Fan cells in lateral entorhinal cortex directly influence medial entorhinal cortex through synaptic connections in layer 1
Figures
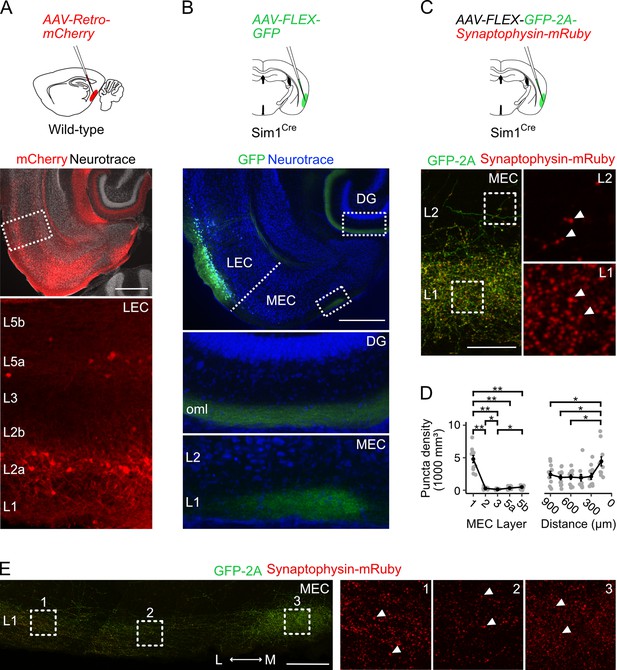
Fan cells in lateral entorhinal cortex send projections to medial entorhinal cortex that terminate in layer 1.
(A) Schematic of strategy for targeting AAV-Retro-mCherry to the medial entorhinal cortex (MEC) of wild-type mice (top) and a horizontal brain section (bottom) showing retrograde labelling of neurons with mCherry in layers (L) 2a and 5a of lateral entorhinal cortex (LEC). Scale bar represents 500 µm. (B) Schematic of strategy for targeting AAV-FLEX-GFP to fan cells in Sim1Cre mice (top) and horizontal brain section (bottom) showing labelling of fan cell axons with GFP (green) in the outer molecular layer (oml) of the dentate gyrus (DG) and L1 of the MEC. Neurons are counterstained with Neurotrace (blue). Scale bar represents 500 µm. (C) Schematic of strategy for targeting AAV-FLEX-GFP-2A-Synaptophysin-mRuby to fan cells in Sim1Cre mice (top) and horizontal brain section (bottom) showing fan cell axons (green) and their terminals (red) in L1 and L2 of the MEC. Insets show synaptic puncta, indicated by white arrows. Scale bar represents 50 µm. (D) Line plots showing the volumetric density of axon terminals across L1-5b (left) and the mediolateral axis of the MEC (right). The mediolateral (M-L) axis of MEC was binned in 150 µm steps from the parasubiculum border. Black line shows population averages, and grey circles are values for single brain slices. Error bars represent SEM. There was a significant effect of MEC layer (Χ2(4)=32.8, p=0.000001, Kendall W=0.745, Friedman test) and location (Χ2(5)=21.6, p=0.0006, Kendall W=0.394) on puncta density. There was a higher density of puncta in L1 (4790±545 mm3) compared to all other layers (L2: W=66, p=0.01; L3: W=66, p=0.01; L5a: W=66, p=0.01; L5b: W=66, p=0.01, pairwise Wilcoxon tests with Bonferroni correction) and a higher density of puncta in L2 and L5b compared to L3 (L2: W=63, p=0.049; L5b: W=1, p=0.02). Further, there was a higher puncta density in the region of MEC closest to parasubiculum as compared to the regions laterally distanced 900 µm (W=66, p=0.015), 750 µm (W=66, p=0.015), and 600 µm (W=66, p=0.015). Asterisks indicate significance (**p<0.01, ***p<0.001, ****p<0.00001). (E) Same as C, but showing fan cell axons and axon terminals across the mediolateral axis of MEC L1. Scale bar represents 100 µm.
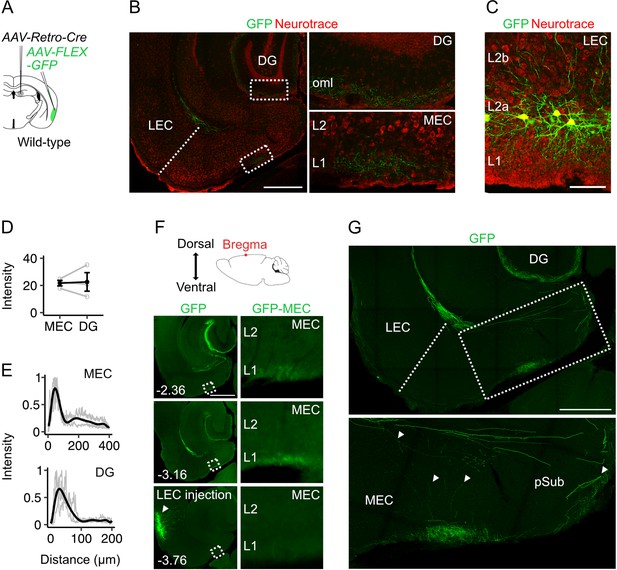
Projections to the medial entorhinal cortex from lateral entorhinal cortex arise from fan cells that also send projections to the hippocampus.
(A) Schematic of strategy for targeting GFP specifically to fan cells in the lateral entorhinal cortex (LEC) that project to the hippocampal dentate gyrus (DG). In a wild-type mouse, adeno-associated virus (AAV) encoding retrograde Cre (AAV-Retro-Cre, grey) was injected into the DG and Cre-dependent AAV encoding GFP (AAV-FLEX-GFP, green) was injected into the LEC. (B) Horizontal brain section from a wild-type mouse showing GFP labelling of fan cell axons (green) in the outer molecular layer (oml) of the DG and layer (L) 1 of the medial entorhinal cortex (MEC). Neurons are counterstained with Neurotrace (red). Scale bar represents 500 µm. (C) Horizontal brain section from the same mouse shown in B, showing retrograde labelling of fan cell bodies with GFP (green) in L2a of the LEC. Scale bar represents 100 µm. (D) Fluorescence intensity of fan cell axons in L1 of MEC and the oml of DG. Intensity was quantified as the mean gray value of pixels in regions of interest (ROIs), where the possible range of values was 1–255 and a value of 255 corresponds to white. Mean intensity values were quantified for ROIs in MEC and DG, and were adjusted to baseline by subtracting the intensity value of an ROI from the same slice that did not contain fan cell axons. Fluorescence intensities were similar for DG and MEC (W=4, p=1, Mann Whitney U test). Black line shows the population average and grey circles are values for single brain slices. Error bars are SEM. (E) Fluorescence intensity as a function of distance across layers of MEC (top) and DG (bottom). For MEC, intensity was sampled from the edge of the slice (L1) towards the hippocampus. For DG, intensity was sampled from the outer edge of the oml towards the hilus. Plots are normalised to the minimum and maximum intensity values. Black line is a polynomial fit to the population data, and gray lines are values for single brain slices. (F) The dorsal-ventral position of the horizontal sections was estimated relative to bregma (top). Epifluorescent images of horizontal brain sections show GFP expression at dorsoventral locations containing the MEC and extending to the injection site in LEC. Zoomed in images of the ROIs highlighted by boxes in the left panels are shown in the panels to the right. Numbers indicate depth of slice from bregma in mm. Scale bar represents 500 µm. (G) Confocal images of a horizontal brain section showing fan cell axons expressing GFP in the MEC and parasubiculum (pSub)(upper). The zoomed in image (lower) shows the region highlighted by the rectangle, which contains axon collaterals in the deep MEC and the pSub. Putative branching points are indicated by white arrows. Scale bar represents 500 µm.
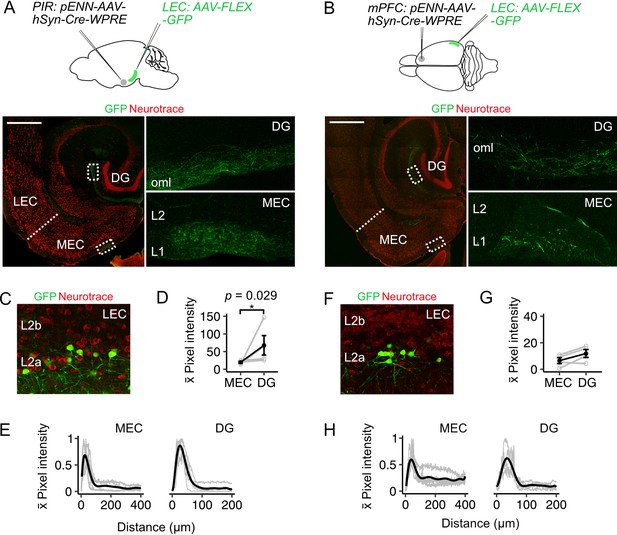
Fan cells that project to medial entorhinal cortex receive inputs from piriform and prefrontal cortices (A-B) (Top).
Schematic of strategy for targeting GFP specifically to fan cells in the lateral entorhinal cortex (LEC) that receive projections from piriform cortex (PIR, A) or medial prefrontal cortex (mPFC, B). AAV that anterogradely transports Cre (pENN-AAV-hSyn-Cre-WPRE, grey) was injected into the PIR or mPFC of a wild-type mouse. Cre-dependent AAV encoding GFP (AAV-FLEX-GFP, green) was injected into the LEC of the same mouse to label Cre-expressing fan cells and their axons. Horizontal brain section showing labelling of fan cell axons with GFP in the outer molecular layer (oml) of the dentate gyrus (DG) and layer (L) 1 of the MEC (right panels show zoomed in images of the regions of interest highlighted in the left panels). Scale bar is 500 µm. (C) Expression of GFP in fan cells at the injection site in the same animal as A. Neurons are counterstained with Neurotrace (red). (D) Fluorescence intensity in L1 of MEC and the oml of DG of axons from fan cells receiving inputs from PIR. The fluorescence intensity of fan cell axons was higher in the DG (W=16, p=0.029, Mann Whitney U test). Black line shows population average and grey circles are values for single brain slices. Error bars are SEM. (E) Fluorescence intensity of axons from fan cells that receive PIR inputs as a function of distance across layers of MEC (left) and DG (right). For MEC, intensity was sampled from the edge of the slice (L1) towards the hippocampus. For DG, intensity was sampled from the outer edge of the oml towards the hilus. ROIs were oriented perpendicular to the layers of MEC and DG. Plots are normalised to the minimum and maximum intensity values. Black line is population data fit with a polynomial model and gray lines are values for single brain slices. (F) Same as C but for fan cells labelled on the basis of receiving projections from medial prefrontal cortex (mPFC). (G-H) Same as (D-E), but for axons from fan cells that receive inputs for mPFC. Fluorescence intensities of axons from fan cells that receive inputs from mPFC was similar for DG and MEC (W=12, p=0.343).
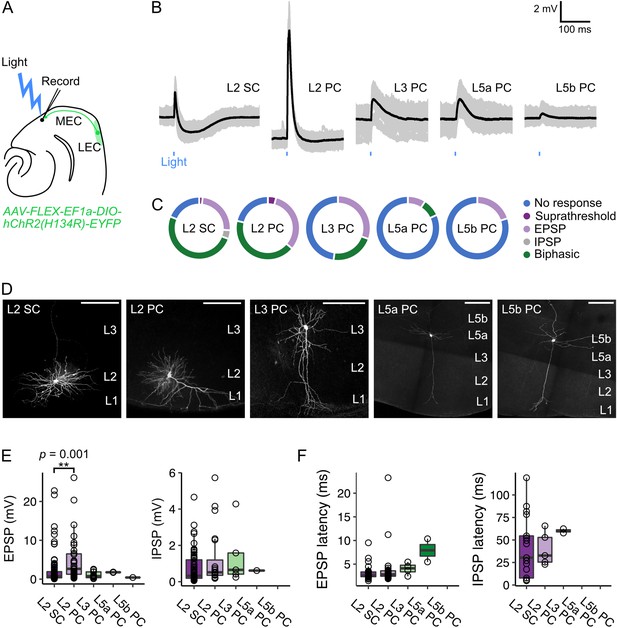
Optogenetic activation of fan cell inputs evokes postsynaptic responses in medial entorhinal cortex principal neurons.
(A) Schematic of recording experiment to evaluate the postsynaptic responses in medial entorhinal cortex (MEC) to stimulation of fan cell axons. AAV-EF1a-DIO-ChR2(H134R)-EYFP was injected into the LEC of Sim1Cre mice to enable optical activation of fan cells. Synaptic output from fan cells was evaluated by recording light-evoked responses of principal neurons in different layers of MEC. (B) Representative examples of membrane potential responses evoked by optical activation of fan cell axons in principal neurons in layers (L) 2, 3, 5a, and 5b of the MEC (SC = stellate cell, PC = pyramidal cell). Blue bar indicates the 3 ms period of stimulation. Individual traces (grey) are overlaid with an average of all traces (black). (C) Charts showing the proportion of each type of neuron that demonstrated different types of membrane potential responses to activation of fan cell inputs, including no response (blue), suprathreshold (dark purple), subthreshold excitatory (EPSP, light purple), inhibitory (IPSP, grey), and biphasic (green). (D) Representative examples of neurons that were recorded from each layer of MEC and filled with Biocytin (white) in horizontal brain sections. Scale bars represent 200 µm. (E) Boxplots showing amplitudes for excitatory (EPSP) and inhibitory (IPSP) membrane potential responses to activation of fan cell inputs in principal neurons in L2, L3, L5a, and L5b of the MEC. Circles represent values for individual neurons. Comparison of response amplitudes across cell-types using a Kruskall-Wallis test revealed a significant effect of cell type on EPSP amplitude (H(4) = 17.261, p=0.002, η²=0.097), but not IPSP amplitude (H(4) = 3.624, p=0.305). Post-hoc pairwise comparisons revealed that EPSPs were larger in L2 PCs compared to L2 SCs (Z=3.89, p=0.001; Figure 4—source data 2). (F) Boxplots showing response latencies of principal neurons in L2, L3, L5a, and L5b with an average membrane response amplitude of > 1 mV. Latency was measured as the time from stimulus onset to 10% deviation from baseline membrane potential. There was no effect of cell type on the latency of EPSPs or IPSPs from stimulus (EPSP: H(3) = 7.550, p=0.056, IPSP: H(2) = 1.663, p=0.435).
-
Figure 4—source data 1
Summary of subthreshold and suprathreshold membrane properties of recorded neurons.
- https://cdn.elifesciences.org/articles/83008/elife-83008-fig4-data1-v2.docx
-
Figure 4—source data 2
Summary of pairwise comparisons of membrane potential responses.
- https://cdn.elifesciences.org/articles/83008/elife-83008-fig4-data2-v2.docx
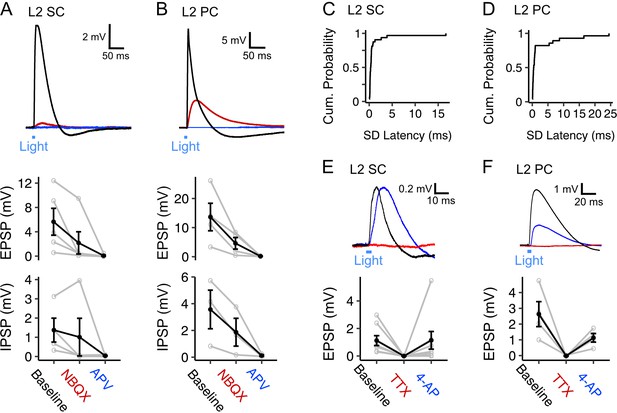
Fan cell inputs to stellate and pyramidal cells in layer 2 of the medial entorhinal cortex are glutamatergic and monosynaptic (A, B) (Top) Membrane potential response of a layer 2 stellate cell (L2 SC, A) and a layer 2 pyramidal cell (L2 PC, B) after optogenetic activation of fan cell inputs.
The membrane potential response was abolished by application of ionotropic glutamate receptor antagonists NBQX (10 µM, red) and APV (50 µM, blue). Blue line indicates the 3 ms period of optical stimulation. (Bottom) Quantification of the light-evoked membrane potential response in stellate (n=5) and pyramidal cells (n=4) after application of NBQX and APV. Data is shown for excitatory (EPSP) and inhibitory (IPSP) components. Black lines indicate population average and grey lines indicate individual neurons. Error bars are SEM. (C-D) Cumulative probability of the standard deviation of EPSP latencies for layer 2 stellate (C, x̄=1.04 ± 0.312, IQR = 0.311) and pyramidal cells (D, x̄=2.55 ± 0.918, IQR = 0.693). (E-F) (Top) Similar to (A-B), but showing effects on a layer 2 stellate (E) and pyramidal cell (F) of application of TTX (500 nM, red) and its recovery with application of 4-AP (200 µM, blue). (Bottom) Quantification of light-evoked EPSPs in stellate (n=8) and pyramidal cells (n=4) after application of TTX and 4-AP.
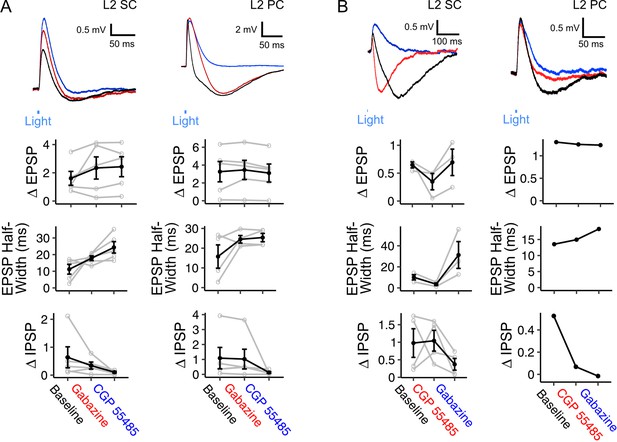
Inhibition of principal neurons in medial entorhinal cortex by inputs from fan cells is mediated by GABAA and GABAB receptors.
(A) (Top) Membrane potential response of a layer 2 stellate cell (L2 SC, left) and pyramidal cell (L2 PC, right) after optogenetic activation of inputs from fan cells. Different inhibitory components of the membrane potential response were abolished by application of GABAA receptor antagonist Gabazine (10 µM, red) and GABAB receptor antagonist CGP55845 (100 µM, blue). Blue line indicates the 3 ms period of optical stimulation. (Bottom) Quantification of the light-evoked membrane potential response in layer 2 stellate (n=5) and pyramidal cells (n=5) after application of Gabazine and CGP55845. Amplitude measurements are shown for excitatory (EPSP) and inhibitory (IPSP) postsynaptic potentials and half-width measurements are also shown for EPSPs. At a population level, GABA receptor antagonists causes a significant change in the halfwidth of EPSPs for L2 SCs (Χ2(2)=8.4, p=0.015, Friedman test), but not L2 PCs (Χ2(2)=1.2, p=0.472), and did not have significant effects on E/IPSP amplitudes for either cell type (L2 SC: EPSP, Χ2(2)=2.8, p=0.247; IPSP: Χ2(2)=5.2, p=0.074; L2 PC: EPSP, Χ2(2)=4.8, p=0.091; IPSP: Χ2(2)=5.2, p=0.074). Analyses at the level of individual cells revealed effects of antagonist application on measurements of E/IPSP amplitude and EPSP halfwidth for almost all neurons (Figure 6—source data 1, Figure 6—source data 2, Figure 6—source data 3). Black lines indicate population average and grey lines indicate an individual neuron. Error bars are SEM. (B) Same as A, but showing application of GABAB receptor antagonist CGP55845 (red) followed by application of GABAA receptor antagonist Gabazine (blue) for layer 2 stellate (n=4) and pyramidal cells (n=1). At a population level GABA-receptor antagonists caused significant changes in the halfwidth of EPSPs (Χ2(2)=6.0, p=0.0498), but did not their amplitude(Χ2(2)=4.67, p=0.097) or IPSPs (Χ2(2)=2.67, p=0.264). As for A, analysis for individual neurons revealed a significant effect of GABA-receptor antagonists on measurements of E/IPSP amplitude and EPSP half-width for almost all neurons (Figure 6—source data 3).
-
Figure 6—source data 1
Summary of cell-level analysis of effects of GABA receptor antagonists on synaptic response properties.
- https://cdn.elifesciences.org/articles/83008/elife-83008-fig6-data1-v2.docx
-
Figure 6—source data 2
Summary of cell-level pair-wise comparisons of effects of GABA receptor antagonists on synaptic response properties.
- https://cdn.elifesciences.org/articles/83008/elife-83008-fig6-data2-v2.docx
-
Figure 6—source data 3
Summary of cell-level pair-wise comparisons of effects of GABA receptor antagonists on synaptic response properties.
- https://cdn.elifesciences.org/articles/83008/elife-83008-fig6-data3-v2.docx
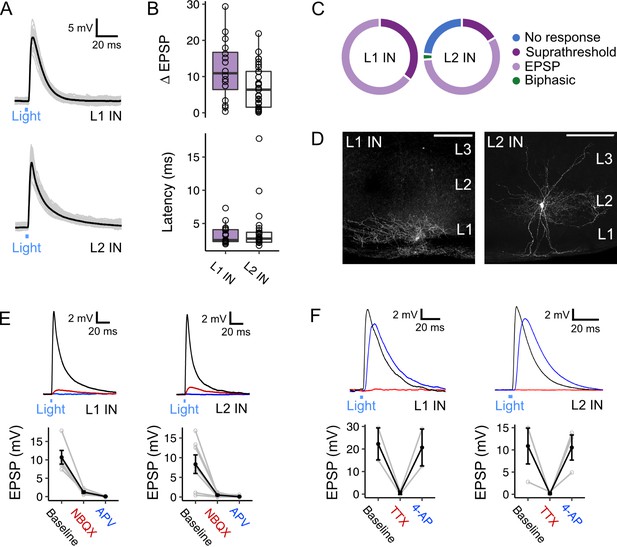
Activation of fan cell inputs to medial entorhinal cortex evokes monosynaptic glutamatergic postsynaptic responses in layer 1 and layer 2 interneurons.
(A) Representative examples of membrane potential responses evoked by optogenetic activation of fan cell inputs for interneurons in layer 1 (L1 IN, top) and layer 2 (L2 IN, bottom) of medial entorhinal cortex (MEC). Blue bar indicates the 3 ms period of optical stimulation. Individual traces (grey) are overlaid by an average of all traces (black). (B) Boxplots showing the amplitude (top) and latency (bottom) of excitatory responses (EPSP) for interneurons in L1 (purple) and L2 (white). Circles represent single neurons. There was no difference in the amplitude (W=314, p=0.053, Mann Whitney U test) or latency (W=185, p=0.898) across cell-types. (C) Charts showing the proportion interneurons in L1 (left) and L2 (right) that demonstrated different types of responses to activation of fan cell inputs, including no response (blue), suprathreshold (dark purple), subthreshold excitatory (light purple) and biphasic (green). Low-threshold spiking (LTS) and fast-spiking (FS) interneurons both responded to stimulation of fan cell inputs (LTS: 7/12 cells, 2 suprathreshold; FS: 25/30 cells, 5 suprathreshold). (D) Representative examples of interneurons in L1 (left) and L2 (right) that were filled with biocytin (white) in horizontal brain sections. Scale bars represent 200 µm. (E) (Top) Membrane potential response of interneurons in L1 (left) and L2 (right) after optogenetic activation of fan cell inputs. The membrane response was abolished by application of ionotropic glutamate receptor antagonists NBQX (10 µM, red) and APV (50 µM, blue). Blue line indicates the 3 ms period of optical stimulation. (Bottom) Quantification of light-evoked EPSPs in L1 (n=7) and L2 interneurons (n=6) after application of NBQX and APV. Black lines indicate population average and grey lines indicate individual neurons. Error bars are SEM. (F) Same as E, but showing abolishment of EPSPs evoked in an L1 (left, n=2) and L2 (right, n=3) interneuron by application of TTX (500 nM, red) and their recovery with 4-AP (200 µM, blue).
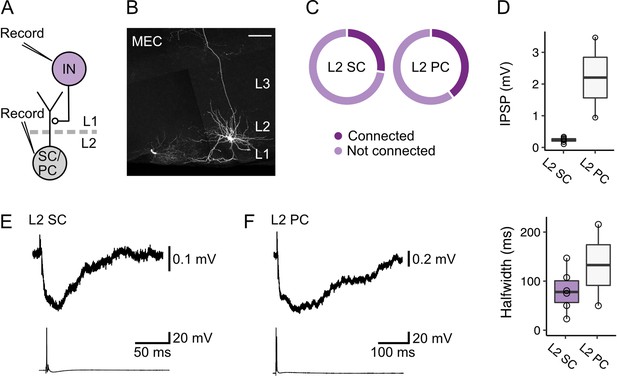
Layer 1 interneurons in the medial entorhinal cortex directly inhibit stellate and pyramidal cells in layer 2.
(A) Schematic of recording configuration to evaluate postsynaptic responses in medial entorhinal cortex (MEC) layer 2 (L2) stellate (SC) and pyramidal cells (PC) to stimulation of interneurons (IN) in layer 1 (L1). Connectivity between L1 interneurons and principal cells was established with simultaneous measurement of membrane potentials in L2 stellate or pyramidal cells and activation of an interneuron in L1. (B) Representative image of a slice containing a simultaneously recorded interneuron in L1 (lower left) and an L2 stellate cell (right) in the MEC. Both recorded neurons were filled with biocytin (white). Scale bar represents 100 µm. (C) Charts show proportions of stellate (left) and pyramidal cells (right) that were hyperpolarized by (connected, dark purple) or did not demonstrate a membrane potential response (not connected, light purple) to activation of inputs from an interneuron in L1 of the MEC. (D) Quantification of the amplitude (top) and half-width (bottom) of inhibitory membrane potential response (IPSP) in layer 2 stellate (purple) and pyramidal cells (white) to activation of inputs from an interneuron in L1. Circles represent single neurons. (E-F) Representative traces of membrane potentials during the stimulation experiments for stellate (E) and pyramidal cells (F). Traces from the stellate or pyramidal cell are at the top, and action potentials fired by the interneuron in L1 with injection of current are shown on the bottom.
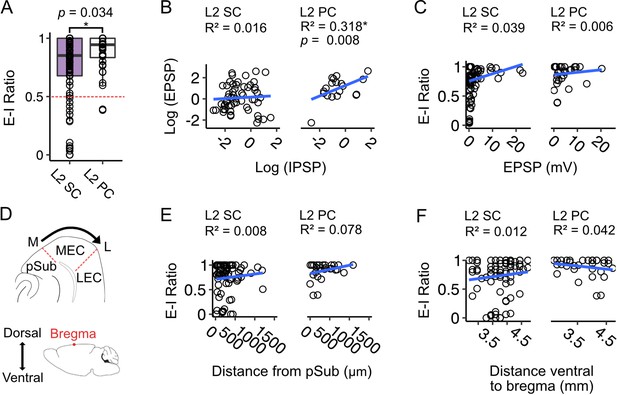
Excitation-inhibition bias of responses to fan cell inputs differs between stellate and pyramidal cells, is highly variable and is independent of location.
(A) Boxplot comparing ratios of excitation to inhibition (E-I ratio) = EPSP amplitude/(EPSP amplitude +IPSP amplitude) for responses of layer 2 stellate (L2 SC, purple) and pyramidal cells (L2 PC, white) to activation of fan cell inputs.
Dashed red line indicates equal amplitudes of excitation and inhibition. The bias to excitation was larger in pyramidal cells (W=1233.5, p=0.034, Mann Whitney U test). Each dot represents a single neuron. (B) EPSP amplitude as a function of IPSP amplitude for stellate (right) and pyramidal cells (left). Raw values were log transformed (see Figure 9—figure supplement 1B for raw data). EPSP amplitude increased with IPSP amplitude for pyramidal cells (F(1,19) = 8.877, p=0.008, F-test of overall significance), but not stellate cells (F(1,57) = 0.894, p=0.348). Blue line is fit of a linear regression model with the equation y = β0 + β1x + ϵ. (C) Same as B, but with E-I ratio plotted as a function of EPSP amplitude. We did not find a relationship between E-I ratio and EPSP amplitude for either cell type (L2 SC: F(1,84) = 3.365, p=0.070; L2 PC: F(1,34) = 0.193, p=0.665). (D) Schematics illustrating estimation of the mediolateral (M-L, top) and dorsoventral (bottom) positions of neurons within the medial entorhinal cortex (MEC). On the mediolateral axis, neuron position was measured as distance of the cell body from the MEC border with parasubiculum (pSub), where larger values indicate proximity to the lateral border of MEC. On the dorsoventral axis, neuron position was measured as distance of the cell body from bregma (indicated by a red dot). (E-F) E-I ratio plotted as a function of mediolateral (E) or dorsoventral (F) position for stellate (left) and pyramidal cells (right). We did not find a relationship between E-I ratio and position for either cell type (Mediolateral position, L2 SC: F(1,72) = 0.564, p=0.455, L2 PC: F(1, 26)=2.203, p=0.150; Dorsoventral position, L2 SC: F(1,77) = 0.901, p=0.346, L2 PC: F(1, 31)=1.352, p=0.254).
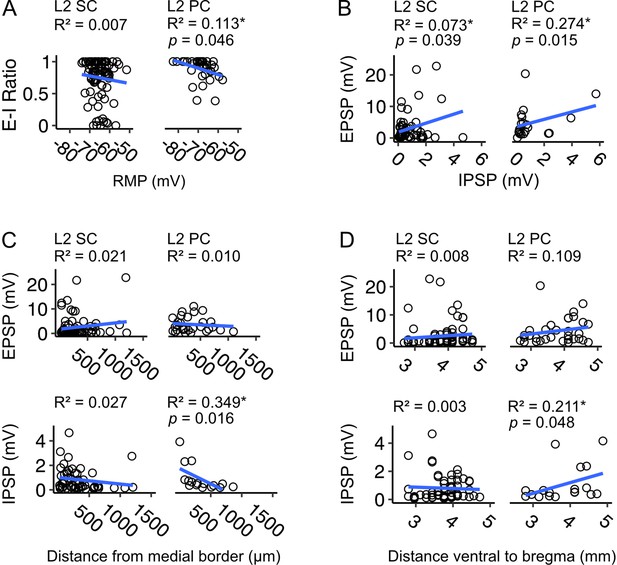
Relationships between excitatory and inhibitory components and the resting membrane potential or position of neuron in medial entorhinal cortex.
(A) Ratios of excitation to inhibition (E-I) of postsynaptic membrane potential responses plotted against resting membrane potential (RMP) for layer 2 stellate cells (L2 SCs, right) and pyramidal cells (L2 PCs, left) in medial entorhinal cortex (MEC). Each circle represents the value for a single neuron. We did not find a relationship between E-I ratio and RMP for stellate cells (F(1,88) = 0.581, p=0.448, F-test of overall significance), but in pyramidal cells the E-I ratio was higher in hyperpolarised neurons (F(1,34) = 4.31, p=0.046). Blue line is the fit of a linear model with the equation y = β0 + β1x + ϵ. (B) Same as A, but amplitudes of excitatory postsynaptic potentials (EPSP) are plotted against amplitudes of inhibitory postsynaptic potentials (IPSP). These are raw values that have not undergone log transformation (Figure 9B). There was a relationship between EPSP and IPSP amplitude in stellate (F(1,57) = 4.46, p=0.039) and pyramidal cells (F(1,19) = 7.16, p=0.015). (C-D) Same as A, but E-I ratios are plotted against neuron position on the mediolateral (C) and dorsoventral (D) axes of the MEC. There was no relationship between the neuron distance from the border with parasubiculum (pSub) and E/IPSP amplitude for stellate cells (EPSP: F(1,69) = 1.509, p=0.224, IPSP: F(1, 50)=1.375, p=0.247), but for pyramidal cells IPSP amplitude decreased with neuron distance from pSub (F(1,14) = 7.513, p=0.016), with no corresponding change in EPSP amplitude (F(1, 26)=0.254, p=0.619). Similarly, we did not find a relationship between dorsoventral (DV) position and E/IPSP amplitude for stellate cells (EPSP: F(1, 73)=0.581, p=0.448, IPSP: F(1, 54)=0.133, p=0.717), but for pyramidal cells IPSP amplitude increased with distance from bregma (F(1,17) = 4.534, p=0.048), with no corresponding change in EPSP amplitude (F(1,31) = 3.809, p=0.060).
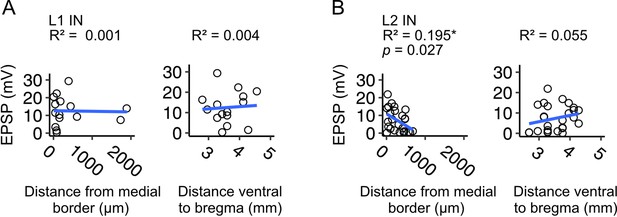
Relationships between response amplitudes in layer 1 and layer 2 interneurons and neuron position in medial entorhinal cortex.
(A-B) Excitatory postsynaptic potential (EPSP) amplitudes plotted against mediolateral (left) and dorsoventral (left) cell body position for interneurons in layer 1 (L1 IN) (A) and layer 2 (L2 IN) (B). Each circle represents a single neuron. We did not find a relationship between EPSP amplitude and distance of the cell body from the MEC border with parasubiculum (pSub) for interneurons in layer 1 (F(1,13) = 0.008, p=0.929, F-test for overall significance), but EPSP amplitude decreased with lateral distance for layer 2 interneurons (F(1,23) = 5.566, p=0.027, F-statistics for overall significance). There was no relationship between EPSP amplitude and dorsoventral position for either cell type (L1 IN: F(1,13) = 0.057, p=0.815, L2 IN: F(1, 24)=0.055, p=0.247). Blue line is fit of a linear model with the equation y = β0 + β1x + ϵ.
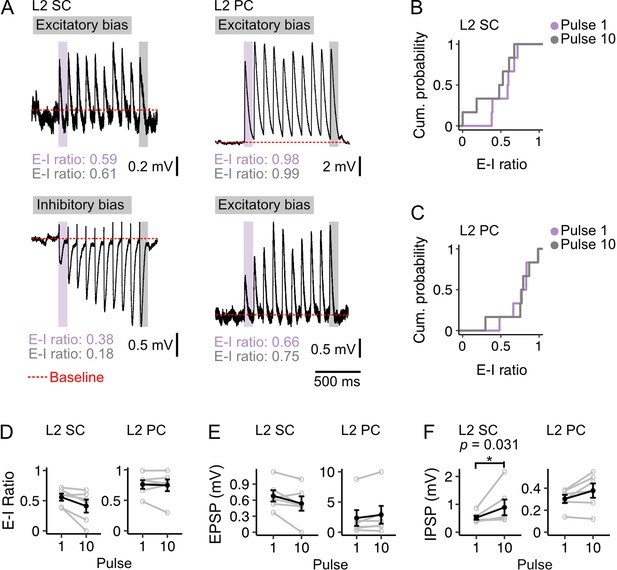
Diversity of excitation-inhibition bias is maintained during theta frequency stimulation.
(A) Example membrane potential responses of layer 2 stellate (L2 SC, left) and pyramidal cells (L2 PC, right) to 10 Hz optical stimulation of fan cell inputs. Examples illustrate relatively high (upper) and low (lower) E-I values for each cell type. Traces are averages of multiple responses (10–30 repetitions). Red dotted line indicates baseline membrane potential (average of the 990 ms window prior to stimulation, with the 10 ms immediately preceding stimulation excluded). (B-C) Cumulative probability of the ratios of excitation to inhibition (E-I ratio) of membrane potential responses for pulse 1 (purple) and pulse 10 (grey) for stellate (B; Pulse 1: x̄=0.558, IQR = 0.216, Pulse 10: x̄=0.411, IQR = 0.331) and pyramidal cells (C; Pulse 1: x̄=0.759, IQR = 0.147, Pulse 10: x̄=0.746, IQR = 0.145). There was more variance in the distribution of E-I ratios for stellate cells at pulse 10 (D=0.833, p=0.026, Kolmogorov Smirnov test), but the distributions were not different between cell-types at pulse 1 (D=0.667, p=0.143). (D-F) Average E-I ratios (D), and amplitudes of excitatory (E, EPSP) and inhibitory components (F, IPSP) of responses evoked in stellate (left) and pyramidal (right) cells by the first (1) and last (10) pulse. IPSP amplitudes were larger at pulse 10 for stellate cells (V=0, p=0.031, paired samples Wilcoxon test). There were no other differences between pulse 1 and 10 for stellate (EPSP: V=19, p=0.094; E-I ratio: V=20, p=0.063) or pyramidal cells (EPSP: V=4, p=0.219; IPSP: V=3, p=0.156; E-I ratio: V=10, p=1.00). Black line is the population average and grey lines indicate single neurons. Values for single neurons are calculated as the average of pulses 1 or 10 across all stimulations. Error bars are SEM.
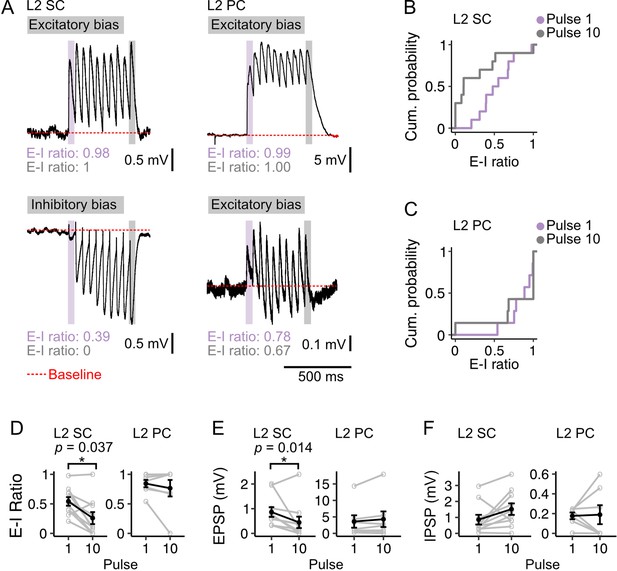
Optogenetic stimulation of fan cell axons at a frequency of 20 Hz.
(A) Examples of membrane potential responses in layer 2 stellate cells (L2 SC, left) and pyramidal cells (L2 PC, right) to 20 Hz optical stimulation of fan cell axons. Examples illustrate relatively high (upper) and low (lower) E-I values for each cell type. Black trace is the average of responses (10–30 repetitions). Red dotted line indicates baseline membrane potential (average of the 990 ms window prior to stimulation, with the 10 ms immediately preceding stimulation excluded). (B-C) Plots showing cumulative probability of the ratios of excitation to inhibition (E-I) of membrane potential responses to pulse 1 (purple) and pulse 10 (grey) for stellate (B; Pulse 1: x̄=0.541, IQR = 0.287, Pulse 10: x̄=0.259, IQR = 0.415) and pyramidal cells (C); Pulse 1: x̄=0.843, IQR = 0.204, Pulse 10: x̄=0.765, IQR = 0.323. Membrane responses of L2 stellate cells were more heterogeneous than pyramidal cells at pulse 1 (D=0.757, p=0.010, Kolgomorov-Smirnov test) and pulse 10 (D=0.757, p=0.018). (D-F) Line plots showing average E-I ratios (D), and amplitudes of excitatory (E, EPSP) and inhibitory components (F, IPSP) of the membrane potential responses evoked in stellate (left) and pyramidal (right) cells by the first (1) and last (10) pulse. For stellate cells, EPSP amplitude And E-I ratio decreased between the first and last pulse (EPSP: V=48, p=0.037; E-I: V=51, p=0.014; paired samples Wilcoxon test), with no corresponding change in IPSP amplitude (V=9, p=0.065). There was no change across pulses for any measure in pyramidal cells (EPSP: V=9, p=0.469; IPSP: V=14, p=1.00; E-I ratio: V=16, p=0.813). Black line is the population average and grey lines indicate single neurons. Values for single neurons are calculated as the average amplitude of pulses 1 or 10 across all stimulations. Error bars are SEM.
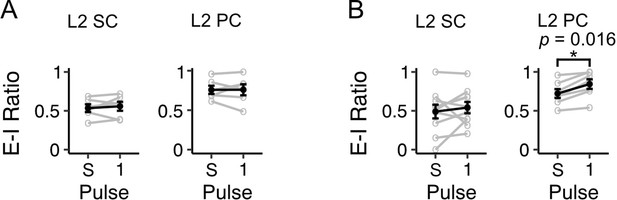
Ratios of excitation to inhibition are similar for potentials evoked by a single stimulus and the first pulse in a train of stimuli.
(A) To account for the possibility that our data reflect errors introduced by the technical challenge of measuring separate excitatory and inhibitory components for single pulses evoked by stimuli trains, we compared E-I ratios calculated for single pulses of optical stimulation (Figure 4) to the E-I ratios calculated for the membrane response to the first stimulus of a 10 Hz train for neurons in our dataset. Line plot shows the average ratio of excitation to inhibition (E-I) for stellate (L2 SC, left) and pyramidal (L2 PC, right) cells as calculated for a single 3ms optical stimulus (S) and for the first pulse of a 10 Hz stimulus train (1). The ratio was calculated using the following formula: E-I ratio = peak EPSP/ peak EPSP + peak IPSP. The resultant value indicates the proportion of the postsynaptic response that is excitatory. There was no difference in the E-I ratio calculated for the single pulse versus the first pulse for stellate (V=9, p=0.844, paired samples Wilcoxon test) or pyramidal cells (V=11, p=1.00). Black line is the average value across all neurons, and grey lines indicate values for single neurons. Values for single neurons are calculated as the average amplitude of pulses 1 and 10 across all trains of stimuli. Error bars are SEM. (B) Same as A, but for stimulations at a frequency of 20 Hz. There was no difference in E-I ratio as calculated from a single pulse versus from the first pulse of a 20 Hz stimulation train for stellate cells (V=17, p=0.322) but E-I ratio was found to be higher as calculated for the first pulse in a stimulus train for pyramidal cells (V=0, p=0.016). This may reflect the dominant excitation in this cell type and challenge of detecting inhibitory components with optical stimulation at a high frequency in neurons with low inhibition at the level of a single pulse (Figure 9A).
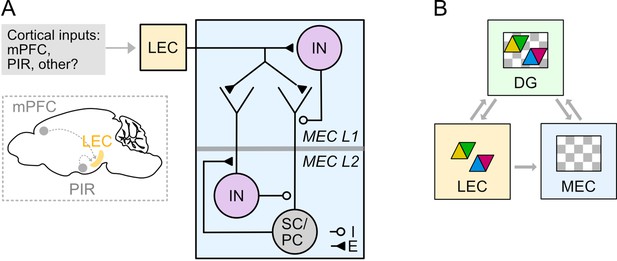
Circuitry for higher order and sensory signals to reach medial entorhinal cortex through connectivity with lateral entorhinal cortex.
(A) Circuit organisation through which neocortical neurons may influence stellate cells (SC), pyramidal cells (PC) and inhibitory interneurons (IN) in the MEC via their projections to LEC. (B) A model for entorhinal-hippocampal integration. Grey arrows indicate flow of information about features (coloured triangles) and spatio-temporal context (grey and white chequered triangles) between fan cells in the LEC (yellow) and the hippocampal dentate gyrus (DG, green) and the MEC (blue). Classic models suggest the hippocampus is the main point of convergence of these two types of information. Our data further suggest that feature information could be integrated with spatio-temporal contextual information in the MEC prior to reaching the hippocampus.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (mouse) | Sim1Cre | Gen-Sat, MMRC | RRID: MMRRC_034614-UCD | NA |
| Chemical compound, drug | Biocytin | Sigma-Aldrich | B4261 | Biotin-lysine compound used for neuron reconstruction |
| Chemical compound, drug | Gabazine | Hello Bio | HB0901 | GABAA receptor antagonist, diluted to 10 µM |
| Chemical compound, drug | CGP55845 | Hello Bio | HB0960 | GABAB receptor antagonist,diluted to 100 µM |
| Chemical compound, drug | D-AP5 | Hello Bio | HB0225 | NMDA receptor antagonist, diluted to 50 µM |
| Chemical compound, drug | NBQX disodium salt | Hello Bio | HB0442 | AMPA receptor antagonist, diluted to 10 µM |
| Chemical compound, drug | Tetrodotoxin | Hello Bio | HB1034 | Na +channel-blocker, diluted to 500 nM |
| Chemical compound, drug | 4-Aminopyridine (AP) | Hello Bio | HB1073 | Kv channel-blocker, diluted to 200 µM |
| Software, algorithm | R | NA | Version: 3.6.0 | https://www.r-project.org/ |
| Software, algorithm | Matlab | Mathworks | Version: 2013 a | https://www.mathworks.com |
| Software, algorithm | Igor Pro | Wavemetrics | Version: 6.3 | https://www.wavemetrics.com/ |
| Software, algorithm | AxoGraph | AxoGraph | Version 1.7.6 | https://www.axograph.com |
| Software, algorithm | ImageJ | Fiji | NA | https://fiji.sc |
| Other (Stains) | AlexaFluor Streptavidin 647 | Invitrogen | Cat #: S21374 | 1:500 dilution, biotin-binding |
| Other (Stains) | Neurotrace 435/55 (Nissl stain) | Invitrogen | Cat#: N21479 | 1: 500 |
| Other (Stains) | Neurotrace 640/660 (Nissl stain) | Invitrogen | Cat#: N21479 | 1:500 |
| Other (AAV) | AAV1/2-Retro-Ef1a-Cre-WPRE | Addgene | Plasmid #: 51502 | titer: 2.4x1011 |
| Other (AAV) | AAV1/2-FLEX-GFP | Addgene | Plasmid #: 28304 | titer: 2.44x1013 |
| Other (AAV) | AAV1/2-FLEX-GFP-2A-Syn-mRuby | Addgene | Plasmid #: 71760 | titer: 2.96x1012 |
| Other (AAV) | AAV2-FLEX-EF1a-DIO-hChR2(H134R)-EYFP | UNC Vector Core; Zhang et al., 2006 | titer: 4x1012 | |
| Other (AAV) | AAV2-Retro-Syn-mCherry | Addgene | Plasmid #: 114472 | titer: 1.9x1013 |
| Other (AAV) | pENN-AAV1-hSyn-Cre-WPRE | Addgene | Plasmid #: 105553 | titer: 1.8x1013 |





