Efficacy and safety of endocrine therapy after mastectomy in patients with hormone receptor positive breast ductal carcinoma in situ: Retrospective cohort study
Figures
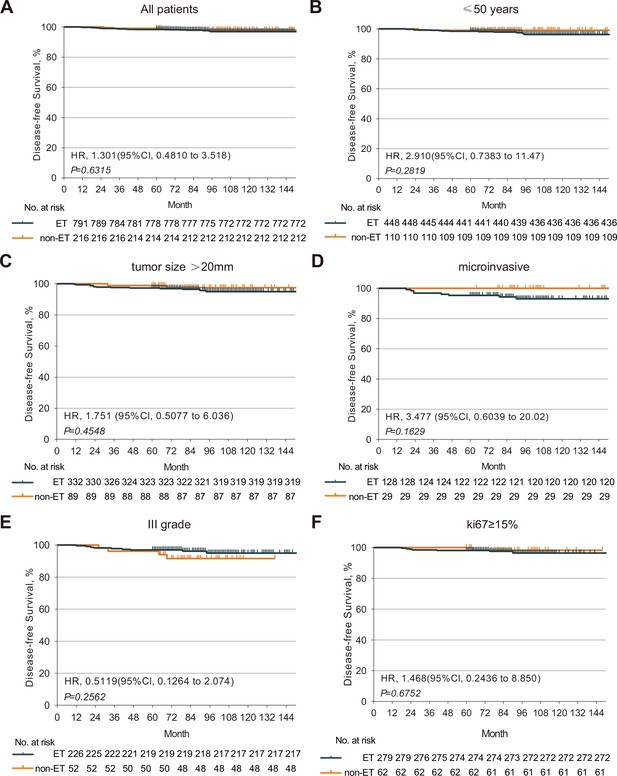
The DFS of HR+ DCIS patients with or without post-mastectomy ET.
Kaplan-Meier analysis indicated that there was no significant difference in the DFS of HR+ DCIS patients between those with and without post-mastectomy ET. (A) There was no significant difference in the DFS of HR+ DCIS patients with age <50, (B) a larger tumor, (C) positive microinvasive, (D) higher tumor grade, (E) higher Ki67 level, (F) between those with and without post-mastectomy ET. HR, hormone receptor; DCIS, ductal carcinoma in situ; ET, endocrine therapy; DFS, disease-free survival. Source files available in Figure 2—source data 1.
-
Figure 2—source data 1
The disease-free survival of hormone receptor positive (HR+) ductal carcinoma in situ (DCIS) patients between endocrine therapy (ET) and non-ET groups.
- https://cdn.elifesciences.org/articles/83045/elife-83045-fig2-data1-v1.xlsx
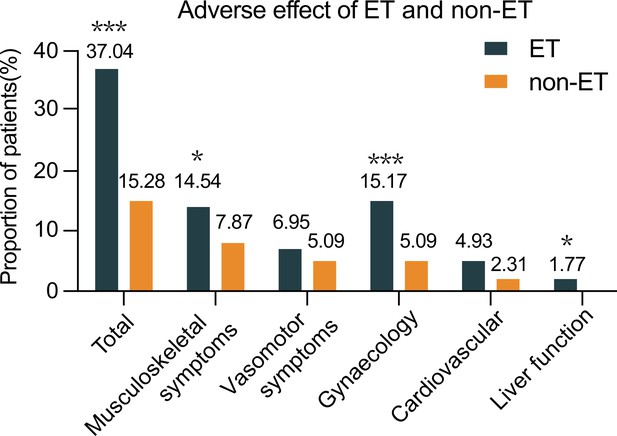
The frequency of patients with adverse effects between the ET and non-ET groups.
Data are expressed as % of cases with adverse events and real case numbers labeled and analyzed by Chi-squared test. *p<0.05, ***p<0.001. ET, endocrine therapy. Source files available in Figure 3—source data 1.
-
Figure 3—source data 1
Adverse events between the endocrine therapy (ET) and non-ET groups.
- https://cdn.elifesciences.org/articles/83045/elife-83045-fig3-data1-v1.xlsx
Tables
The demographic and clinical characteristics of patients.
| ET (N=791) | Non-ET (N=216) | p-Value | |
|---|---|---|---|
| Age (n, %) | 0.134 | ||
| ≤50 | 448 (57%) | 110 (51%) | |
| >50 | 343 (43%) | 106 (49%) | |
| Tumor size (n, %) | 0.839 | ||
| ≤20 mm | 459 (58%) | 127 (59%) | |
| >20 mm | 332 (42%) | 89 (41%) | |
| Microinvasive (n, %) | 0.322 | ||
| Yes | 128 (16%) | 29 (13%) | |
| No | 663 (84%) | 187 (87%) | |
| Tumor grade (n, %) | 0.190 | ||
| I-II | 565 (71%) | 164 (76%) | |
| III | 226 (29%) | 52 (24%) | |
| Ki67 (n, %) | 0.071 | ||
| ≥15% | 279 (35%) | 62 (29%) | |
| <15% | 512 (65%) | 154 (71%) | |
| Multifocal (n, %) | 0.310 | ||
| Yes | 64 (8%) | 13 (6%) | |
| No | 727 (92%) | 203 (94%) |
-
Notes: Data are n (%). ET, endocrine therapy. Source files available in Table 1—source data 1.
-
Table 1—source data 1
The demographic and clinical characteristics of patients.
- https://cdn.elifesciences.org/articles/83045/elife-83045-table1-data1-v1.xlsx
Tumor recurrence rates in patients with HR+ DCIS after mastectomy.
| Tumor recurrence | ET (N=19) | Non-ET (N=4) |
|---|---|---|
| Invasive local recurrence | 4 (21%) | 0 (0%) |
| Contralateral breast cancer | 3 (16%) | 0 (0%) |
| Distant metastasis | ||
| Bone | 6 (32%) | 1 (25%) |
| Liver | 2 (11%) | 2 (50%) |
| Lung | 1 (5%) | 0 (0%) |
| Brain | 1 (5%) | 0 (0%) |
| Abdominal cavity | 1 (5%) | 1 (25%) |
| Lymph nodes | 1 (5%) | 0 (0%) |
-
Notes: Data are n (%). HR, hormone receptor; DCIS, ductal carcinoma in situ; ET, endocrine therapy.
-
Source files available in Table 2—source data 1.
-
Table 2—source data 1
Tumor recurrences in patients with hormone receptor positive (HR+) ductal carcinoma in situ (DCIS) after mastectomy.
- https://cdn.elifesciences.org/articles/83045/elife-83045-table2-data1-v1.xlsx
Stratification analysis of tumor recurrence rates in patients with HR+ DCIS after mastectomy.
| Characteristic | ET (N=791) | Non-ET (N=216) | HR (95% CI) | p-Value |
|---|---|---|---|---|
| Total | 19 (791) | 4 (216) | 1.30 (0.48–3.52) | 0.64 |
| Age | ||||
| ≤50 | 12 (448) | 1 (110) | 2.91 (0.74–11.47) | 0.28 |
| >50 | 7 (343) | 3 (106) | 0.75 (0.18–3.17) | 0.67 |
| Tumor size | ||||
| ≤20 mm | 6 (459) | 2 (127) | 0.82 (0.15–4.44) | 0.81 |
| >20 mm | 13 (332) | 2 (89) | 1.75 (0.51–6.04) | 0.45 |
| Microinvasive | ||||
| Yes | 8 (128) | 0 (29) | 3.48 (0.60–20.02) | 0.16 |
| No | 11 (663) | 4 (187) | 0.76 (0.22–2.59) | 0.64 |
| Tumor grade | ||||
| I-II | 10 (565) | 0 (164) | 3.64 (0.82–16.06) | 0.09 |
| III | 9 (226) | 4 (52) | 0.51 (0.13–2.07) | 0.26 |
| ER | ||||
| 1–10% | 4 (165) | 2 (40) | 0.47 (0.08–2.67) | 0.386 |
| >10% | 15 (626) | 2 (176) | 2.14 (0.48–9.43) | 0.305 |
| Ki67 | ||||
| ≥15% | 7 (279) | 1 (62) | 0.88 (0.09–8.29) | 0.67 |
| <15% | 12 (512) | 3 (154) | 1.42 (0.46–4.38) | 0.74 |
| Multifocal | ||||
| Yes | 3 (64) | 0 (13) | 3.34 (0.17–67.46) | 0.43 |
| No | 16 (727) | 4 (203) | 1.11 (0.38–3.22) | 0.85 |
| Surgery | ||||
| Unilateral mastectomy | 19 (776) | 4 (215) | 1.32 (0.45–3.93) | 0.61 |
| Bilateral mastectomy | 0 (15) | 0 (1) | – | – |
-
Notes: HR, hormone receptor; DCIS, ductal carcinoma in situ; ET, endocrine therapy.
-
Source files available in Table 3—source data 1.
-
Table 3—source data 1
Tumor recurrences in subgroups.
- https://cdn.elifesciences.org/articles/83045/elife-83045-table3-data1-v1.xlsx
Adverse events between TAM and AI in the ET group.
| Adverse events | TAM (N=551) | AI (N=223) |
|---|---|---|
| Total | 212 (38%) | 76 (34%) |
| Musculoskeletal symptoms | 61 (11%) | 52 (23%) |
| Vasomotor symptoms | 42 (8%) | 12 (5%) |
| Gynecological events | 113 (21%) | 5 (2%) |
| Cardiovascular events | 26 (5%) | 12 (5%) |
| Abnormal liver function | 10 (2%) | 4 (2%) |
-
Notes: TAM, tamoxifen; AI, aromatase inhibitor; ET, endocrine therapy.
-
Source files available in Table 4—source data 1.
-
Table 4—source data 1
Adverse events in the endocrine therapy (ET) group.
- https://cdn.elifesciences.org/articles/83045/elife-83045-table4-data1-v1.xlsx