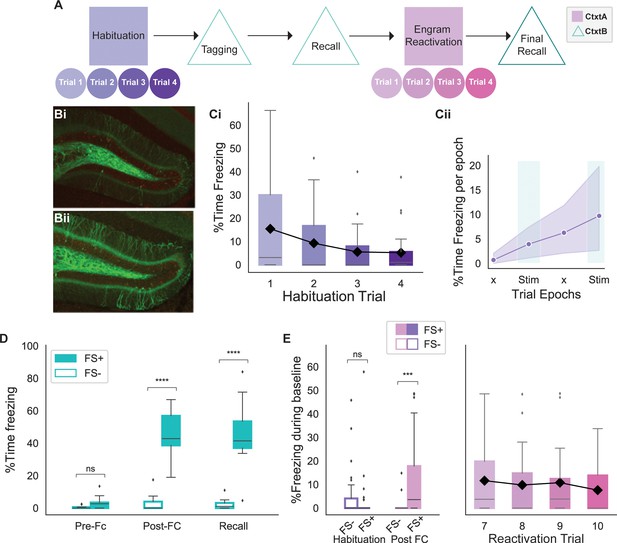
Behavioral experiment design.
(A) Schematic of the behavioral experiment. Animals were habituated in context A for 4 days prior to the tagging of engram neurons in context B. During tagging, some animals underwent a foot shock (FS+) while others were placed in context B but without any stimulus (FS-). Following recall, animals were re-exposed to context A and underwent reactivation of the engram neurons with four different stimulation strategies on distinct days (trials). The experiment concluded with a final recall in context B. (B) FS+ (Bi) and FS- (Bii) animals had a similar number of dentate gyrus (DG) cells tagged as shown by the number of granule cells expressing EYFP-ChR2. FS+ animals received a foot shock in context A, while FS- animals did not. (C) Trends of mouse baseline freezing during habituation. (Ci) Habituation of animals over 4 days in context A resulted in decreased in freezing, indicating comfort with the setup. Black line shows the trend for the mean (n=26 animals). (Cii) Average of the percent time freezing over four epochs of trials on the last day of habituation (day 4) indicates 5% increase in percent time freezing due to fatigue later in the trial. This value serves as a baseline for subsequent analysis. ‘x’ indicates No Stim epochs. Shaded area indicates 95% confidence interval (n=26 animals). (D) Both FS+ and FS- animals showed minimal freezing in context B prior to the foot shocks (FS+ n = 17 animals, FS- n=9 animals; independent t-test with Bonferroni correction, p=0.7). However, FS+ animals showed significantly higher freezing post foot shock that persisted on the following day, indicating recall (FS+ n = 17, FS- n=9; independent t-test with Bonferroni correction, ****p<0.00001). (E) On the last day of habituation, both FS+ and FS- groups exhibited minimal baseline freezing (FS+ n = 17, FS- n=9; independent t-test with Bonferroni correction, p=0.6). However, post fear conditioning (FC), the FS+ group showed significantly higher baseline freezing (FS+ n = 17 animals, FS- n=9 animals; independent t-test with Bonferroni correction, ***p<0.0001). The elevated baseline freezing for the FS+ animals is sustained throughout all 4 days of the experiment. The black line shows the trend for the mean freezing. (For all figures, box shows the quartiles of the dataset, while whiskers show the rest of the distribution. Outliers are shown using diamonds.)
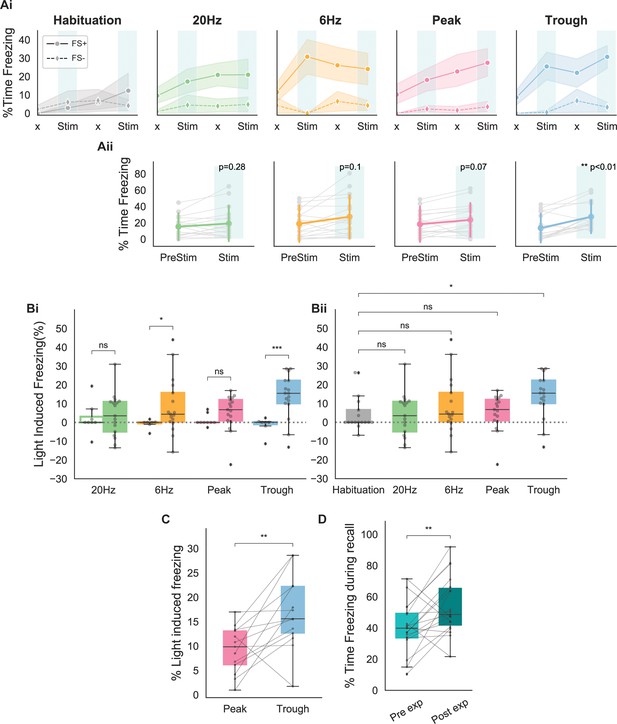
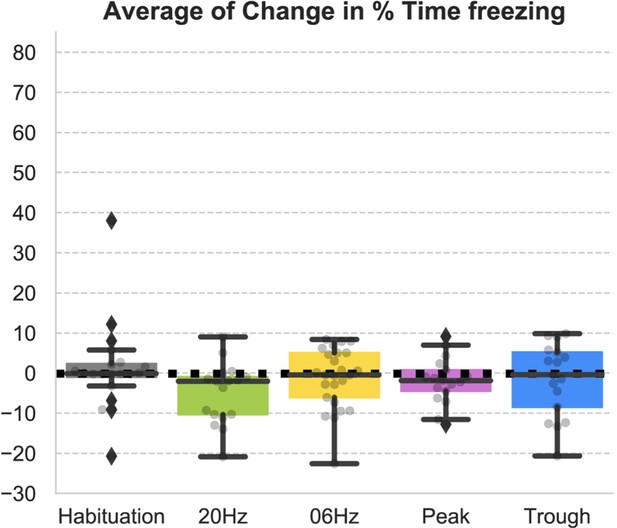
Behavioral responses indicate that recall is largest when using stimulating at the trough of theta.
(A) (Ai) Average freezing per epoch for FS+ (solid line) and FS- (dashed line) animals during habituation (gray) and during the four modes of stimulation (20 Hz: green, 6 Hz: yellow, peak: pink, trough: blue). ‘x’ indicates No Stim epochs. Shaded region represents 95% confidence interval. (Aii) Average increase in freezing using no stimulation (epochs 1 and 3) and stimulation (epochs 2 and 4). Only trough stimulation reliably caused increased freezing that resulted from activation of engram neurons (n=17 animals, paired t-test with Bonferroni correction). (B) (Bi) Average light-induced freezing was calculated for both experimental (FS+, shaded boxes) and control (FS-, open boxes) animals by subtracting epochs 2 and 4 from epochs 1 and 3, respectively, and averaging the value. Only 6 Hz stimulation and trough stimulation showed light-induced freezing that differed significantly from the non-foot shocked group. Light-induced freezing of using peak and 20 Hz stimulation was not significantly different than the control group (n=17 animals, independent t-test with Bonferroni correction; 6 Hz: *p=0.02 < 0.05; 20 Hz: p=0.6; peak: p=0.07; trough: ***p=0.0002 < 0.0001). (Bii) Light-induced freezing on the last day of habituation prior to the experiment only differed significantly for trough stimulation (n=17 animals, independent t-test with Bonferroni correction; 20 Hz: p=0.8, 6 Hz: p=0.4, peak: p=0.8, trough: *p=0.02 < 0.05). (C) Paired comparison between trough and peak stimulation for animals that exhibited light-induced freezing indicated significantly higher levels of freezing induced by trough stimulation (n=13 animals, **p=0.007 < 0.001). (D) Significantly higher freezing was observed upon exposure to the fearful context B 4 days after artificial reactivation of engram neurons in context A (n=17 animals, paired t-test, **p=0.01).
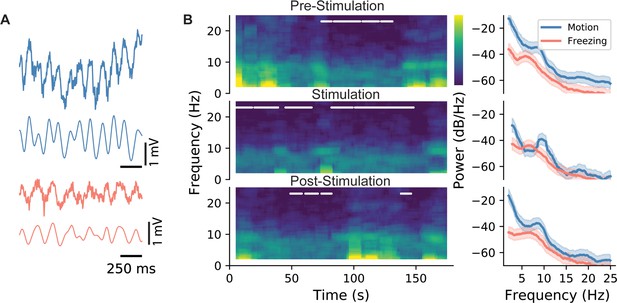
LFP characteristics during locomotion and stimulation.
(A) Sample LFP recordings from CA1 during active exploration (blue) and freezing (red). (B) Spectrogram for the whole duration of an epoch (left), and the associated power spectral density graph. In spectrograms, freezing episodes are marked with a white line. As shown, during all three epochs (pre-stimulation: epoch 1, stimulation: epoch 2, and post-stimulation: epoch 3), power and the peak frequency of theta were lower during freezing episodes. This is indicated more clearly in the PSD graphs on the right as evidenced by the shifts in the peak of theta oscillations. Shaded areas represent 95% confidence intervals.
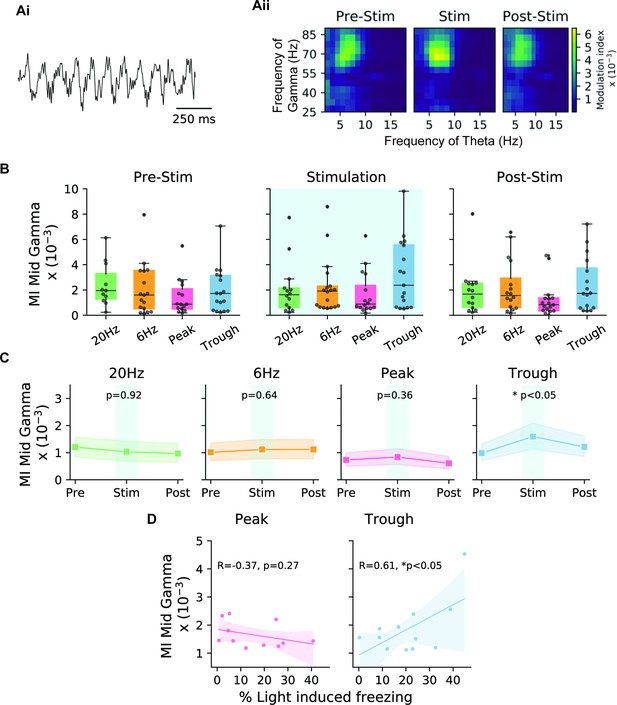
Theta-gamma cross-frequency coupling correlates with memory recall performance during trough stimulation.
(A) (Ai) Sample LFP recording indicating theta-nested gamma oscillations. (Aii) The modulation index (MI) was calculated for cross-frequency coupling between the mid-gamma (55–85 Hz) amplitude and the phase of theta for trough stimulation during epochs 1–3 corresponding to pre-stimulation, stimulation, and post-stimulation. Comodulograms only showed an increase in the MI during the stimulation epoch. (B) Boxplots show the MI during the three different epochs for the four stimulation modes (20 Hz: green, 6 Hz: yellow, peak: pink, trough: blue) (n=17 animals). (C) Direct comparison of the three epochs for the different stimulation modes indicates that only trough stimulation significantly increased the MI during the stimulation epoch (n=17 animals, paired t-test with Bonferroni correction). Shaded areas represent 95% confidence intervals. (D) Correlation of the stimulation efficacy (% light-induced freezing) and MI was only correlated significantly in the case of trough stimulation, and not during peak stimulation. Note, only mice that showed light-induced freezing were included in the analysis. Shaded areas represent 95% confidence intervals (peak: n=11 animals, trough: n=12 animals, independent t-test).
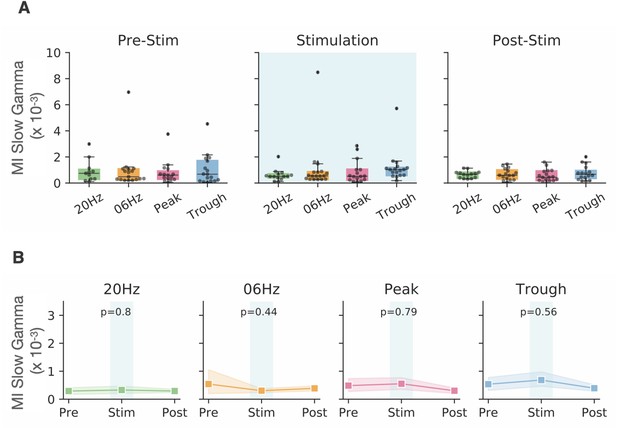
Analysis of change in cross-frequency coupling between theta and slow gamma (30–55 Hz).
(A) The modulation index (MI, see main paper for details) between theta and slow gamma, plotted before (left), during (middle), and after (right) optogenetic stimulation for four stimulation conditions (20 Hz, 6 Hz, peak, trough). (B) For all four stimulation conditions, stimulation did not have a statistically significant effect on MI for slow gamma. Shaded areas show 95% confidence intervals. Same data set as for Figure 5.
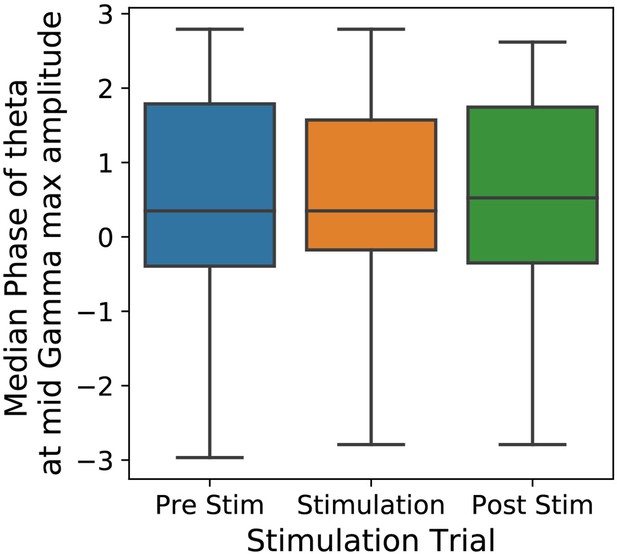
Median phase of theta at maximum amplitude of mid-gamma oscillation.
The phase of theta at maximum amplitude of gamma during epochs of 2 s was calculated. Before and during stimulation, the median theta phase of maximum gamma amplitude was +0.35 (π/9) radians. Post stimulation, the median phase was +0.52 (π/6) radians.





