Differential processing of decision information in subregions of rodent medial prefrontal cortex
Figures
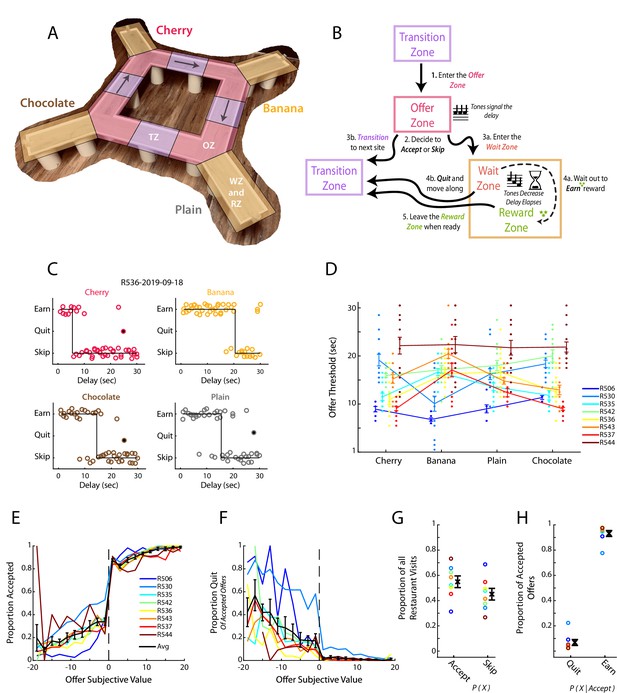
Restaurant Row as a decision-making task.
(A) Illustration of the Restaurant Row (RRow) maze. (B) Schematic of the task structure and the decision progression through an individual reward site (restaurant). (C) Example choice responding and thresholds across one behavioral session on RRow. Each circle represents one restaurant visit and is plotted according to the presented delay and the behavioral response. Offers that were quit are shown as filled circles. (D) Thresholds across the four restaurants for each rat in each session (n=8 Rats; 104 Total session). Threshold Means ± SEM for each rat are shown as error bars. (E, F) Proportion of offers that were accepted (E) or Quit (F) as a function of the subjective value of the offer (Value = Threshold – Offer Delay). Data are presented for each rat with the Mean ± SEM across rats shown in black. Note that Quits (F) are calculated as the proportion of offers that were initially accepted. (G) Proportion of all restaurant visits that were Accepted or Skipped. (H) Proportion of Accepted offers that were subsequently Quit or Earned.
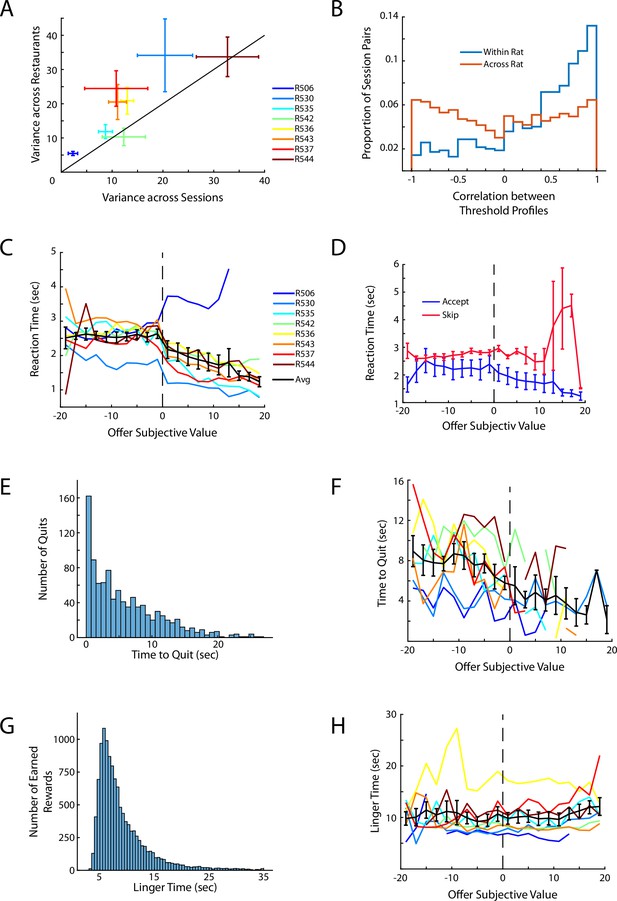
Additional behavior on Row.
(A) Average variance in threshold across sessions plotted against the average variance in threshold across restaurants. Data are plotted as Mean ± SEM of the threshold variance for each rat. Rats showed more variance in thresholds across restaurants than across sessions indicating that rats exhibited preferences across reward flavors but that the subjective assessment of each flavor was comparably stable across successive sessions. (B) Correlation of restaurant thresholds across behavioral sessions. Threshold profiles across sessions for a given rat tended to be strongly correlated whereas thresholds between rats were unrelated. (C) Average time in the Offer Zone (reaction time) before making a decision and exiting the OZ. Data are presented for each rat with the Mean ± SEM across rats shown in black. (D) Reaction time in the OZ split between accept and skip decisions. (E) Histogram of the time to quit an accepted offer in the WZ. Note that, by definition, time to quit must be less than accepted delay. (F) Average time to quit as a function of the subjective value of the accepted offer. (G) Histogram of the total time spent in the RZ (linger time) after earning a reward before rats left the restaurant and transitioned to the next site. (H) Linger time as a function of subjective value.
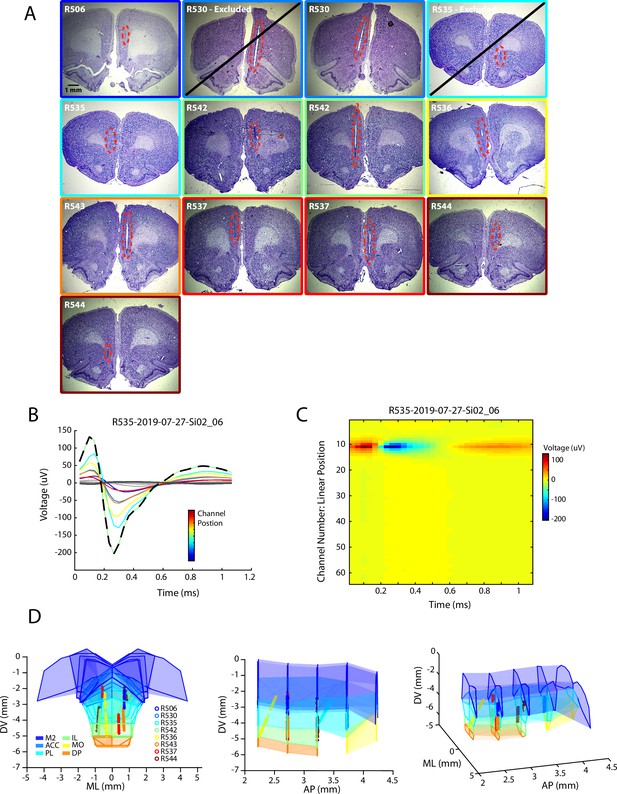
Localization of single-unit recordings from medial prefrontal cortex.
(A) Histological sections of silicon probe trajectories through the mPFC. Note that two probes were located outside of the mPFC and were excluded from subsequent analysis. (B,C) Line and image plots of the waveform of an example cell recorded across the 64 linear channels of a silicon probe. For the line plot, the eight largest amplitude channels are colored according to their sequential positions on the probe. The channel with the largest amplitude waveform (black dashed line) can be identified and treated as the location most proximal to the cell body of the recorded unit. (D) Static views of the three-dimensional reconstruction of the mPFC and the anatomical location of all cells included in the study. Each dot corresponds to a single unit (n=3017) and are colored according to rat identity (n=8). Shaded regions indicate subregions of the mPFC as defined in the Paxinos and Watson atlas.
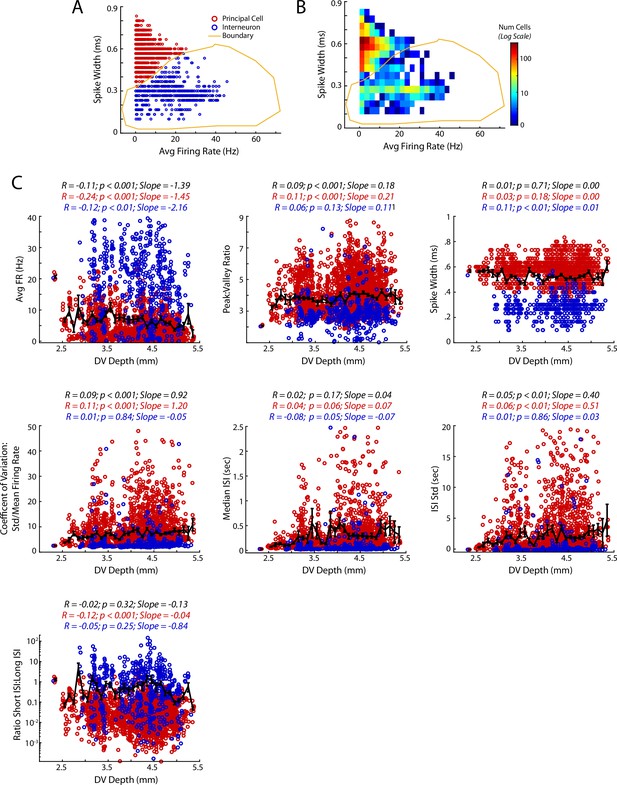
Basic spiking characteristics of medial prefrontal cells.
(A,B) Segregation of Principal cells and Interneurons in mPFC based on firing rate and waveform shape. Data are shown as points for individual cells (A) or as a density heat map (B) (C) Basic spiking parameters of mPFC cells as a function of DV location of each cell (see Methods for details about each metric). Each cell is colored according to the principal/interneuron designation derived in panel A. For each spiking parameter, the correlation coefficient between the metric and DV location, its p-value, and linear slope are reported above the respective plot. Statistics for each plot are reported for all cells (black), and for principal cells (red) and interneurons (blue) separately. Black lines show mean and standard errors.
3D Anatomical location of recorded mPFC cells.
Three-dimensional reconstruction of the mPFC and the anatomical location of all cells included in the study. Each dot corresponds to a single unit (n=3017) and are colored according to rat identity (n=8). Shaded regions indicate subregions of the mPFC as defined in the Paxinos and Watson atlas. See Figure 1—figure supplement 2, D for legend keys.
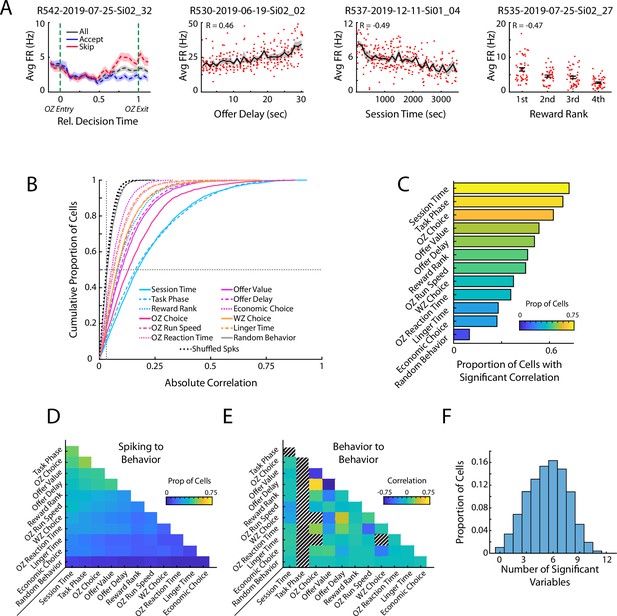
Prefrontal cells respond to task relevant variables.
(A) Four example mPFC cells that respond to offer zone choice, offer delay, session time, and reward rank. Mean and standard errors are shown. (B) Cumulative density functions of the unsigned correlation between firing rate of mPFC cells (n=3017) and various task relevant behaviors. Chance values are computed by correlating firing rates to randomly drawn values (Random Behavior) and by time shifting spiking of each cell and correlating the dissociated spiking data to the behaviors of interest (Shuffled Spks). Firing rates in mPFC were significantly more correlated to each behavioral variable than to the respective chance distributions. (D) Proportion of the mPFC cell population that was significantly correlated to both of a pair of behavioral variables. (E) Different pairs of behavioral variables were correlated to each other. The average correlation across the 104 behavioral sessions is shown for each pair of variables. Note that Task Phase varied within each restaurant visit and thus could not be compared to other variables. Additionally, some combinations of variables were conditionally impossible to co-occur and thus could not be correlated (e.g. OZ Choice and WZ Choice). (F) Individual mPFC cells were often significantly correlated to multiple behaviors. Distribution of multimodal coding across the mPFC cell population. Each bar indicates the proportion of mPFC cells with firing rates that were significantly correlated to the indicated number of behavioral variables.
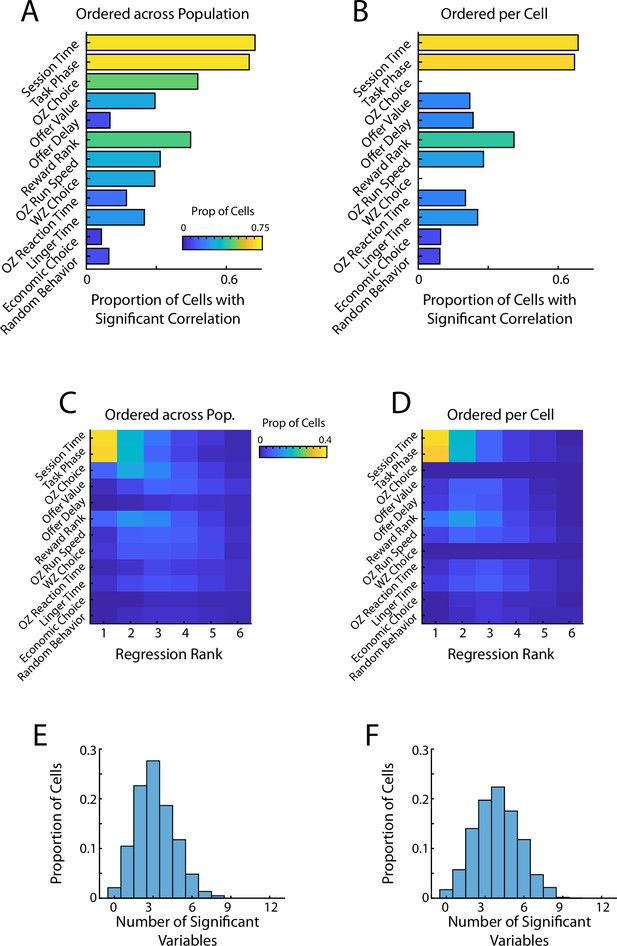
Stepwise regression of spiking to behavior.
Detection of behavioral coding using two stepwise regression approaches (A, C, E) The stepwise order of behavioral regressors was dictated externally based on the overall prevalence in the baseline correlation analysis (Figure 2C). The same ordering was used for all cells in the population. (B, D, F) The stepwise order of behavioral regressors was determined independently for each cell in order of explanatory power. (A, B) Proportion of cells with a significant regression weight for each behavioral variable when using a stepwise regression based approach. (C, D) Breakdown of significant behavioral regressors split by the explanatory rank in the stepwise regressor. A variable of rank 1 would be the most explanatory and a rank 2 would be the next most explanatory. Note that the regressor rank is not always equivalent to the step of the regression at which the variable was added. (E, F) Number of significant explanatory variables (multimodal coding) for mPFC cells when using a stepwise regression. Note that by using a regression based approach this represents mathematically orthogonal variables.
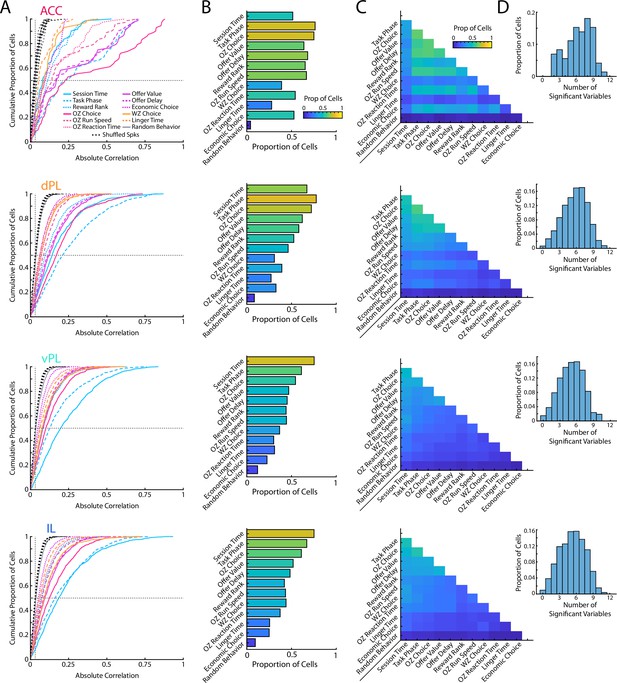
Segregation of prefrontal responses by subregion.
Data are presented as in Figure 2B, C, D and F but here segregated by mPFC subregion (ACC, dPL, vPL, IL) defined using functional communication and TE. The first column (A) shows cumulative density functions of the unsigned correlation between firing rate of mPFC cells and various task relevant behaviors. The second column (B) shows the proportion of cells that were significantly correlated to each behavioral variables. The third column (C) shows the proportion of cells from each subregion that were significantly correlated to pairs of behavioral variables. The fourth column (D) shows the distribution of multimodal coding for cells in each subregion.
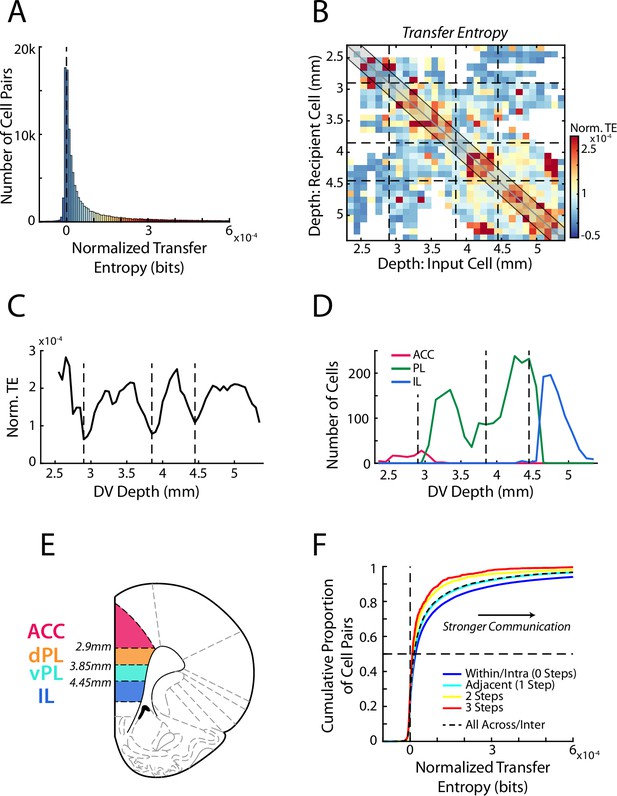
Transfer entropy reveals subregions within mPFC.
(A) Transfer entropy (TE) between simultaneously recorded pairs of cells (n=107,418 pairs) while rats performed RRow. Data were normalized based on shuffled spike times of the input cell meaning that TE values above zero (dashed line) reflect cell interactions that are stronger than expected by chance. Across the population, TE was significantly higher than chance. Data along the x-axis are color coded according to the scale in panel B. (B) Mean normalized TE (norm. TE) between pairs of cells as a function of the DV location of the input and recipient cells. Black dashed lines represent the functional boundaries between mPFC subregions computed in panel C. (C) Quantification of TE between cell pairs within 200 µm of the identity diagonal (shaded region in B). Data are the mean TE of bins within 200 µm of each DV depth. Dashed lines denote the local minima of the curve and represent derived transition bounds between subregions. (D) Number of recorded ACC, PL, and IL cells at each DV position, as classified based purely on anatomical information as defined in the Paxinos and Watson atlas. TE derived bounds are repeated from panel C. (E) A schematic of four subdivisions of mPFC based on the boundaries derived from analysis of functional communication (TE). (F) Cumulative distributions of normalized TE values between pairs of cells. Data are grouped according to the relative proximity (number of steps) between the subregions of the two cells. Black dashed line groups all inter-regional pairs regardless of distance. Note that a more rightward distribution corresponds to overall stronger TE.
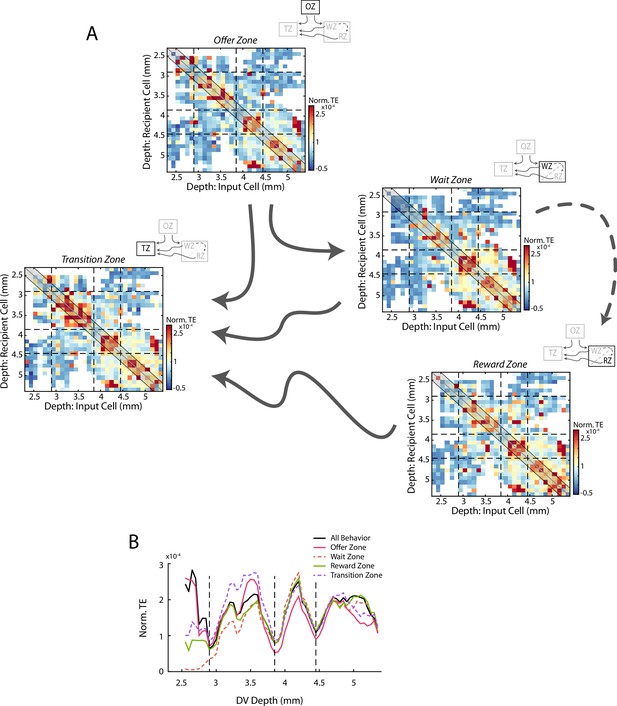
Transfer entropy analyses during different task phases.
(A) As in Figure 3B: Mean normalized transfer entropy (norm TE) between pairs of cells as a function of the DV location of the input and recipient cells. Data are segregated according to task phase (Offer Zone, Wait Zone, Reward Zone, Transition Zone) including only time points during which rats were in the respective task phase. Black dashed lines represent the functional boundaries between mPFC subregions and are reproduced here from Figure 3 and calculations from the full data set. TE profiles were largely comparable across task phases (all pairwise correlations r>0.66). (B) Quantification of TE between cells pairs around the identity diagonal as in Figure 3C. Data from the full task (Figure 3C) is reproduced here in black.
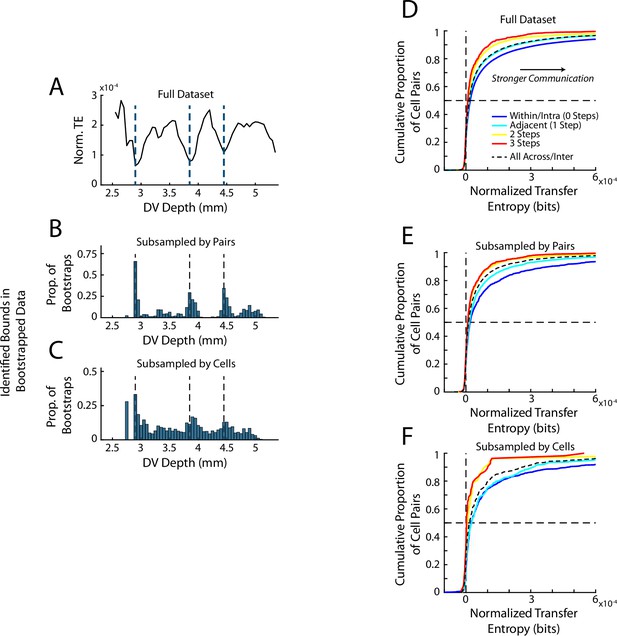
Subsampling of mPFC recordings for TE calculations.
(A) Plot of the TE quantification by depth derived from the full data set, repeated from Figure 3C. (B) The number of cell pairs included in the analysis was down sampled to match those of the ACC subregion. For each subregion the total number of pairs within the subregion (334 pairs) and the average number of pairs across subregions (397 pairs) was equated. Quantifications of TE were then computed and subregion bounds were identified from this quantification as for the full data. This process was repeated 500 times. The histogram shows the proportion of these 500 iterations in which a subregion boundary was detected at each respective depth. Note that the majority of subsamples yielded detected bounds at the same anatomical position as those of the full data set. (C) Data are presented as in B but here the total number of cells in each subregion was subsampled to equal that of ACC (72 cells). Note that because TE requires simultaneously recorded cells and our subsampling did not consider this, subsampling by cells yields a more stringent control. However, again the same anatomical bounds are largely detected in the subsampled data. (D, E, F) Quantification of the normalized transfer entropy within and between subregions. (D) The full data is reproduced from Figure 3F. (E) Data are subsampled according to number of cell pairs. (F) Data are subsampled according to total number of included cells.
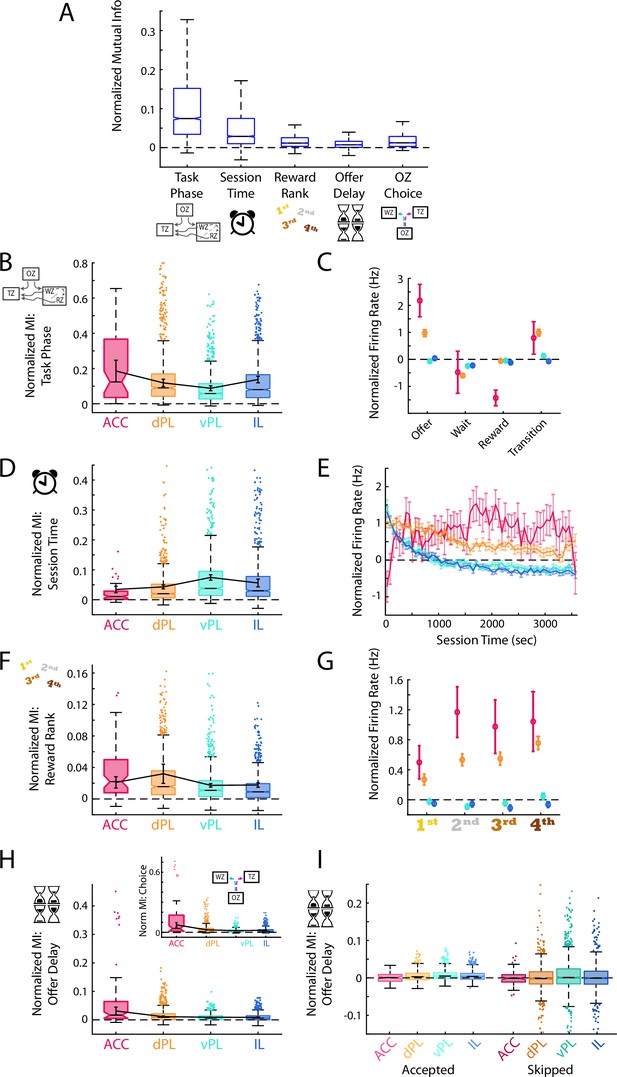
Generalized task variables are differentially represented.
(A) Mutual information (MI) calculations for mPFC cells between the task phase, elapsed session time, reward site ranking, offer delay, and choice made in the offer zone. For each cell, MI values were normalized based on data generated with shuffled spike times. For clarity, distribution outliers (beyond 1.5 x IQR) are not displayed. (B, D, F, H) Normalized MI to task phase (B), session time (D), reward rank (F), and offer delay (H) for each mPFC subregion. Boxplots with outliers show the data for all recorded cells in each subregion. Data were also averaged within each rat and the Mean ± SEM across rats is shown overlayed in black. The inset in panel (H) shows the corresponding MI data for offer zone choice. Note that because choice is based largely on the presented delay these two data are highly correlated. (C, E, G) Mean ± SEM firing rate of cells in each mPFC subregion across the four task phases (C), over the session duration (E), and for each restaurant rank (G). Note that firing rates were normalized based on shuffled spike times. Reward rank was determined in each session by sorting restaurants by decision threshold with the largest threshold (willing to wait longest to earn a reward) ranked 1st. (I) MI for offer delay split by whether rats accepted or skipped in the OZ. Note that after accounting for the choice made, MI for delay is largely eliminated suggesting that the neural responses are more associated with choice than delay.
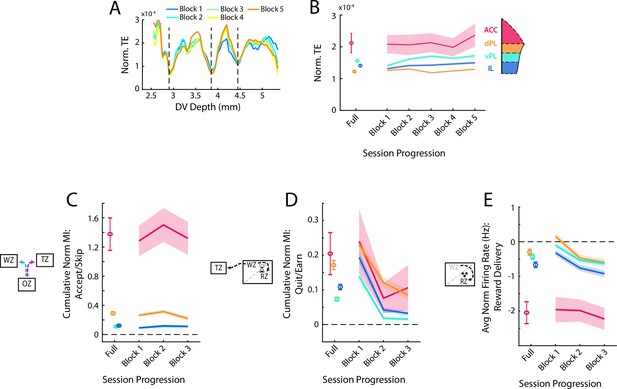
Analysis of main functional effects over the session.
(A) Transfer entropy calculations for deriving subregion bounds subdivided by portion of the behavioral session. Data were divided into five equal portions of 12 min each (Blocks) and the TE analysis was repeated independently for each time block. Block 1 is the beginning of the session and Block 5 is the end. (B) Average within subregion TE coupling over the course of the session. Data are shown as Mean ± SEM of normalized TE taken only from those cell pairs within the respective subregion. Data from the full session are shown first and from each block of 12 min in sequence. (C, D, E) Quantification of the main response patterns in the OZ (C), WZ (D), and RZ (E) split by progression through the session. Note that here the session is divided into three equal portions of 20 min each. In the OZ and WZ, these correspond to the cumulative MI during the decision period. In the RZ this corresponds to the average firing during reward delivery.
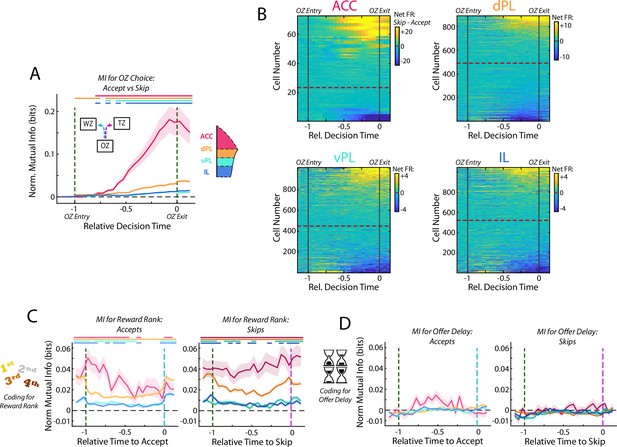
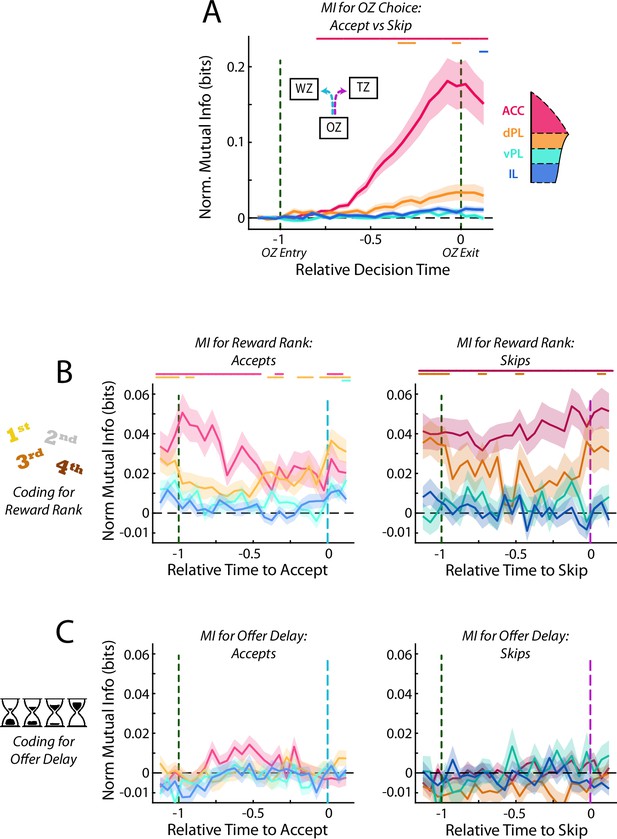
Activity in mPFC strongly encodes the identity of the upcoming choice.
(A) Mutual information (MI) between firing rate and OZ choice (accept vs skip) for cells in each mPFC subregion. Note that durations in the OZ are normalized for each lap to yield relative duration for analysis. (B) Net firing rate of mPFC cells between accept and skip decisions (Skip FR – Accept FR). For each subregion, cells are sorted according to the net firing rate after entering the OZ. Color scales are identified for each subregion but note the change in scale between subregions. (C) Mutual information between firing rate of mPFC cells and restaurant ranking and (D) between firing rate and the presented offer delay. Data are segregated according to the decision that was made. For (A,C,D), data are shown as the Mean ± SEM across cells in each mPFC subregion. All data were normalized against analyses performed using shuffled spike times. Time bins with a significant MI response (one-tailed Wilcoxon Signed-rank, α=0.05 after multiple comparison correction; n = 26 time bins) are identified by colored bars above each plot.
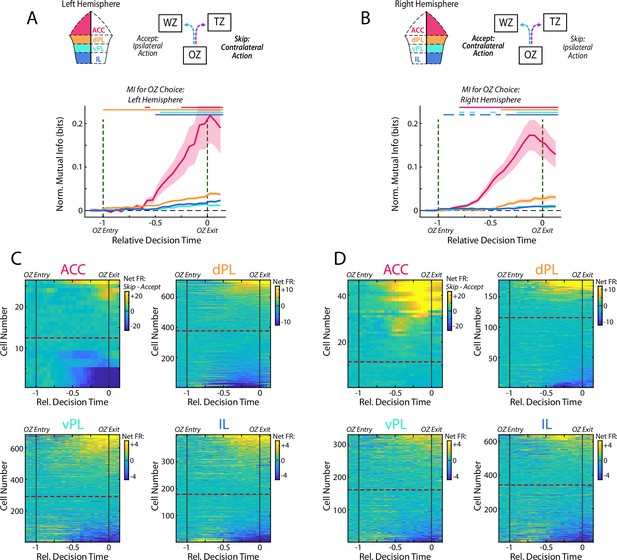
Offer Zone responses by recording hemisphere.
Cell firing in the Offer Zone was segregated according to which hemisphere each cell was recorded on to distinguish between ipsilateral and contralateral direction of movement. The left column (A, C) denotes cells that were recorded from the left hemisphere and the right column (B, D) is cells recorded from the right. (A, B) Normalized MI during decision making in the Offer Zone. Data are presented as in Figure 5A. (C, D) Net firing rates for each cell between Skip decisions and Accept decisions. Data are presented as in Figure 5B. Note that if neural responses were strongly related to motor action, we would expect a bias toward increased firing during contralateral movement. We did not observe such a bias in our OZ data.
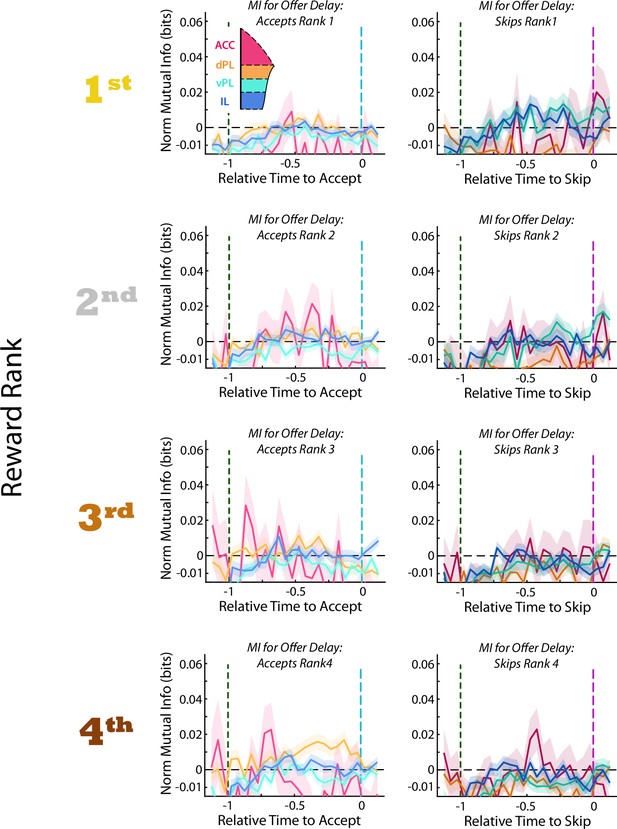
Multiplexed coding of Rank and Delay.
Mutual information between firing rate and offer delay. Data are segregated according to choice made in the Offer Zone (Accept or Skip) and by restaurant at which that decision was made. Data as presented in Figure 5D. Under no conditions did we observe evidence for coding of offer delay in any mPFC subregions.
Responses in the Offer Zone from subsampled data.
Populations of dPL, vPL, and IL cells were subsampled to match that of the ACC data set (72 Cells). Analyses from Figure 5 were repeated using the subsampled data and results are presented as in the main figure. (A) Mutual information between firing rate and choice in the OZ. (B) MI between firing rate and reward ranking. (C) MI between firing rate and offer delay.
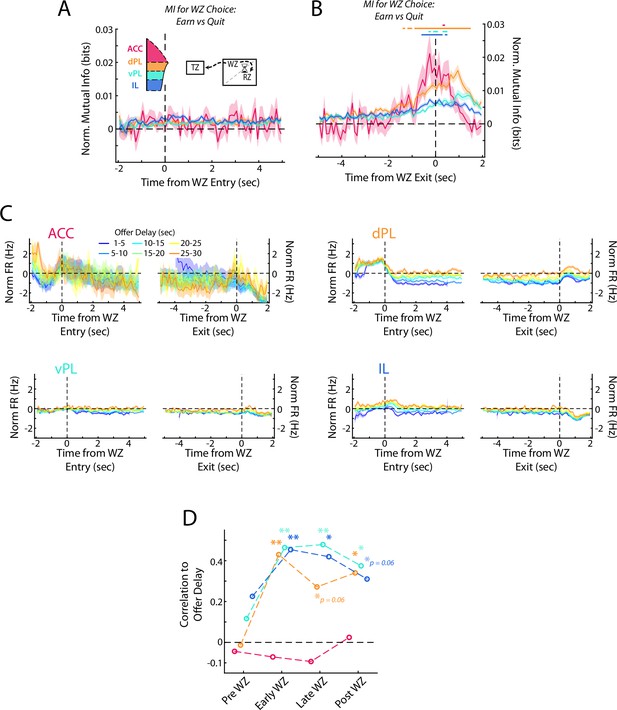
Re-evaluative decisions are represented in mPFC.
(A, B) Mutual information (MI) between firing rate and WZ choice (earn vs quit) for cells in each mPFC subregion at the beginning and end of delay period. Data are show in (A) aligned to WZ entry and in (B) aligned to WZ exit. Time bins with a significant MI response (one-tailed Wilcoxon Signed-rank, α=0.05 after multiple comparison correction; n=70 time bins) are identified by colored bars above each plot. (C) Average firing rate of mPFC cells for each subregion separated by the offer delay that had been accepted. Data are aligned to the beginning of the WZ and the end of the WZ. (D) Correlation between average firing rate across mPFC cells and the offer delay that had been accepted. Average firing rate profiles were computed across all cells for each rat independently in each offer delay bin (up to 48 values: 8 rats x 6 delay bins). These firing rates were then correlated against offer delay during four time windows: Pre WZ, Early WZ, Late WZ, and Post WZ. See Methods for additional details. For (A–C), data are shown as the Mean ± SEM across cells in each mPFC subregion. All data were normalized against analyses performed using shuffled spike times.
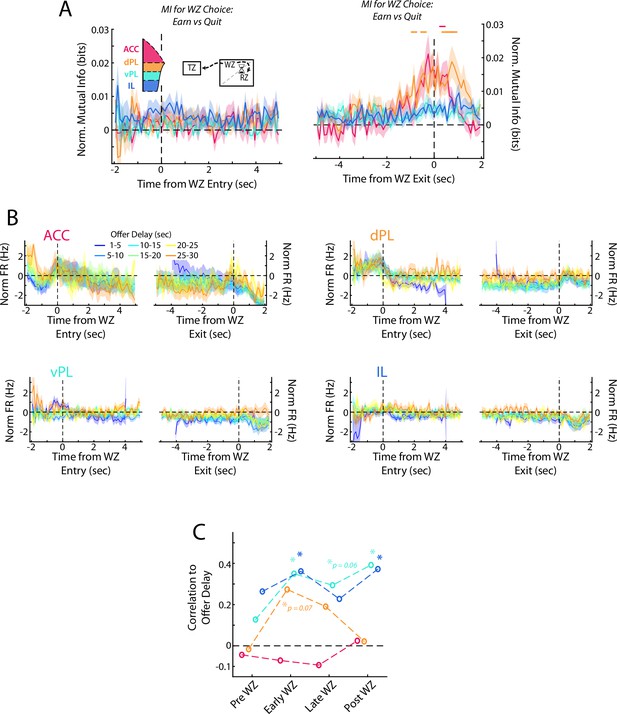
Responses in the Wait Zone from subsampled data.
Populations of dPL, vPL, and IL cells were subsampled to match that of the ACC data set (72 Cells). Analyses from Figure 6 were repeated using the subsampled data and results are presented as in the main figure. (A) Mutual information between firing rate and choice at the beginning and end of the WZ. (B) Average firing rate separated by the offer delay that had been accepted (C) Correlation between average firing rate across mPFC cells and offer delay that was accepted.
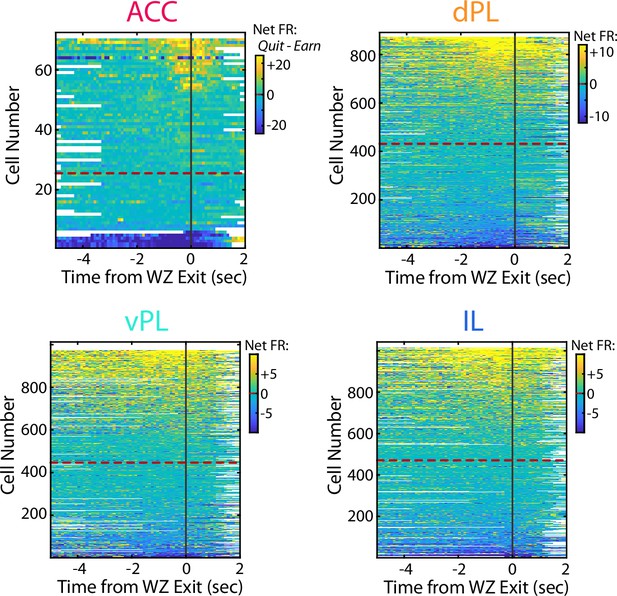
Net firing rate between Quit and Earn responses.
Net firing rate of mPFC cells between quit and earn decisions in the Wait Zone (Quit FR – Earn FR). For each subregion, cells are sorted according to net firing rate in the last 2 s in the WZ. Color scales are shown for each subregion but note the differences in scale between subregions.
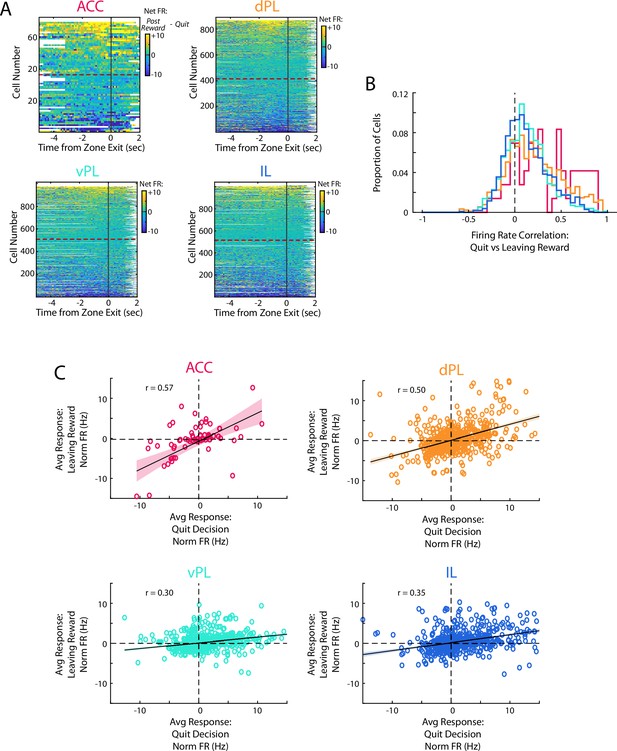
Similarity of quit decisions and leaving after reward.
(A) Net firing rate of mPFC cells between the end of the time in the WZ when rats quit and offer and the time when they leave the RZ after consuming a reward (Post Reward FR - Quit FR). Note that these reflect comparable motor actions but occur during different cognitive situations. Cells are sorted according to net firing in the last 2 s in the zone. (B) Correlation of the PETHs between quit events and rats leaving the RZ after consuming a reward. (C) Scatter plot of the average firing rate response as rats make a quit decision and when they leave the RZ. Data are fit with a linear regression, shown with 95% confidence bounds, and the correlation between the two periods is noted for each plot.
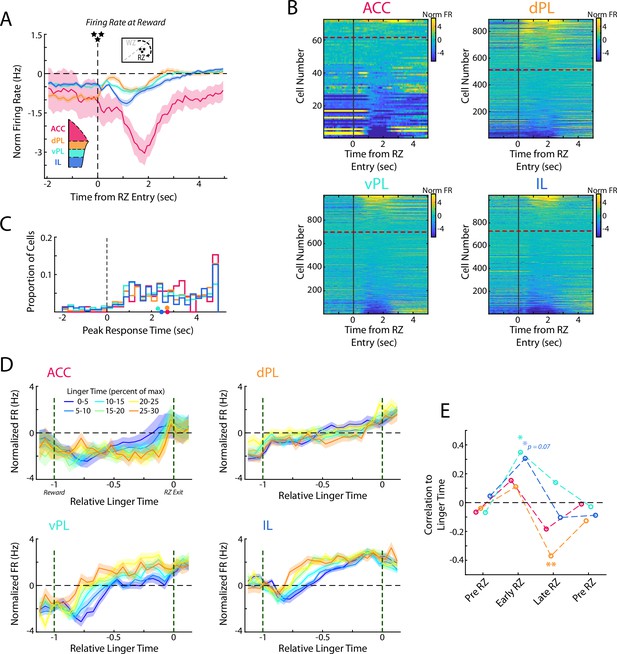
Responses to reward delivery differ across mPFC subregions.
(A) Average firing rate for cells in each mPFC subregion as rats entered the Reward Zone and received their reward. (B) Distribution of all cell responses for each subregion at RZ entry. Cells are sorted according to the average activity from 0.5 to 3 s after RZ entry. (C) Time of the peak cellular response in the first portion of the RZ period (−2–5 s from RZ entry). Distributions of all cells are shown as histograms and mean response times for each subregion are indicated with a colored dot. (D) Average firing rate of mPFC cells for each subregion separated by the amount of time spent in the RZ (linger time). Data are grouped according to the percentage of maximal linger time computed independently for each session. Note that duration in the RZ is normalized across laps to produce a relative duration for analysis. (E) As for Figure 6D, correlations between average firing rate across mPFC cells and the linger time percentage. Average rate profiles were computed for each rat in each linger time bin (up to 48 values) and correlated to percentage of linger time. Average firings rates and correlations were computed in four time windows: Pre RZ, Early RZ, Late RZ, Post RZ. See methods for additional details. For (A, D, E), data are shown as the Mean ± SEM across cells in each mPFC subregion. All data were normalized against analyses performed using shuffled spike times.
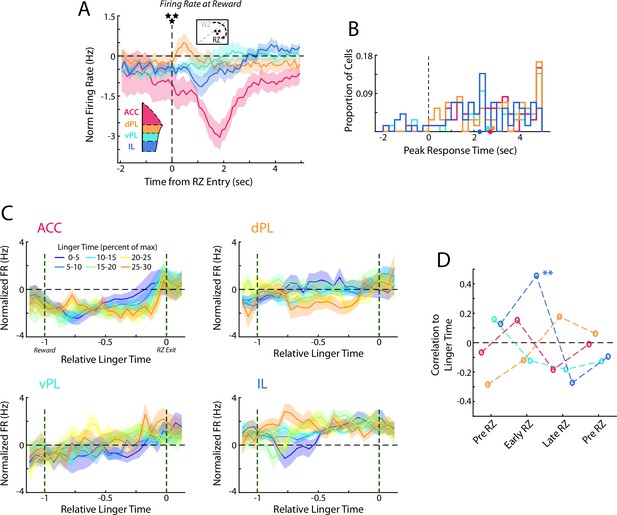
Responses in the Reward Zone from subsampled data.
Populations of dPL, vPL, and IL cells were subsampled to match that of the ACC data set (72 Cells). Analyses from Figure 7 were repeated using the subsampled data and results are presented as in the main figure. (A) Average normalized firing rate as rats enter the RZ and earn their reward. (B) Histogram of peak responses of mPFC cells. (C) Average firing rates of mPFC cells segregated by the time spend lingering in the RZ. (D) Correlation between average firing rate across mPFC cells and the time that was spent lingering in the RZ.
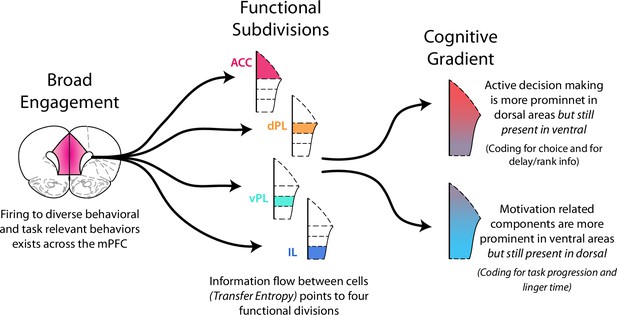
Functional activity in the medial prefrontal cortex.
Functional activity in mPFC can be described in multiple ways. There was broad, distributed engagement across subregions for many behavioral variables. Analysis of functional coupling between cells using TE revealed distinct subregions. In many cases functional activity exhibited a gradient along the dorso-ventral axis. Neural engagement during active decision processing was more prominent in dorsal regions while motivation related components were more strongly represented in ventral areas.
Tables
Probe counts by rat.
Breakdown of the sex and allocation of probe targeting across rats.
| Rat ID | Sex | Number of Probes | Dorsal Probe (ACC/PL) Hemisphere | Ventral Probe (PL/IL) Hemisphere |
|---|---|---|---|---|
| R506 | Male | 1 | Right | N/A |
| R530 | Female | 2 | Left | Right (In MO; Excluded) |
| R535 | Female | 2 | Left | Right (In VO; Excluded) |
| R542 | Male | 2 | Right | Left |
| R536 | Female | 1 | Left* Single probe moved from dorsal to ventral | |
| R543 | Male | 1 | N/A | Right |
| R537 | Female | 2 | Right | Left |
| R544 | Male | 2 | Right | Left |
Cell counts by rat.
Breakdown of the number of cells recorded from each rat. Blue shaded cells denote the sum across a respective column and green shaded cells denote the mean. Note that while a large proportion of ACC and dPL cells come from one rat each, for all analyses we confirmed that results were qualitatively similar across the remaining animals (data not shown).
| Per Session Counts | Totals Counts | Per Session Averages | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rat ID | Total Sessions | Total Cells | Avg Cells | Min Cells | Max Cells | Std Cells | ACC Cells | dPL Cells | vPL Cells | IL Cells | ACC Cells | dPL Cells | vPL Cells | IL Cells |
| R506 | 7 | 109 | 15.6 | 7 | 20 | 4.6 | 41 | 68 | 0 | 0 | 5.9 | 9.7 | 0.0 | 0.0 |
| R530 | 14 | 206 | 14.7 | 3 | 34 | 10.6 | 0 | 92 | 102 | 12 | 0.0 | 6.6 | 7.3 | 0.9 |
| R535 | 13 | 611 | 47.0 | 35 | 63 | 9.9 | 0 | 563 | 48 | 0 | 0.0 | 43.3 | 3.7 | 0.0 |
| R542 | 14 | 562 | 40.1 | 29 | 50 | 5.7 | 1 | 40 | 310 | 211 | 0.1 | 2.9 | 22.1 | 15.1 |
| R536 | 14 | 145 | 10.4 | 3 | 24 | 5.8 | 12 | 44 | 46 | 43 | 0.9 | 3.1 | 3.3 | 3.1 |
| R543 | 14 | 464 | 33.1 | 19 | 47 | 8.0 | 0 | 9 | 172 | 283 | 0.0 | 0.6 | 12.3 | 20.2 |
| R537 | 14 | 530 | 37.9 | 20 | 54 | 10.8 | 14 | 2 | 157 | 357 | 1.0 | 0.1 | 11.2 | 25.5 |
| R544 | 14 | 390 | 27.9 | 18 | 41 | 7.1 | 4 | 73 | 177 | 136 | 0.3 | 5.2 | 12.6 | 9.7 |
| TOTAL | 104 | 3017 | 28.3 | 16.8 | 41.6 | 7.8 | 72 | 891 | 1012 | 1042 | 1.0 | 8.9 | 9.1 | 9.3 |
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Rattus norvegicus, 4 M, 4 F) | Fisher-Brown Norway F1 Hybrid | Bred in House | Aged 9–15 months | |
| Chemical compound, drug | Isoflurane | UMN Research Animal Resources (RAR) | ||
| Chemical compound, drug | Carprofin | UMN RAR | ||
| Chemical compound, drug | Dual-Cillin | UMN RAR | ||
| Chemical compound, drug | Baytril | UMN RAR | ||
| Chemical compound, drug | C&B Metabond | Patterson Dental | Cat #: 553–3484 | |
| Chemical compound, drug | Pentobarbitol | UMN RAR | ||
| Chemical compound, drug | Paraformaldehyde | Sigma-Aldrich | Cat #: 158127 | |
| Software, algorithm | Matlab v2017b | Mathworks | RRID: SCR_001622 | For Formal Analysis |
| Software, algorithm | Matlab v2015b | Mathworks | RRID: SCR_001622 | For Running Task |
| Software, algorithm | Kilosort v2.0 | Marius Pachitariu | https://github.com/MouseLand/Kilosort (Pachitariu et al., 2016; Stringer et al., 2019) | Downloaded Jan 2020 |
| Software, algorithm | Phy v2.0 Beta 1 | Cyrille Rossant | https://github.com/cortex-lab/phy (Rossant, 2022). | Downloaded Feb 2020 |
| Software, algorithm | Analysis Code | This Paper | https://osf.io/s5xqm/ | Code written for the study |
| Other | Physiology and Behavioral Data | This Paper | https://osf.io/s5xqm/ | Data collected for the study |
| Other | Food Pellets (45 mg, 5TUL) | TestDiet | Cat #: 1811155; 1811443; 1812298; 1811645 | Reward food pellets; Flavors: Plain; Chocolate; Fruit “Cherry”; Banana |
| Other | 64 Ch Silicon Probe | Cambridge Neurotech | ASSY-156-H3 | Recording probe; H3 Model |
| Other | 256 Ch Intan RHD Recording System | Intan | Cat #: C3100 | Recording Hardware |
| Other | 64 Ch Intan RHD Headstage | Intan | Cat #: C3325 | Recording Headstage |





