Three-dimensional imaging of vascular development in the mouse epididymis
Figures
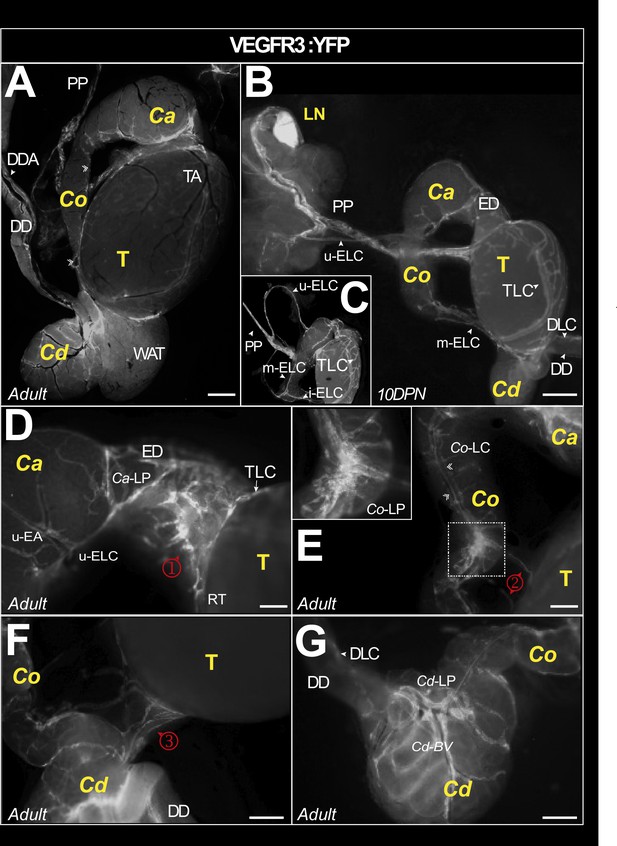
Macroscopic view of the lymphatic vasculature of the epididymis and testis in the VEGFR3:YFP model.
Representative image of the lymphatic vasculature of the adult epididymis and testis observed with a Leica binocular loupe (A). VEGFR3:YFPpos lymphatics are visible in the caput, corpus, and cauda regions. Lymphatics responsible for epididymal and testicular drainage follow the pampiniform plexus (PP) before reaching a lymph node (B). The superior and lateral epididymal lymphatic collectors (upperELC and medianELC, respectively) drain lymph from the caput and corpus, respectively, before joining the main testicular lymphatic collector at the PP. The latter is connected to a main collector that surrounds the testis (arrowhead) and branches into a rich network (A–C). There are numerous lymphatic connections between the lymphatics of the epididymis and the main lymphatic collector of the testis, notably through a lymphatic network at the level of the efferent ducts (φ in D), corpus (κ in E), and cauda (λ in F). Through the capsule covering the epididymal duct, there is also fluorescence that outlines the tubules, weakly in the caput (D) and more intense in the cauda (F and G). The scale bar corresponds to 1 mm. AT = adipose tissue; Ca = caput; Cd = cauda; Co = corpus; DD = deferent duct; DDA = deferent duct artery; DLC = deferent lymphatic collector; DPN = days postnatal; ED = efferent duct; LELC = lower epididymal lymphatic collector; LN; lymph node; P=pampiniform plexus; SEA = superior epididymal artery; Cd-BV = caudal blood vessel; SELC = superior epididymal lymphatic collector; T = testis; TA = testis artery; TLC = testicular lymphatic collector. ①=caput–testis lymphatic connection; ②=corpus–testis lymphatic connection; ③=cauda–testis lymphatic connections between testis and epididymis.
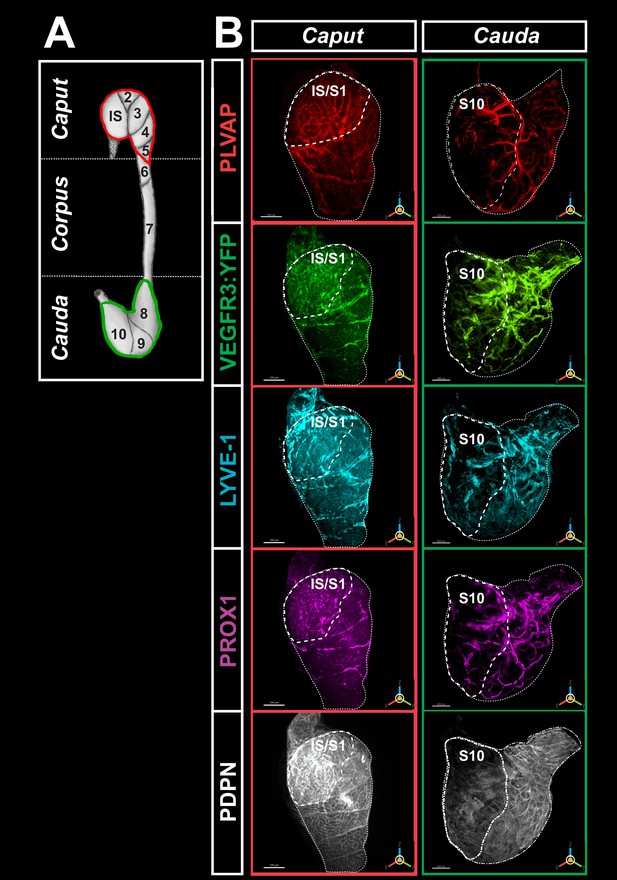
High-resolution three-dimensional (3D) imaging of the blood and lymphatic vasculature of the mouse epididymis after organ clearing.
(A) shows a schematic representation of the caput (red) and cauda (green) regionalization and segmentation commonly used to describe the mouse epididymis. (B) Representative multiplex immunostaining of the caput (left) and cauda (right) of the epididymis using five markers recognizing blood and lymphatic vessels is shown. An anti-MECA32 antibody (red) reveals PLVAPpos blood vessels, particularly fenestrated vessels. An anti-GFP antibody revealing the VEGFR3:YFP transgene (green) and an anti-LYVE1 antibody (cyan). Anti-LYVE1 (cyan), anti-PROX1 (magenta), and anti-PDPN (white) antibodies were used as additional lymphatic markers. After organ clearance, high-resolution 3D imaging was performed with a light-sheet ultramicroscope (LaVision BioTec). The two extreme segments, IS (initial segment or S1) and S10, are delineated by a large dashed line, whereas the caput (left) and cauda (right) regions are delineated by a small dashed line. The scale bar is 500 µm. The PLVAP image corresponds to the contro-lateral epididymis used for the four lymphatic markers since it is not possible to use all five markers on the same organ.
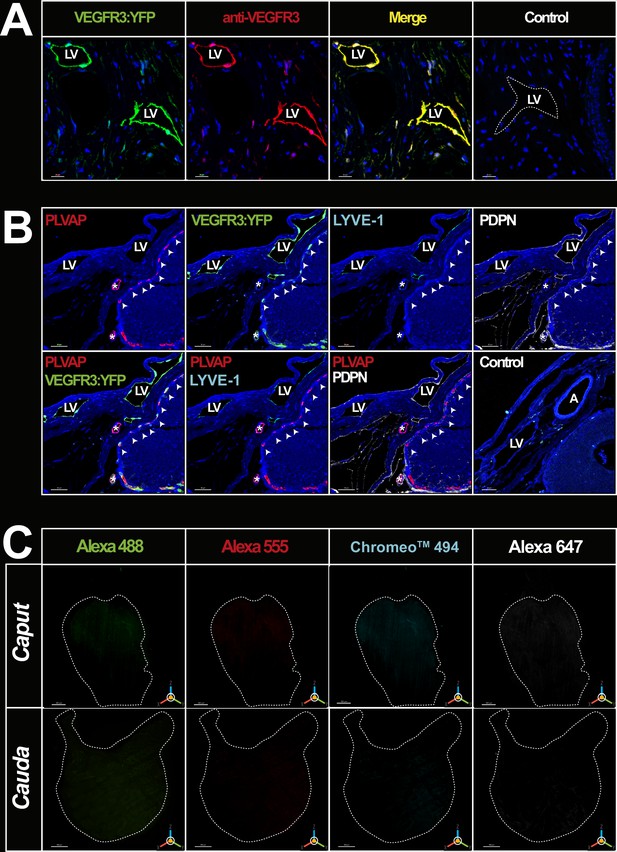
Positive and negative immunohistochemical controls.
Panel A shows confocal views of multiplex labeling with the anti-GFP antibody (green) and the anti-mouse VEGFR3 antibody (red)on paraffin sections of an adult mouse epididymis, attesting that the two antibodies recognize the same structures. The scale bar is 20 µm. Panel B shows the compatibility of the four markers/dyes chosen for imaging blood and lymphatic vessels. Multiplex paraffin immunodetection of PLVAP/Alexa A555 (red), VEGFR3:YFP/GFP/Alexa A488 (green), LYVE1/Chromeo 494 (cyan), and PDPN/Alexa A647 (white) at the junction between the initial segment (IS)/S1 and the epididymal capsule. We noted the presence of fenestrated PLVAPpos blood vessels at the periphery of the epididymal tubule of the IS (white arrowheads). These are also positive for VEGFR3:YFP/GFP but negative for LYVE1 and PDPN, demonstrating that there is no cross-detection between the labeling revealed with Chromeo 494 and those revealed by Alexa 488, Alexa 555, and Alexa 647. The peripheral lymphatic vessels of the organ located in the capsule (LV) are negative for PLVAP and positive for the three lymphatic markers (VEGFR3:YFP/GFP, LYVE1, and PDPN). We noted the presence of intertubular blood vessels (*), which present heterogeneous labeling. The upper one is only PLVAPpos while the lower one is PLVAPpos and VEGFR3:YFPpos. In addition, note that the latter shows peripheral labeling for PDPN, while that is not the case for the upper one. A=artery. Panel C shows three-dimensional (3D) imaging obtained with a light-sheet ultramicroscope (LaVision Biotech) of the clarified epididymis caput and cauda after whole-mount incubation with the different dyes used to reveal the blood and lymphatic markers (Alexa 488 [green], Alexa 555 [red] Chromeo 494 [cyan], and Alexa 647 [white]).
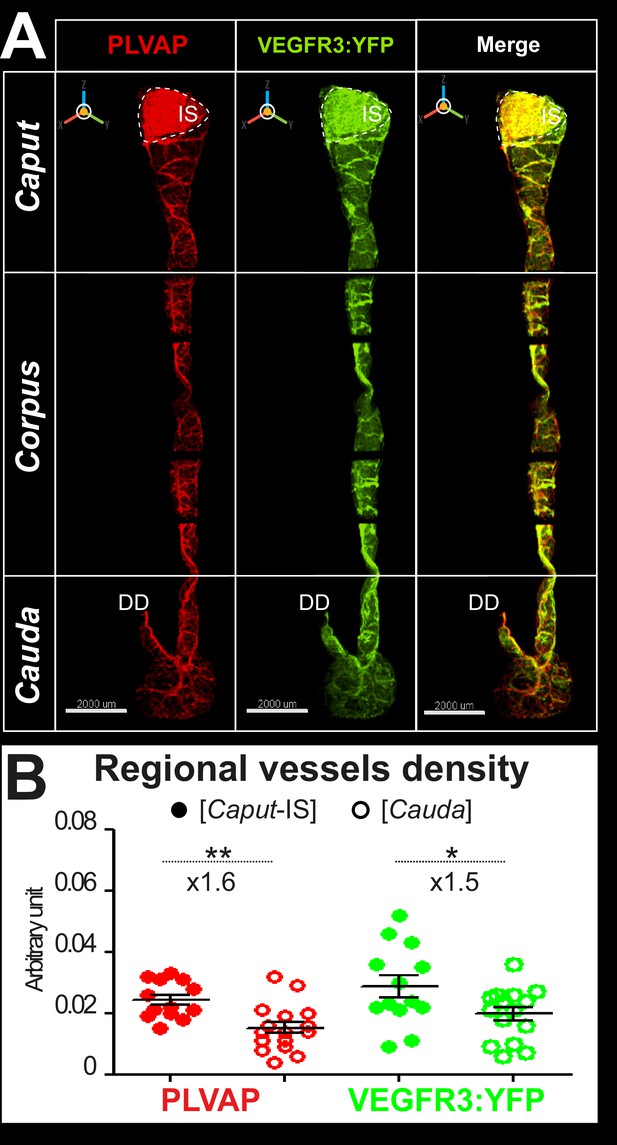
Three-dimensional (3D) view of blood and lymphatic vascularization of whole epididymis after 3DISCO clarification.
Panel A shows representative 3D views of an epididymis of an adult VEGFR3:YFP transgenic mouse obtained by light-sheet ultramicroscopy (LaVision Biotech, 2× resolution) after immunodetection using the blood marker Meca32/PLVAP (red, left panel) or the lymphatic VEGFR3:YFP transgene, revealed using an anti-GFP antibody (green, middle panel). The right panel presents a merged view. Panel B presents a comparison of blood and lymphatic vessel density between the caput and cauda as described in Figure 2 legend. The Mann–Whitney test was used to determine statistical significance (**p<0.001, *p<0.01).
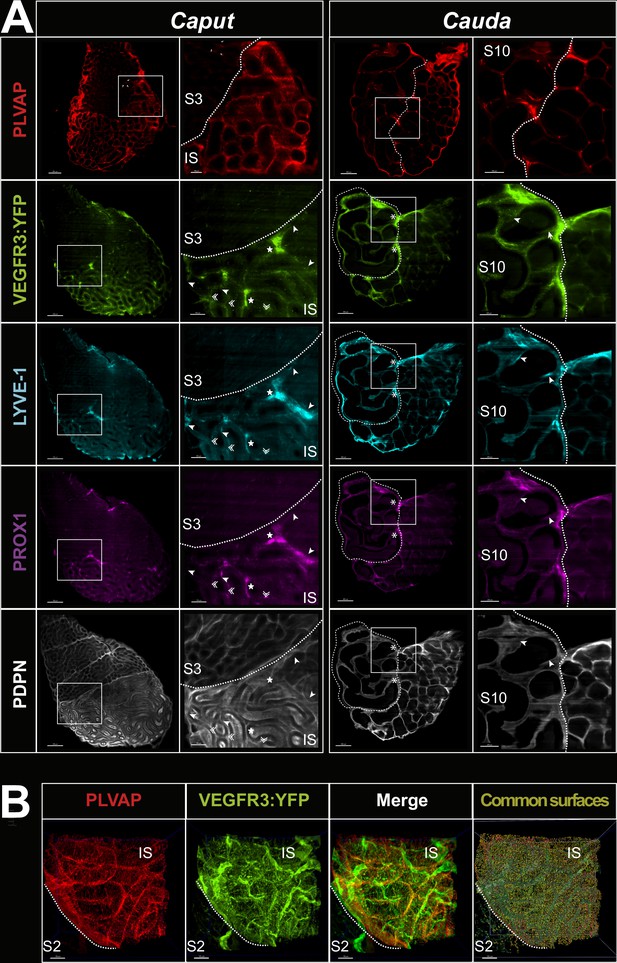
Cross-sectional views of the clarified caput and cauda epididymides.
Panel A presents median slices of the caput (left) and cauda (right) shown in Figure 2 for the PLVAP blood marker (red) and the lymphatic markers (VEGFR3:YFP, LYVE1, PROX1, and PDPN in green, cyan, magenta, and white, respectively). The epididymis used for PLVAP detection is the contralateral organ of the one used for lymphatic markers. The white-squared regions in each photograph (left in caput and cauda) are enlarged in the right photographs. PLVAP+ micro-vascularization (red) is visible at the peritubular epithelium level of the initial segment (IS)/S1 and its expression markedly decreases in the other segments. At higher magnification, a network of blood capillaries surrounds each tubule mainly at the level of the IS/S1 while intertubular vessels can be observed in segment 3 (S3). In the cauda (S10), PLVAPpos vasculature is less dense and is mostly located in the intertubular space. The VEGFR3:YFP transgene (green), revealed by an anti-GFP antibody, is abundant in the caput and in particular in the IS/S1. Enlargements show punctiform labeling at the peritubular level and in the intertubular zone. On can note the presence of very large lymphatics at the intertubular level (white star). In the cauda (S10), reactivity is mainly interstitial, stringy, and compatible with flattened lymphatic vessels trapped in the extracellular matrix. We also noted at the S9/S10 boundaries (delimited by the dotted line) the presence of large lymphatic vessels (white asterisk), suggesting that important drainage takes place at this location. The lymphatic marker LYVE1 in the caput is mainly interstitial and clearly stronger in the IS/S1. We also noticed that the lymphatics detected with the transgene (VEGFR3:YFP) and with LYVE1 present differences (white arrows), in agreement with previous studies reporting heterogeneity for these markers at the level of the lymphatics (Pawlak and Caron, 2020; Ulvmar and Mäkinen, 2016). In the cauda, LYVE1 appears less present compared with VEGFR3:YFP. We also noticed differences in localization and intensity between these two lymphatic markers (white arrows). PROX1 (magenta) shows a profile comparable to those obtained for the transgene product and/or LYVE1 in both the caput and cauda. The labeling is punctiform (compatible with a nuclear localization) and filamentous at the level of the IS/S1. The stringy appearance can be explained by the use of a PROX1-biotinylated antibody that generates a fuzzier/coarser signal. PDPN (white) appears abundant in the caput and cauda at the peritubular level of the epididymal epithelium and in the interstitial space. Stereocilia in the initial segment but not in other segments of the caput also show some reactivity with PDPN. Because of the fuzzy PDPN pattern, it is difficult at this resolution to assert that PDPN strictly co-localizes with VEGFR3:YFP, LYVE1, and PROX1. Panel B shows light-sheet confocal (12×) resolution of the caput/IS-S2 region of a VEGFR3:YFP transgenic epididymis (green) or after PLVAP immunodetection (red). The superposition of the two channels (merge) suggests both co-localization but also specific territories. The far right picture shows the superposition of the surface rendering of the two markers, which allows distinguishing the lymphatic vasculature that expresses only the transgene (in dark green) from the PLVAP+/VEGFR3:YFP+ vessels (in light green).
Three-dimensional (3D) imaging of multiplex labeling of blood and lymphatic networks in the adult mouse caput epididymis.
The video shows the overlay of the PLVAPpos blood networks (red) and those obtained for the lymphatic markers VEGFR3:YFP (green), LYVE1 (cyan), and PDPN (white). 3D reconstruction from the light-sheet ultramicroscopy and video data were performed with IMARIS software.
Three-dimensional (3D) imaging of multiplex labeling of blood and lymphatic networks in the adult mouse corpus epididymis.
The video shows the 3D reconstruction, using light-sheet ultramicroscopy data, of the PLVAPpos blood vessel network and VEGFR3:YFP+ lymphatic vasculature in the corpus epididymis of the adult mouse. The PDPNpos grid is shown to highlight the structure of the epididymis. The surface rendering of these two networks shows a difference in distribution. The blood network is evenly distributed in the corpus while the lymphatics show a greater density on the anterior edge (edge adjacent to the testis) with some large external collectors surrounding the organ. A few large lymphatic collectors cross the corpus from side to side at the septa (magenta arrow). Co-CL=corpus lymphatic collector.
Three-dimensional (3D) imaging of multiplex labeling of blood and lymphatic networks in the adult mouse cauda epididymis.
The video shows the overlay of the PLVAPpos blood networks (red) and those obtained for the lymphatic markers VEGFR3:YFP (green), LYVE1 (cyan), and PDPN (white). 3D reconstruction from the light-sheet ultramicroscopy data and video was performed with IMARIS software. The cross-sectional view shows the internal distribution of the different networks and the zoom shows the importance of the lymphatic network at the terminal.
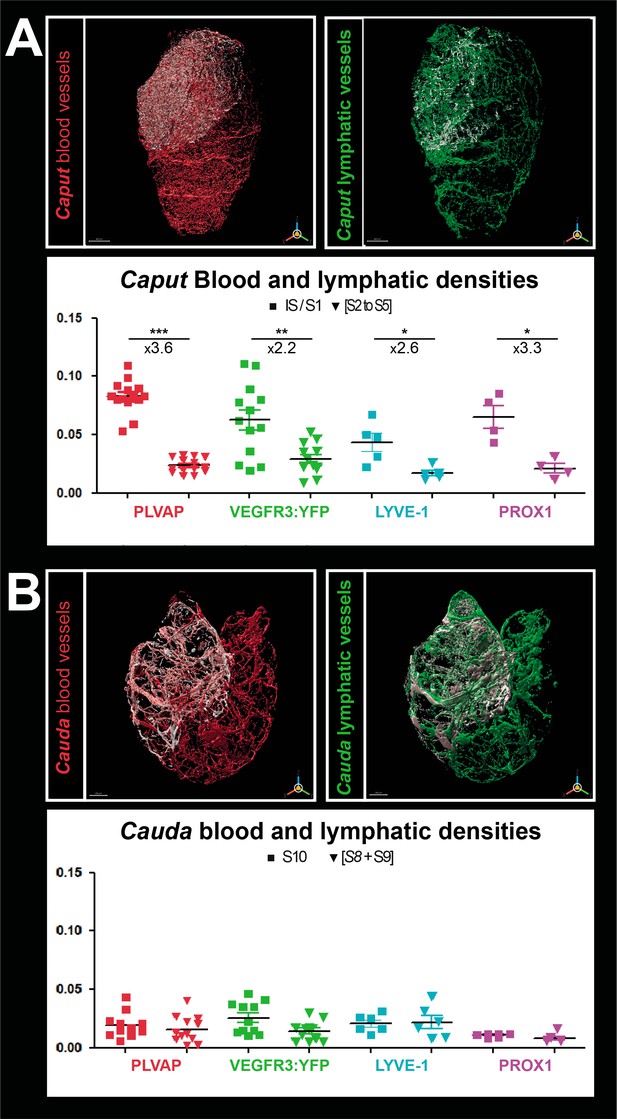
Densitometry of the blood and lymphatic vasculature of the mouse epididymis.
Panel A shows the surface rendering performed with IMARIS software of blood vessels (red, left) and lymphatic vessels (green, right) in the caput. The surface rendering of vessels in S1 is in white in both cases. Vessel densities shown in the graphs below correspond to the ratio of the volume occupied by vessels in the S1 or caput (minus S1) normalized to the total volume of the S1 or caput (minus S1), respectively. Panel B shows the surface rendering of blood (red, left) and lymphatic (green, right) vessels in the cauda. Blood or lymphatic vessel densities are measured as described in A for the caput. The Mann–Whitney test was used to determine statistical significance (***p<0.0001, **p<0.001, *p<0.01, NS = not significant).
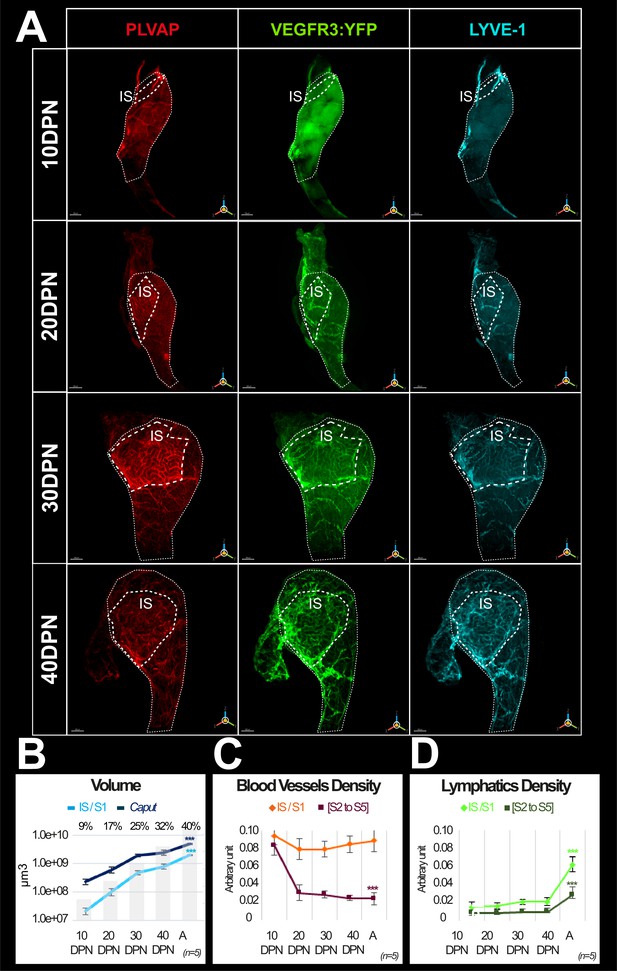
Evolution of the blood and lymphatic vasculature during postnatal epididymal development.
Panel A shows representative three-dimensional (3D) images of blood and lymphatic networks at different postnatal stages during caput ontogenesis. Immunostaining was done with the blood vessel marker MECA32/PLVAP antibody (red) and the lymphatic marker LYVE1 antibody (cyan). The VEGFR3:YFP transgene is revealed by an anti-GFP antibody. Only LYVE1 and VEGFR3 are presented here for better resolution. Immunostaining was also performed with PDPN (not shown in the figure) but PDPN data can be seen in the data sources (figure 4 data sources 1,2 and Figure 4—figure supplement 1 data source 1&2 at https://www.ebi.ac.uk/biostudies/bioimages/studies/S-BIAD618).The dotted line indicates the contours of the initial segment (IS = S1). DPN: days postnatal. The scale bar is 200 µm for 10 DPN, 300 µm for 20 and 30 DPN, and 400 µm for 40 DPN. Panel B shows the evolution of the volume (in log10) of the S1 segment (light blue curve) and the caput region (dark blue) during postnatal development. The superimposed histogram gives the proportion of volume occupied by the S1 relative to the caput region at different stages of postnatal development. Surface rendering of blood vessels and lymphatics was performed using IMARIS software. Blood and lymphatic vessel densities were calculated as described previously (Figures 2 and 3). Quantification was performed on five replicates for the different postnatal developmental stages. Panels C and D are graphs presenting densities (mean and standard error of the mean) of blood and lymphatic vasculature, respectively. The Kruskal–Wallis test with Dunn’s posttest correction was used to determine statistical significance (***p<0.0001, **p<0.001, *p<0.01).
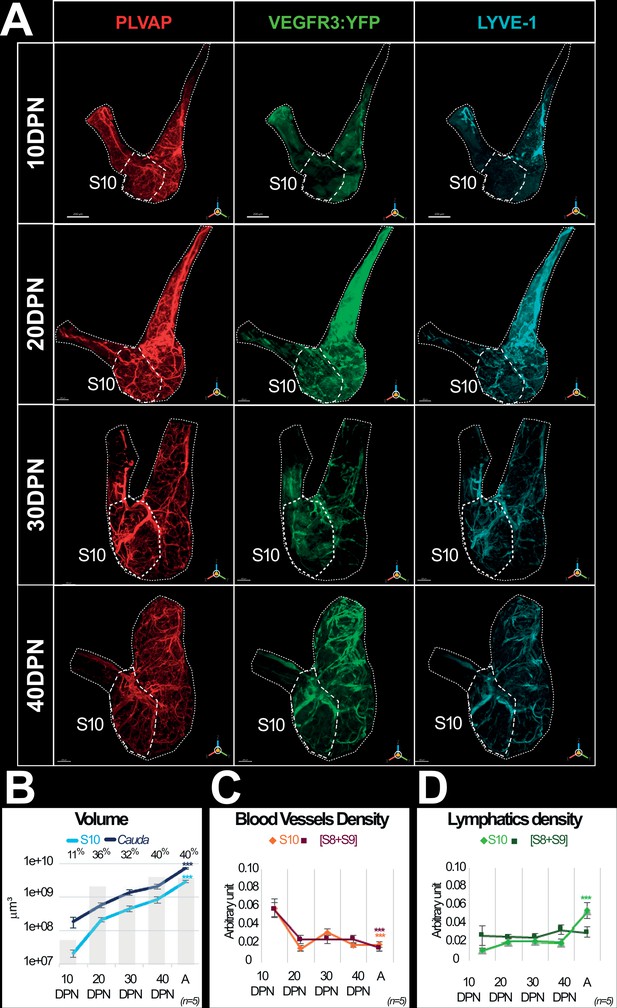
Evolution of the blood and lymphatic vascularization of the epididymal cauda during postnatal development.
Panel A shows representative three-dimensional (3D) views of the blood and lymphatic networks at different postnatal stages of the cauda epididymis ontogenesis from 10 to 40 days postnatal (DPN). Whole-mount immunolabeling of PLVAP+ blood vessels (red) was conducted on the contralateral organ of the one used for the immunolabeling with the lymphatic marker LYVE1 (cyan) and detection of the YFP reporter gene (revealed using an anti-GFP antibody; green). The dotted line indicates the S8–S9/S10 border. The scale bars is 200 µm for 10 and 20 DPN, 300 µm for 30 DPN, and 400 µm for 40 DPN. Panel B shows evolution of the volume (in log10) of the S10 segment (light blue line) and the cauda (S8 to S10; dark blue line) regions during postnatal development. The light gray histogram in the background gives the proportion of volume occupied by the S10 segment within the cauda at different postnatal developmental stages. Panels C and D present surface rendering of blood vessels (C) and lymphatics (D) evaluated using the IMARIS software as described above (see Figures 2 and 3). The curves represent the mean and standard error of the mean of the densities obtained for at least five individuals (for the postnatal development stages) and up to 13 individuals (for the adult stage). The color code in Panel C is orange = S10 and dark red = [S8+S9]. In Panel D, the color code is light green = S10 and dark green = [S8+S9]. The Kruskal–Wallis test with Dunn’s posttest correction was used to determine statistical significance (***p<0.0001).
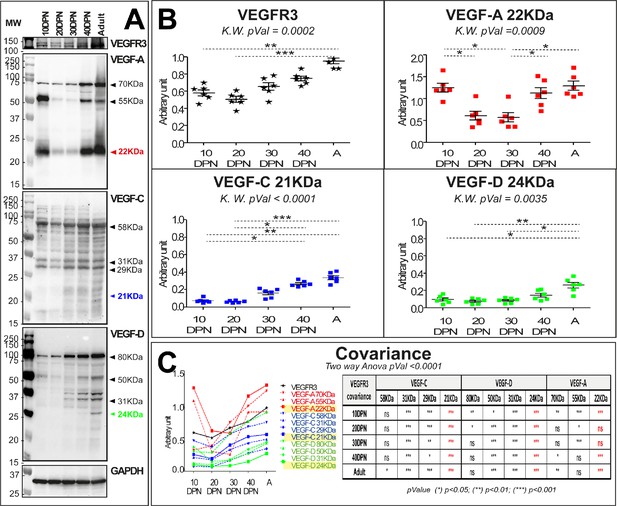
VEGF-A, VEGF-C, and VEGF-D levels vary during postnatal epididymal development.
Panel A shows the expression profile obtained in total extracts of epididymal proteins at different stages of development. The profiles obtained are presented in the following order: VEGFR3, VEGF-A, VEGF-C, and VEGF-D. GAPDH was used for normalization. Panel B shows the quantification of epididymal proteins extracted from six mice. Quantification of VEGFR3 is shown in black, and VEGF-A, VEGF-C, and VEGF-D are shown in red, blue, and green, respectively. The Kruskal–Wallis test and Dunn’s posttest correction were used to determine statistical significance (***p<0.0001, **p<0.001, *p<0.01). Panel C shows the comparison of different isoform profiles. Covariance is assessed by a two-way analysis of variance.

Quantification of all forms of the hemangiogenic and lymphangiogenic ligands VEGF-A, VEGF-C, and VEGF-D.
Different forms of ligands during postnatal development of the epididymis were quantified by optical density measurement with ImageJ software. It was normalized by the level of GAPDH measured in the same organ. The profiles of VEGFR3 (black star) and the active isoforms of VEGF-A (22 kDa, red square), VEGF-C (21 kDa blue square), and VEGF-D (24 kDa, green square) are those presented in Figure 5 and are presented here for comparison with the other isoforms. We noted that both the 55 kDa and 22 kDa VEGF-A profiles behave similarly during postnatal development of the epididymis. There is a drop in these two forms at 20 days postnatal (DPN) followed by a plateau at 30 DPN and then a marked increase from 40 DPN onward VEGF-A could play a different role in the epididymis because the number of PLVAP+ blood vessels do not increase at these stages (see Figure 4—figure supplement 1). Quantification of the different VEGF-C (blue) and VEGF-D (green) isoforms shows a pattern comparable to that observed for lymphatic density during postnatal development (see Figure 4_- and ). However, the present forms are mainly precursors, suggesting progressive lymphangnic activity.
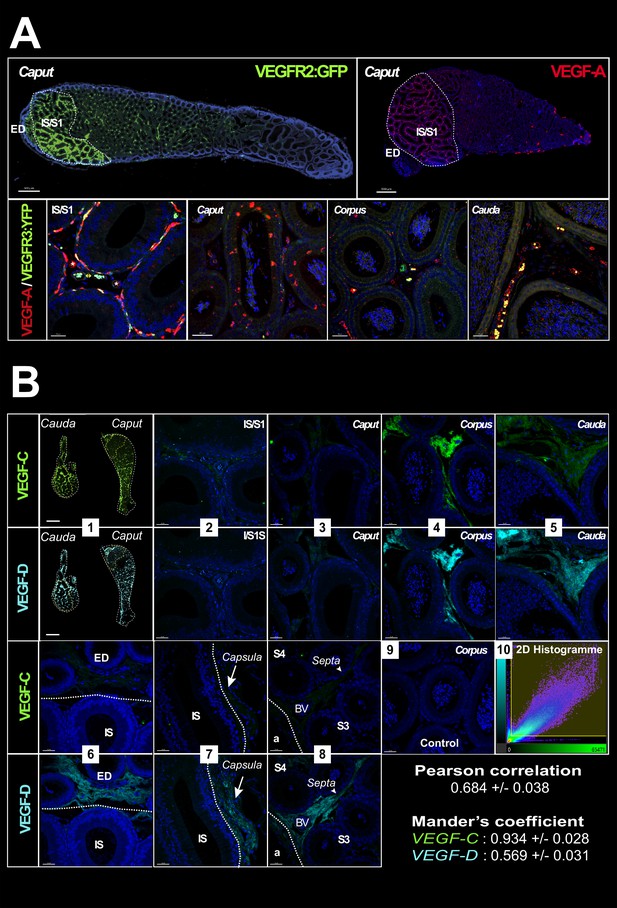
VEGF receptors and ligands in the mouse epididymis.
Panel A (upper row) shows images (Zeiss Axio-Imager) of the expression of the VEGFR2:GFP transgene (green) (used with permission from Prof. A. Medvinsky) and its ligand VEGF-A (red) obtained from paraffin sections of adult epididymis. The lower row shows confocal views (SP8, Leica) of the same VEGF-A labeling. Panel B shows in (1) a mosaic view (AxioVison scanner) of an epididymis after immunolabeling with VEGF-C (green) and VEGF-D (cyan). Photographs 2–9 were taken with a confocal (SP8, Leica) at the level of the initial segment (IS)/S1 (2), caput (3), corpus (4), and cauda (5). Notable differences in expression of these two ligands are shown in photographs 6–8, which respectively concerns the efferent duct and the IS/S1 boundary, the capsule, and a septum. Photograph 9 is a negative control. Pearson correlation and Mander’s co-occurrence were used to analyze the relation between the two ligands with the IMARIS co-localization module. The values shown represent means with standard error of the mean.
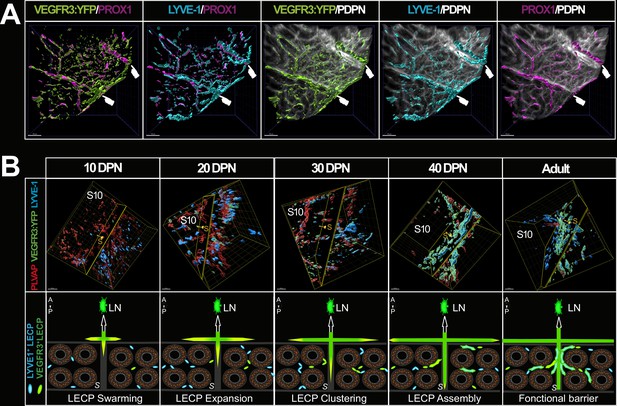
The septa between epididymal segments are intimately linked to lymphatic vasculature.
Panel A shows the intimate connection between the septa and the lymphatic vasculature in a region of interest of the caput shown in Figure 2. Surface rendering of the lymphatics was performed as described previously using IMARIS software. Panel B (top panel) shows a representative three-dimensional (3D) view of the cauda region at the S8–S9/S10 septa during postnatal epididymal development after surface rendering. Images were obtained using the light-sheet ultramicroscope with a 20× objective lens. Putative LYVE1+ lymphatic precursors are in cyan and VEGFR3:YFP lymphatics are in green, whereas PLVAP+ blood vessels are in red. The clipping plane is positioned at the septum level and the yellow arrow-cursor is directed to the S10. The lower panel shows our interpretation of the events observed concomitantly with a progression via sprouting lymphangiogenesis (green to yellow arrows) of peripheral lymphatics that progress and radiate into the organ at the level of the septum, from the anterior side (adjacent to the testis) to the posterior side of the epididymis and via lymphangio-vasculogenesis. It develops in four steps: (1) swarming of precursors mainly LYVE+ (cyan) and VEGFR3+ (green); (2) a stage of LECP expansion; (3) grouping or clustering step; (4) then assembly and fusion with the lymphatics located at the septum from lymphangiogenesis (green to yellow arrow). For clarity, PLVAP+ blood vessels have not been represented here (A=anterior side adjacent to the testis, P=posterior side, S=septum, LN = lymph node). Scale bar = 30 µm.
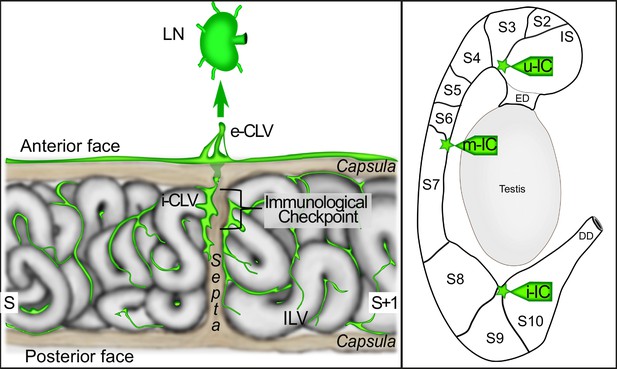
The proposed model of the lympho-septa of the epididymis.
The left scheme presents our view of the lymphatic vasculature at the level of a ‘checkpoint’ septum. The large collectors enter at the septum into the epididymis and radiate within the adjacent segments at the interstitial level, where initial lymphatics are found. The septum where the tubule crosses from one segment to another one would be the most ‘monitored’ site and therefore the richest in lymphatics. The close association of lymphatics with the septa creates both a physical and immunogenic barrier to preserve the organ from ascending infections and thus limited uncontrolled progression of pathogens protecting the epididymis and ultimately the testis from orchitis deleterious to male fertility. e-CLV=external collector lymphatic vessel; i-CLV=internal collector lymphatic vessel; ILV = initial lymphatic vessel; LN = lymph node; S=segment.
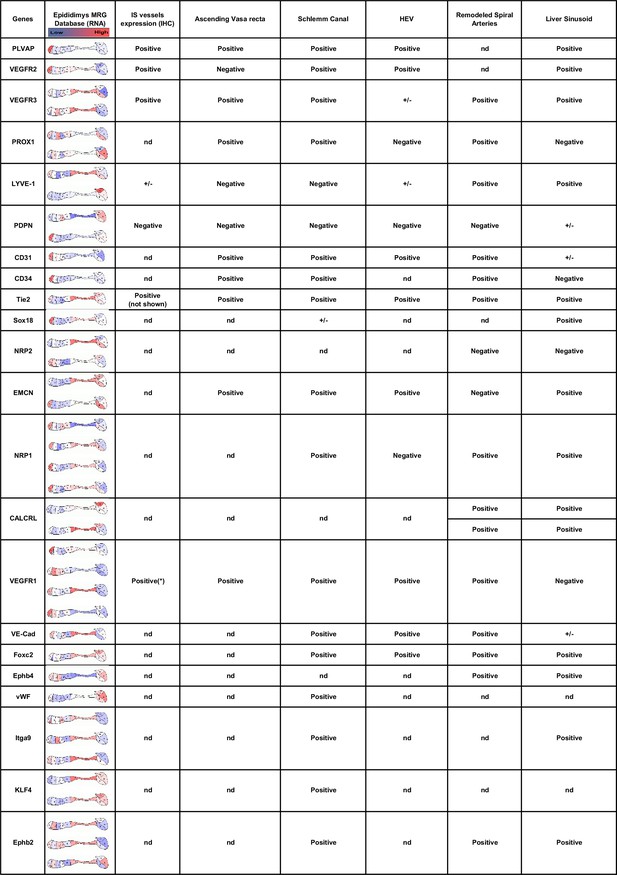
Expression of hybrid lymphatic markers in the mouse epididymis.
This table compares markers associated with hybrid lymphatic vessels described in the literature (for a review, see Pawlak and Caron, 2020) with (column 2) their expression at different segments of the epididymis (source: Mammalian Reproductive Genetic; https://www.mrgd.org). Multiple results for the same gene correspond to the use of different Oligosets in the microarrays used by the MRG. Column 3 summarizes our present results at the initial segment level. The asterisk refers to the publication by Korpelainen et al., 1998.
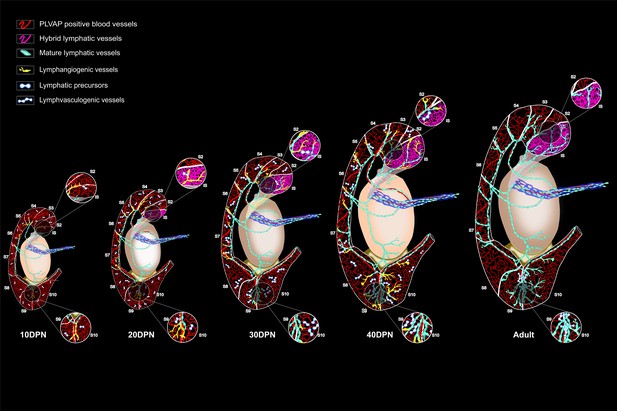
Schematic summary of the expansion of the conventional and hybrid lymphatic vasculature during postnatal development of the murine epididymis.
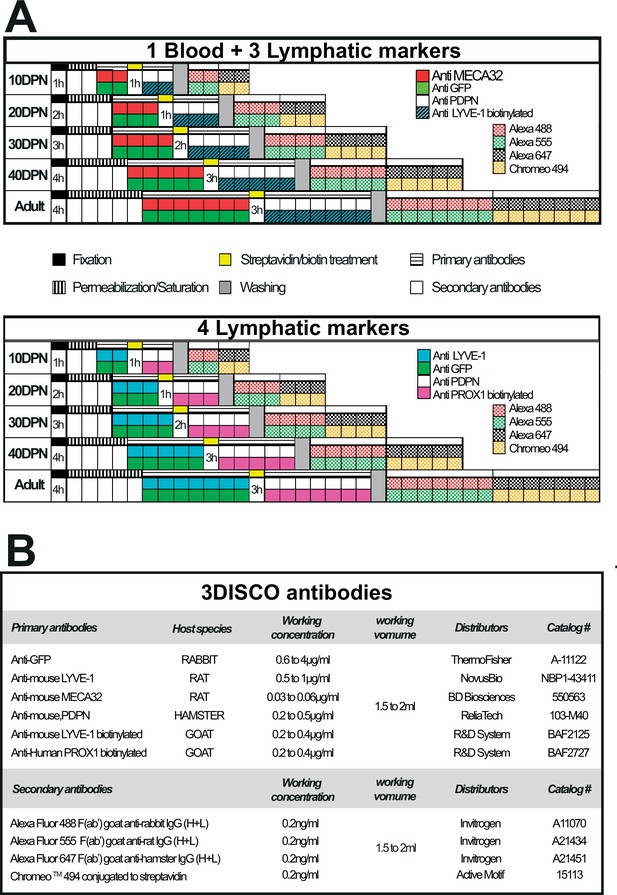
Multiplex labeling workflow used to visualize the blood and lymphatic vasculature of the mouse epididymis clarified by the 3DISCO method.
Panel A presents the multiplex labeling schedule for blood and lymphatic immunodetection. Each square corresponds to 24 hr except for the fixation and streptavidin/biotin treatment, where the time is indicated. Panel B provides detailed information regarding the various antibodies used in the course of the study.
Additional files
-
MDAR checklist
- https://cdn.elifesciences.org/articles/82748/elife-82748-mdarchecklist1-v1.pdf
-
Supplementary file 1
Describes all the data source legends in (*.ims) (Imaris viewer) and (*.czi) (Zeiss AxioScan viewer) format that can be found in the BioImage Archive Biostudies EBI repository.
- https://cdn.elifesciences.org/articles/82748/elife-82748-supp1-v1.docx
-
Supplementary file 2
Is an Excel file listing all the data sources for the figures provided.
- https://cdn.elifesciences.org/articles/82748/elife-82748-supp2-v1.xlsx





