The anti-caspase 1 inhibitor VX-765 reduces immune activation, CD4+ T cell depletion, viral load, and total HIV-1 DNA in HIV-1 infected humanized mice
Figures
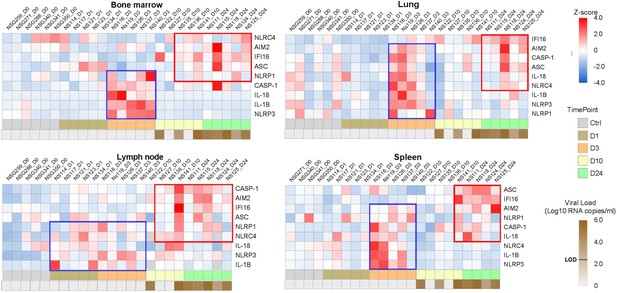
Heatmaps reveal a biphasic inflammasome-related genes transcriptomic upregulation upon HIV-1JRCSF infection of huNSG mice.
Relative mRNA expression of the indicated genes measured by qPCR in hCD45+ cells isolated from bone marrow, lung, lymph nodes and spleen in HIV-1JRCF i.p. infected huNSG mice at day 0 (n=6), day 1 (n=5), day 3 (n=5), day 10 (n=5), and day 24 (n=5). Gene expression was standardized using z-score method. Red and blue colors identify up- and down-regulated genes, respectively.
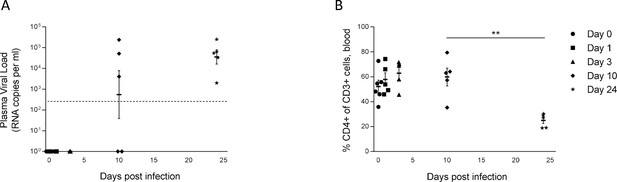
Kinetics of viremia and early CD4+ T cell loss during HIV-1 infection of huNSG mice.
(A) Plasma HIV-1 viral load measured by ddPCR (LOD: 235 copies/ml) and (B) human CD4+ T cells percentage among CD3+ cells measured by flow in HIV-1JRCF i.p. infected NSG humanized mice at day 0 (n=6), day 1 (n=5), day 3 (n=5), day 10 (n=5), and day 24 (n=5). Statistical tests were performed using Mann–Whitney t-tests for comparison of two groups (** p<0.005).
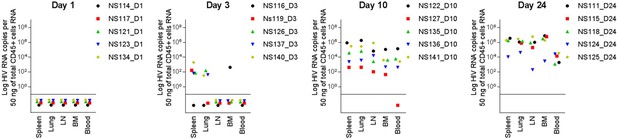
HIV-1 disseminates within 3 days post-infection in huNSG mice.
Viral RNA in the spleen, lung, lymph nodes (LN), bone marrow (BM) and in the blood were measured by RT-PCR (Log HIV RNA copies per 50 ng of total hCD45+ cells RNA) in HIV-1JRCF i.p. infected NSG humanized mice at day 1 (n=5), day 3 (n=5), day 10 (n=5), and day 24 (n=5). Samples in which viral RNA was below 40 copies per 50 ng of total CD45+ cells RNA were plotted below the horizontal line.
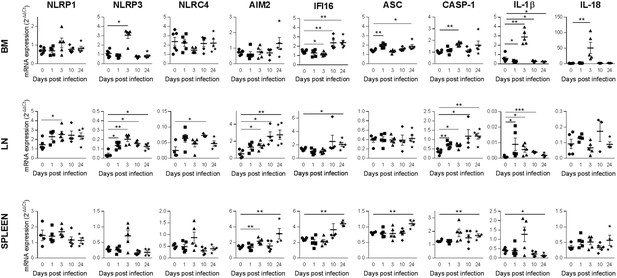
HIV-1 induction of inflammasome related genes expression in tissues.
Relative mRNA expression of the indicated genes measured by qPCR in hCD45+ cells isolated from bone marrow (BM), lymph nodes (LN) and spleen in HIV-1JRCF i.p. infected huNSG mice at day 0 (n=5), day 1 (n=5), day 3 (n=5), day 10 (n=5), and day 24 (n=5). Results were analyzed using the 2-ΔΔCT method. Statistical tests were performed by Mann–Whitney t-tests for comparison of two groups (* p<0.05, ** p<0.005, *** p<0.005).
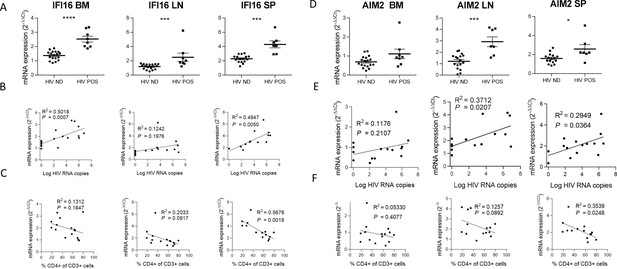
IFI16 and AIM2 expression correlates with HIV-1 replication and pathogenesis.
IFI16 and AIM2 relative mRNA expression comparison between viremic (HIV POS, n=8) and aviremic (HIV ND, n=16) huNSG mice (A and D) in the bone marrow (BM), lymph nodes (LN), and the spleen (SP). Correlation between IFI16 or AIM2 relative mRNA expression with corresponding tissue viral RNA copies (B and E) or CD4 T cells percentage in the blood (C and F) for infected animals showing detectable HIV-1 mRNA in human CD45+ cells (n=15). qPCR results were analyzed using the 2-ΔΔCT method. Statistical tests were performed by Mann–Whitney t-tests for comparison of two groups (* p<0.05, *** p<0.0005, **** p<0.0005) or Spearman correlation tests.
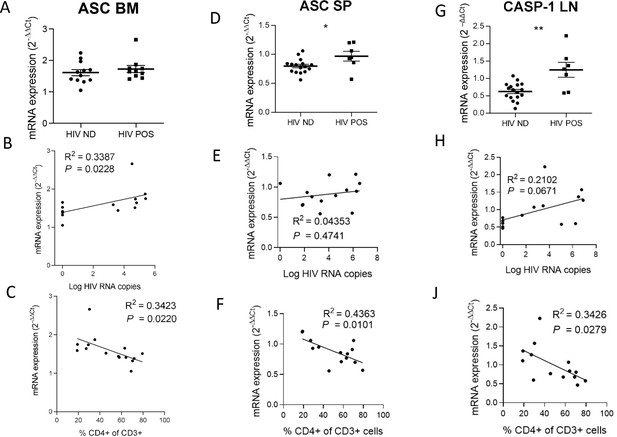
ASC and CASP-1 expression correlates with HIV-1 replication and pathogenesis.
ASC and CASP-1 relative mRNA expression comparison between viremic (HIV POS, n=7) and aviremic (HIV ND, n=16) huNSG mice (A, D, G) in the bone marrow (BM), spleen (SP) and in the lymph nodes (LN). Correlation between ASC and CASP-1 relative mRNA expression with corresponding tissue viral RNA copies (B, E, H) or CD4+ T cells percentage in the blood (C,F, J) for infected animals showing detectable HIV-1 mRNA in human CD45+ cells (n=15). qPCR results were analyzed using the 2-ΔΔCT method. Statistical tests were performed by Mann–Whitney t-tests for comparison of two groups (*p<0.05, ** p<0.005) or Spearman correlation tests.
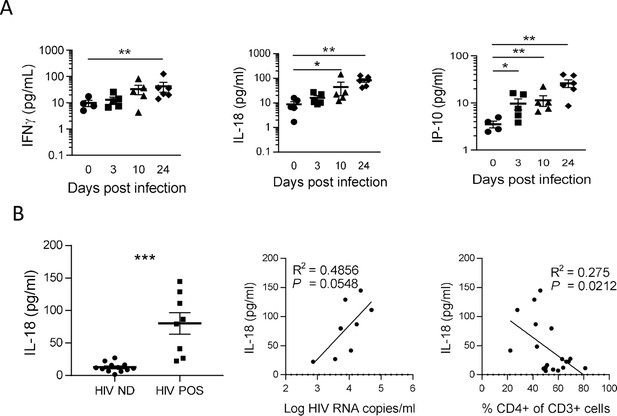
IL-18 is induced by HIV-1 replication and correlates inversely with CD4+ T cells percentage.
(A) Plasma cytokines concentration were measured using a multiplex U-PLEX Biomarker assay kit in the plasma of HIV-1JRCF i.p. infected huNSG mice at day 0 (n=5), day 3 (n=5), day 10 (n=5), and day 24 (n=5). (B) Plasma IL-18 levels comparison between viremic (HIV POS, n=8) and aviremic (HIV ND, n=12) huNSG mice. Correlations between plasma IL-18 levels with corresponding plasma viral load (C) or CD4 +T cells percentage in the blood (D). Statistical tests were performed by Mann–Whitney t-tests for comparison of two groups (* p<0.05, ** p<0.005) or Spearman correlation tests.
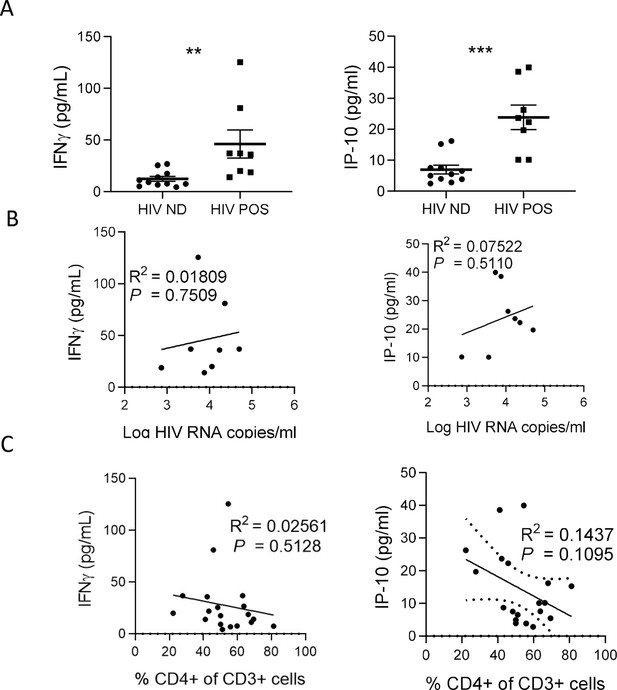
IFN-γ and IP-10 are inducted by HIV-1 replication.
(A) Plasma cytokines levels comparison between viremic (HIV POS, n=8) and aviremic (HIV ND, n=11) huNSG mice. Correlations between plasma cytokines levels with corresponding plasma viral load (B) or CD4 +T cells percentage in the blood (C). Statistical tests were performed by Mann–Whitney t-tests for comparison of two groups (** p<0.005, *** p<0.005) or Spearman correlation tests.
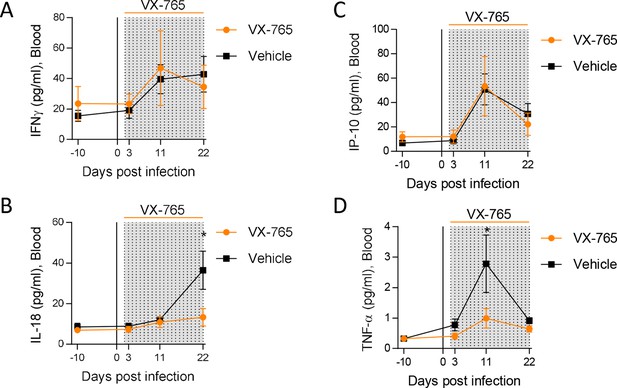
Caspase-1 inhibition reduces circulating levels of IL-18.
Plasma cytokine concentrations were measured using a multiplex U-PLEX Biomarker assay kit in longitudinal plasma samples of HIV-1JRCF i.p. infected huNSG mice treated with VX-765 (VX-765, n=12) or vehicle (Vehicle, n=12) at 3 days, 11 days and 22 days after infection. Statistical tests were analysed by Mann–Whitney t-tests for comparison of two groups (* p<0.05).
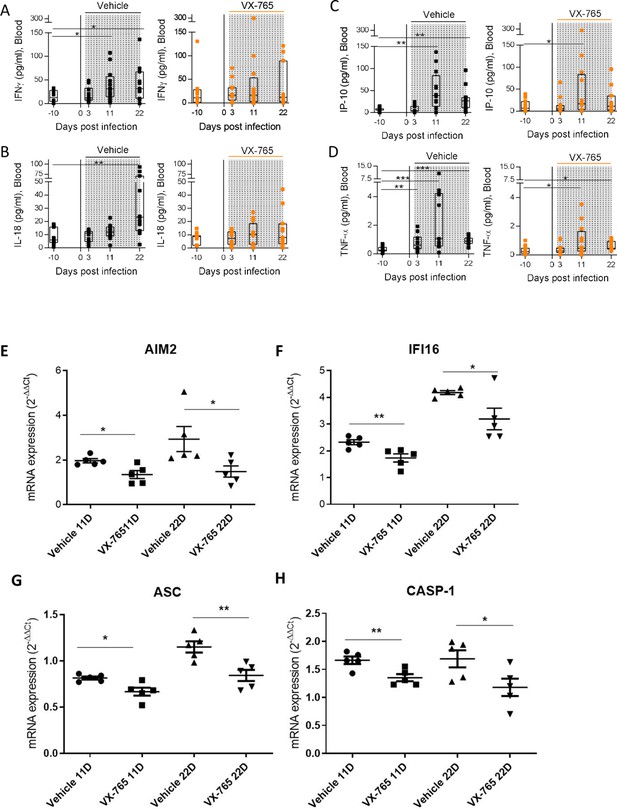
Caspase-1 inhibition reduces levels of plasma cytokines and mRNA expression of inflamasomme genes.
Plasma cytokine concentrations (A to D) weremeasured using a multiplex U-PLEX Biomarker assay kit in longitudinal plasma samples of HIV-1JRCF i.p. infected huNSG mice treated with VX-765 (VX-765, n=12) or vehicle (Vehicle, n=12) at 3 days, 11 days, and 22 days after infection. Statistical tests were analysed by Wilcoxon’s matched pairs signed rank test (* p<0.05, ** p<0.005, *** p<0.0005). mRNA expression of AIM2 (E), IFI16 (F), ASC (G), and Caspase 1 (H) was measured at 11 and 22 days post-infection in the spleen of infected huNSG mice treated with vehicle (Vehicle, n=5) or VX-765 (VX-765, n=5). Statistical tests were analysed by Mann–Whitney t-tests for comparison of two groups (* p<0.05, ** p<0.005).
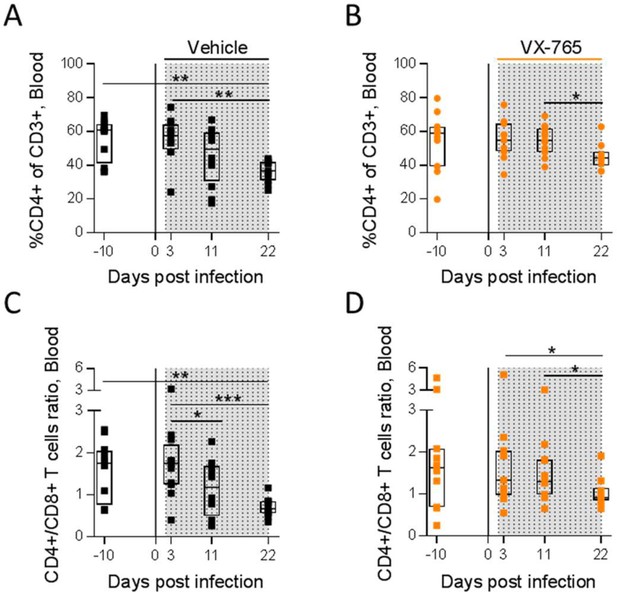
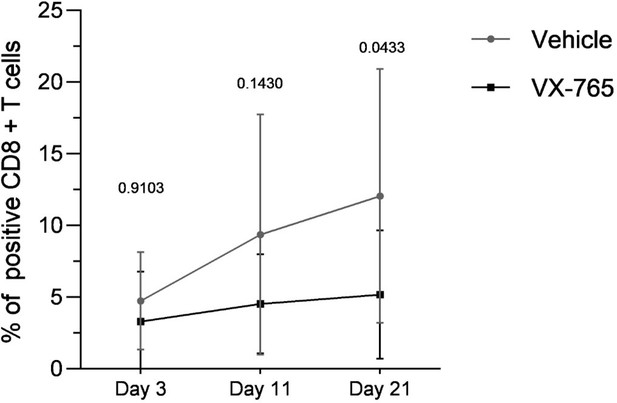
VX-765 treatment prevents CD4+ T cell depletion in the blood.
Blood human CD4+ T cells percentage among CD3+ cells (A and B) and CD4+/CD8+ T cell ratios (C and D) measured longitudinally by flow cytometry in VX-765 (VX-765, n=12) or vehicle (Vehicle, n=12) huNSG mice at 3 days, 11 days, and 22 days after HIV-1 infection. Statistical tests were analysed by Wilcoxon’s matched pairs signed rank test (* p<0.05, ** p<0.005, *** p<0.0005).
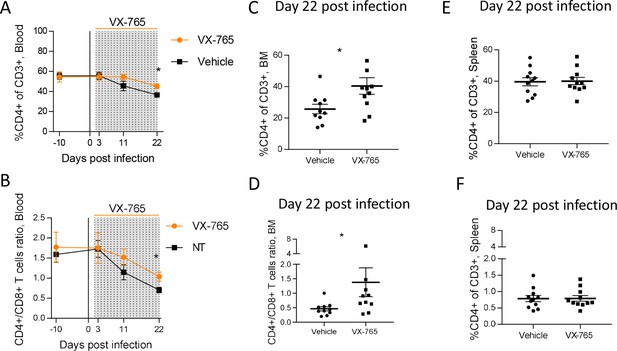
VX-765 treatment prevents CD4+ T cell depletion in the blood and bone marrow (BM) during HIV-1 infection of huNSG mice.
Blood human CD4+ T cells percentage among CD3+ cells (A) and CD4+/CD8+ T cell ratios (B) measured longitudinally by flow cytometry in VX-765 (VX-765, n=12) or vehicle (Vehicle, n=12) huNSG mice at 3 days, 11 days, and 22 days after HIV-1 infection. Bone marrow (BM) (C and D) and spleen (E and F) human CD4+ T cells percentage among CD3+ cells and CD4+/CD8+ T cell ratios, respectively, measured at day 22 post-infection in vehicle or VX-765-treated HIV-1 infected huNSG mice. Statistical tests were analysed by Mann–Whitney t-tests for comparison of two groups (* p<0.05).
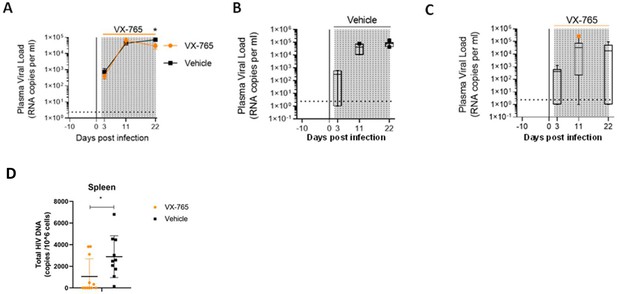
VX-765 treatment reduces plasma viral load and total HIV-1 DNA after HIV-1 infection of huNSG mice.
Plasma viral load (A) was measured longitudinally by ddPCR in vehicle (Vehicle, n=12) (B) or in VX-765 (VX-765, n=12) (C) or in treated HIV-1 infected huNSG mice at 3 days, 11 days, and 22 days after HIV-1 infection. Total HIV-1 DNA was measured by qPCR at day 22 post-infection in the spleen of CD45+ human cells isolated by MACS purification from all HIV-1 infected huNSG mice treated (VX-765, n=12) or not (Vehicle, n=12) with VX-765 (D). Statistical tests were analysed by Mann–Whitney t-tests for comparison of two groups (* p<0.05).
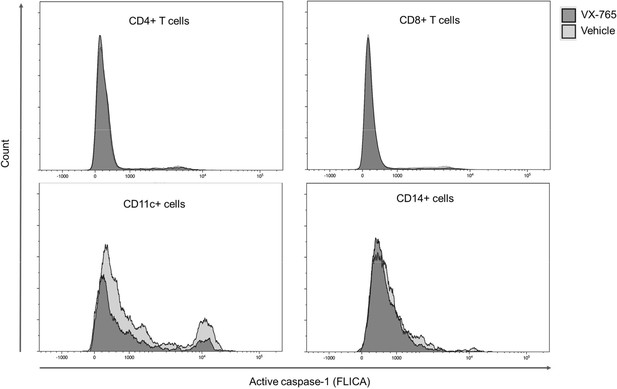
Representative histograms of FLICA staining in splenocytes from VX-765 and vehicle-treated HIV-1 infected huNSG mice.
Splenocytes were stained for active caspase-1 using FAM-FLICA reagent. Representative MFI of FAM-FLICA+ CD4+, CD8+, CD11c+, and CD14+ splenic cells in one HIV-1 infected huNSG mice treated with vehicle or with VX-765-treated mice at day 22 post-infection is shown.
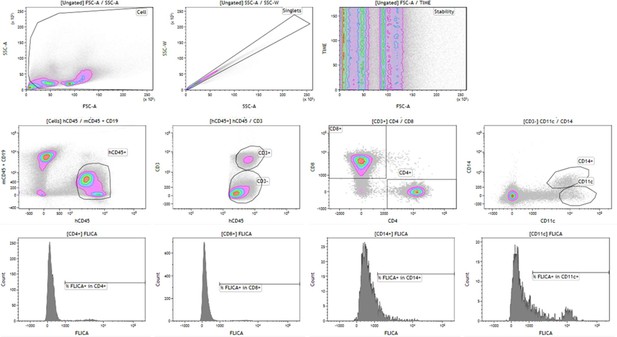
Gating strategy for FLICA analysis.
FAM-Flica staining was analysed in gated human CD4+, CD8+, CD14+ and CD11c+ cells using the anti-hu CD45, anti-hu CD4, anti-hu CD8, anti-hu CD11c, anti-hu CD14 antibodies.
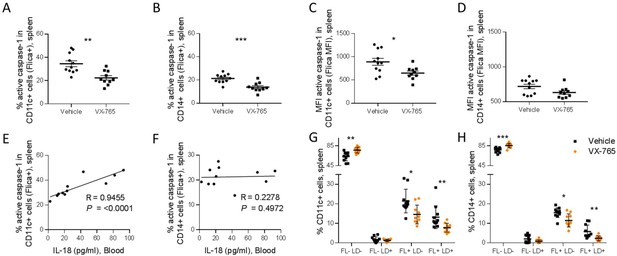
VX-765 treatment reduces caspase-1 activation in splenic CD11c+ and CD14+ in HIV-1 infected huNSG mice.
Splenocytes were stained for active caspase-1 using FAM-FLICA reagent. Percentage and MFI of FAM-FLICA+ CD11c+ (A, C), CD14+ (B, D) splenic cells in vehicle or VX-765 treated HIV-1 infected huNSG mice at day 22 post-infection. Correlation between the percentage of FAM-FLICA+ CD11c+ (E), CD14+ (F) splenic cells and IL-18 plasma levels in vehicle-treated HIV-1 infect group at day 22 post-infection. Percentage of FAM-FLICA-/Live-dead (FL-LD-), FAM-FLICA-/Live-dead+ (FL-LD+), FAM-FLICA+/Live-dead- (FL+LD-) and FAM-FLICA+/Live-dead+ (FL+LD+) CD11c+ (G), CD14+ (H) splenic cells in vehicle (Vehicle, n=12) or VX-765 (VX-765, n=12) treated HIV-1 infected huNSG mice at day 22 post-infection. Statistical tests were performed by Mann–Whitney t-tests for comparison of two groups (* p<0.05, ** p<0.005, *** p<0.0005) or Spearman correlation tests.
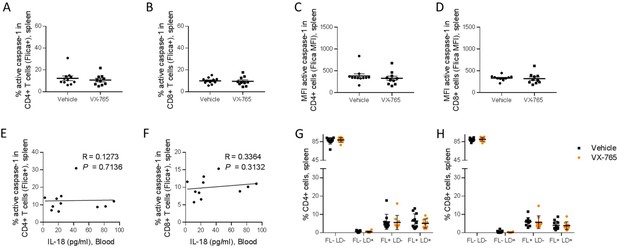
VX-765 treatment does not affect caspase-1 activation in splenic CD4+ and CD8+ T cells in HIV-1 infected huNSG mice.
Splenocytes were stained for active caspase-1 using FAM-FLICA reagent. Percentage and MFI of FAM-FLICA+ CD4+ (A, C), CD8+ (B, D) splenic cells in vehicle or VX-765 treated HIV-1 infected huNSG mice at day 22 post-infection. Correlation between the percentage of FAM-FLICA+ CD4+ (E), CD8+ (F) splenic cells and IL-18 plasma levels in vehicle treated HIV-1 infect group at day 22 post-infection. Percentage of FAM-FLICA-/Live-dead- (FL-LD-), FAM-FLICA-/Live-dead+ (FL-LD+), FAM-FLICA+/Live-dead- (FL+LD-) and FAM-FLICA+/Live-dead+ (FL+LD+) CD4+ (G), CD8+ (H) splenic cells in vehicle (Vehicle, n=12) or VX-765 (VX-765, n=12) treated HIV-1 infected huNSG mice at day 22 post-infection. Statistical tests were performed by Mann–Whitney t-tests for comparison of two groups or Spearman correlation tests.
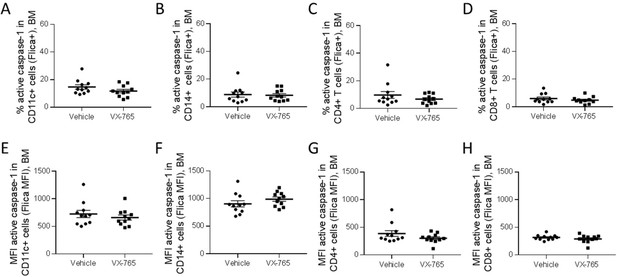
VX-765 treatment does not affect caspase-1 activation in the bone marrow.
Cells from the bone marrow were stained for active caspase-1 using FAM-FLICA reagent. Percentage of FAM-FLICA+ CD11c+ (A), CD14+ (B), CD4+ (C), CD8+ (D) splenic cells in vehicle or VX-765 treated HIV-1 infected huNSG mice at day 22 post-infection. Mean fluorescence intensity (MFI) of FAM-FLICA in CD11c+ (E), CD14+ (F), CD4+ (G), CD8+ (H) splenic cells in vehicle (Vehicle, n=12) or VX-765 (VX-765, n=12) treated HIV-1 infected huNSG mice at day 22 post-infection. Statistical tests were analysed by Mann–Whitney t-tests for comparison of two groups.
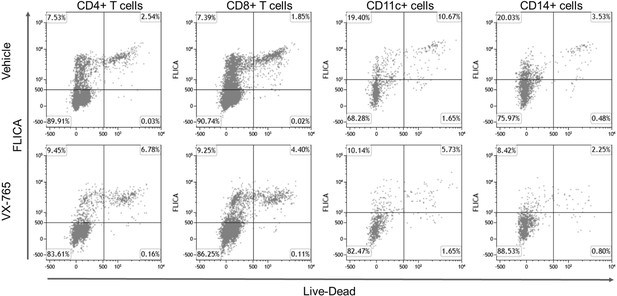
Representative dot plots of FLICA versus LIVE-DEAD staining in splenocytes from VX-765 and vehicle-treated HIV-1 infected huNSG mice.
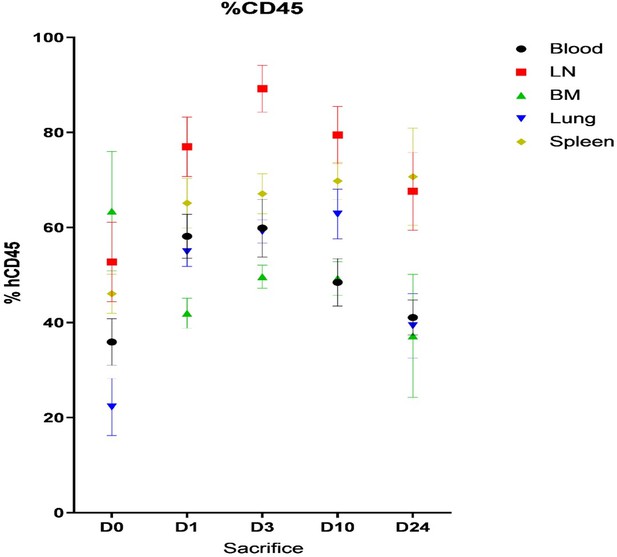
Mean of percentage (± SD) of human CD45+ cells in blood, Lymph node (LN), Bone Marrow (BM), lung and spleen of humanized mice before (day 0) and after HIV infection (day 3, 10 and 24).

Mean percentage (± SD) of human CD4+ T cells in human CD45+CD3+ cells in blood and tissues of humanized mice before (D0) and after HIV infection (D1, D3, D10 and D24).
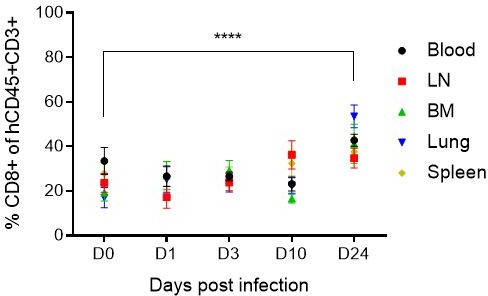
Mean percentage (± SD) of human CD8+ T cells in human CD45+CD3+ cells in blood and tissues of humanized mice before (D0) and after HIV infection (D1, D3, D10 and D24).
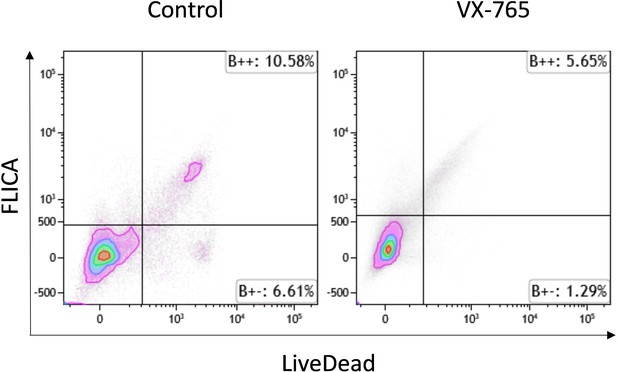
Representative dot plots of FLICA versus LIVE-DEAD staining among debris in splenocytes from vehicle treated (left panel) and VX-765 (right panel) HIV-1 infected huNSG mice.
Tables
LOD of the Mesoscale U-PLEX biomarker assay kit for the different cytokines.
| U-PLEX Assay | LLOD(Sensitivity) | Dynamic Range |
|---|---|---|
| Human IFN-γ | 1.7 pg/mL | 1.7-17,000 pg/mL |
| Human IL-1β | 0.15 pg/mL | 0.15-3,800 pg/mL |
| Human IL-2 | 0.70 pg/mL | 0.70-1,900 pg/mL |
| Human IL-6 | 0.33 pg/mL | 0.33-2,000 pg/mL |
| Human IL-12p70 | 0.69 pg/mL | 0.69-5,3000 pg/mL |
| Human IL-18 | 0.5 pg/mL | 0.5-14,000 pg/mL |
| Human IP-10 | 0.49 pg/mL | 0.49-6,000 pg/mL |
| Human MIP-1β | 1.5 pg/mL | 1.5-1,600 pg/mL |
| Human TNF-α | 0.51 pg/mL | 0.51-3,700 pg/mL |
| Human TRIAL | 0.66 pg/mL | 0.66-10,000 pg/mL |