Shear and hydrostatic stress regulate fetal heart valve remodeling through YAP-mediated mechanotransduction
Figures
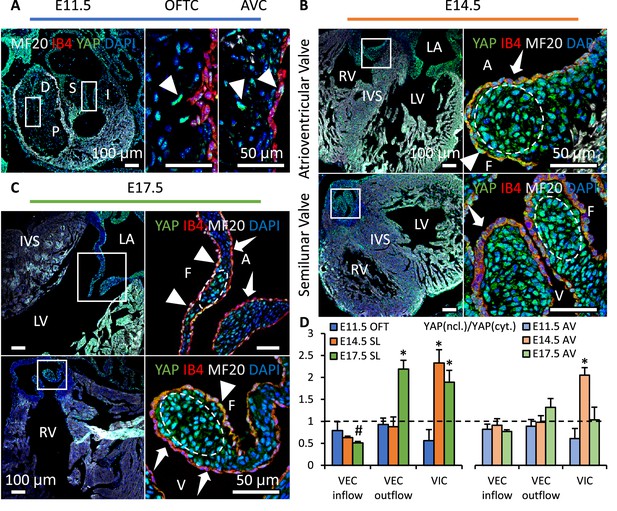
YAP expression is spatiotemporally regulated.
(A). YAP was expressed in both cushion mesenchyme and endothelium (triangles) at E11.5. (B). YAP is intensively expressed in VICs (white circles) at E14.5 but seldom detected in VECs (dashed arrows). (C). YAP expression remains in VICs on the tip regions and in VECs on the fibrosa side at E17.5 (solid arrows), while disappears in VECs on the ventricularis or ventricularis (dashed arrows) (D) Intensity ratios of nuclear vs. cytoplasmic YAP expressions in VECs and VICs of AV and SL valves at different stages. Data are presented by mean ± SEM, n=6 sections from three embryos, *p<0.05, two-way ANOVA tests. OFTC, outflow tract cushion; AVC, atrioventricular cushion; I, inferior cushion; S, superior cushion; D, distal cushion; P, proximal cushion; LA, left atrium; LV, left ventricle; RV, right ventricle; IVS, interventricular septum; V, ventricularis; A, atrialis; F, fibrosa.
-
Figure 1—source data 1
YAP activation measurement for Figure 1D.
- https://cdn.elifesciences.org/articles/83209/elife-83209-fig1-data1-v2.xlsx
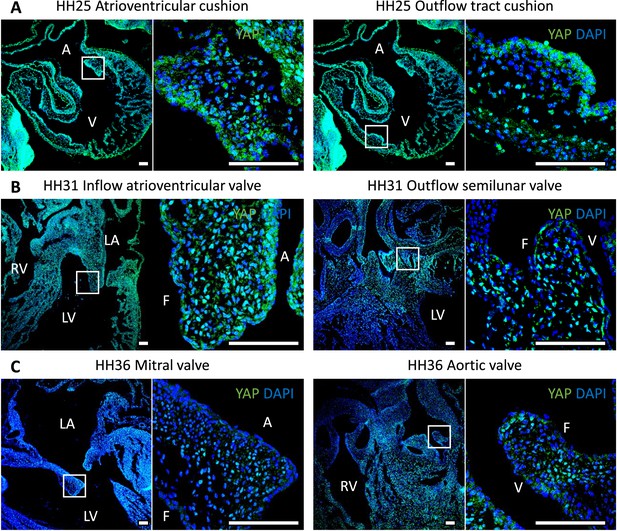
YAP expression in chick hearts during embryonic development.
LA, left atrium; RA, right atrium; LV, left ventricle; RV, right ventricle; V, ventricularis; A, atrialis; F, fibrosa. Scale bar: 100 μm.
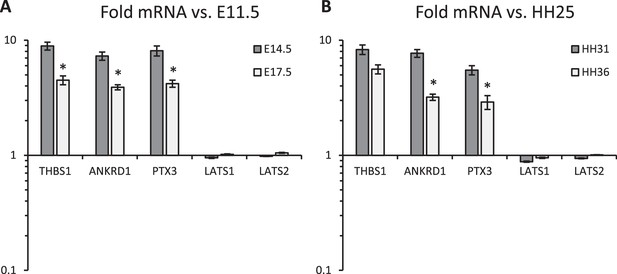
qPCR analysis of (A) mouse and (B).
chick embryonic valves at different developmental stages, measures YAP transcriptional activity and the effect of Hippo pathway on YAP activities. Mouse data were normalized to E11.5 data, chick data were normalized to HH25 data. n>10 isolated valves. *p<0.05, two-way ANOVA tests.
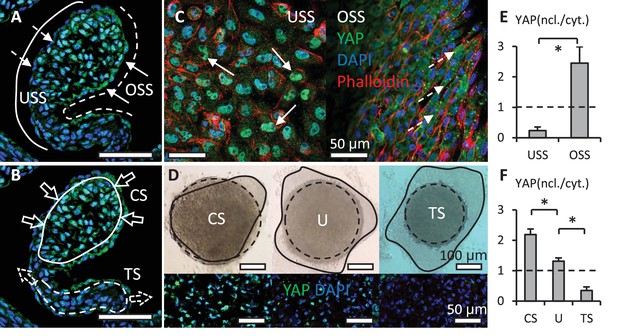
Shear stress and hydrostatic pressure regulate YAP activity.
(A). Unidirectional shear stress (USS) developed on the inflow surface (solid line), where YAP was rarely expressed in the nuclei of VECs (dash arrows). Oscillatory Shear Stress (OSS) developed on the outflow surface (dash line), where VECs with nuclear YAP localized (solid arrows). (B). Compressive Stress (CS) was generated in the tips of valves (solid circle), where VICs with nuclear YAP localized. Tensile Stress (TS) is created in the elongated regions (dash circle), where YAP was absent in VIC nuclei. (C). When applied on a monolayer of VEC, high USS restricted YAP in cytoplasm, low OSS promoted YAP nuclear localization. (D). Cushion explants were cultured under compress stress (CS), tensile stress (TS) and unloaded (U) conditions for 24 hr. Solid outlines describe the explant morphologies at 0 hr, dash outlines describe the explant morphologies at 24 hr. (E). Intensity ratios of nuclear vs. cytoplasmic YAP expressions for experiments in (C, F). Intensity ratios of nuclear vs. cytoplasmic YAP expressions for experiments in (D). Data are presented by mean ± SEM, n=15 explant valves from eight embryos, *p<0.05, two-tailed student t-tests.
-
Figure 2—source data 1
YAP activation measurements for Figure 2E and F.
- https://cdn.elifesciences.org/articles/83209/elife-83209-fig2-data1-v2.xlsx
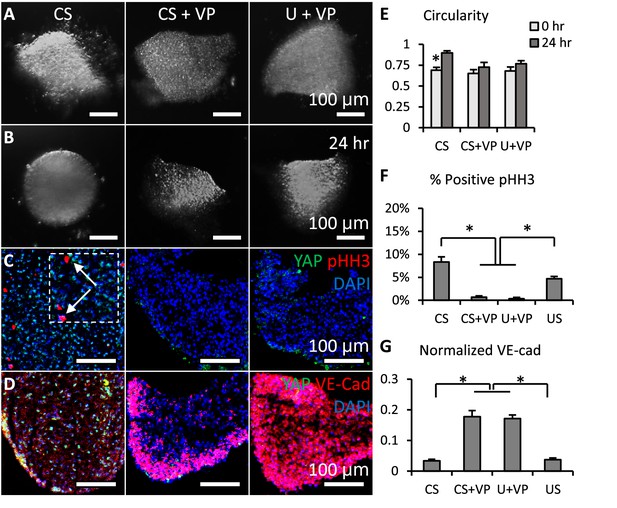
Loss of YAP limited cell proliferation and promoted valve shaping.
(A-B). Cushion explants were cultured under CS (compressive stress), CS +VP (compressive stress +YAP inhibitor), U+VP (unloaded +YAP inhibitor) conditions for 24 hr. (C). After 24-hr-culture, explants were stained for YAP (green) and proliferation marker pHH3 (red, arrows), or (D). YAP (green) and VE-Cadherin (red). (E). Circularity of explants cultured under different stress conditions, which describes how close a valve is to a perfect sphere. (F). Percentages of cells expressing pHH3 under different culture conditions. (G). Average intensities of VE-Cad expression under different culture conditions, the intensities are normalized to maximum intensity. Data are presented by mean ± SEM, n=15 explant valves from eight embryos, *p<0.05, two-tailed student t-tests.
-
Figure 3—source data 1
Data used to generate Figure 3E, F and G.
- https://cdn.elifesciences.org/articles/83209/elife-83209-fig3-data1-v2.xlsx
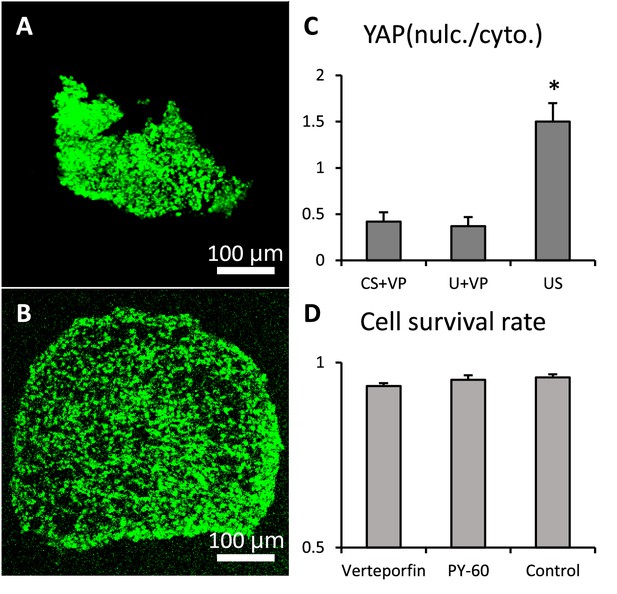
Verteporfin and PY-60 treatments.
Live and dead staining of (A). verteporfin and (B). PY-60 treated cushion explants. (C). Expression ratios of nuclear vs. cytoplasmic YAP show the effective inhibition of YAP by verteporfin. (D). Survival rate was not affected by the treatments.
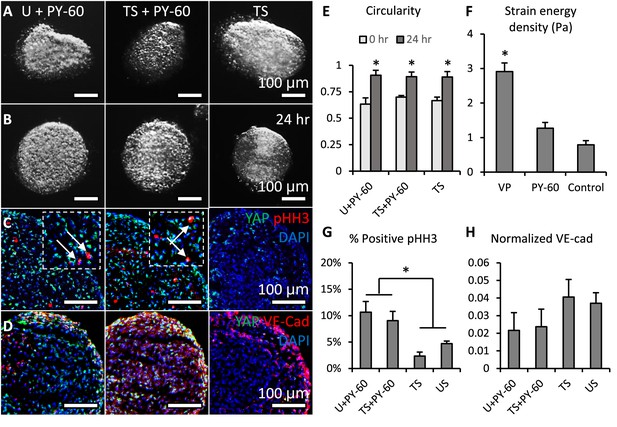
Activation of YAP promoted cell proliferation and inhibited valve elongation.
(A-B). Cushion explants were cultured under U+PY-60 (unloaded +YAP activator), TS +PY-60 (tensile stress +YAP activator), TS (tensile stress) conditions for 24 hr. (C). After 24-hr-culture, explants were stained for YAP (green) and proliferation marker pHH3 (red, arrows), or (D). YAP (green) and endothelial cell-cell junction VE-Cadherin (red). (E). Circularity of explants cultured under different stress conditions, which describes how close a valve is to a perfect sphere. (F). Stiffness of cushion explants cultured with YAP activator and inhibitor, which was measured by micropipette aspiration measurement. (G). Percentages of cells expressing pHH3 under different culture conditions. (H). Average intensities of VE-Cad expression under different culture conditions, the intensities are normalized to maximum intensity. Data are presented by mean ± SEM, n=15 explant valves from eight embryos, *p<0.05, two-tailed student t-tests.
-
Figure 4—source data 1
Data used to generate Figure 4E-H.
- https://cdn.elifesciences.org/articles/83209/elife-83209-fig4-data1-v2.xlsx
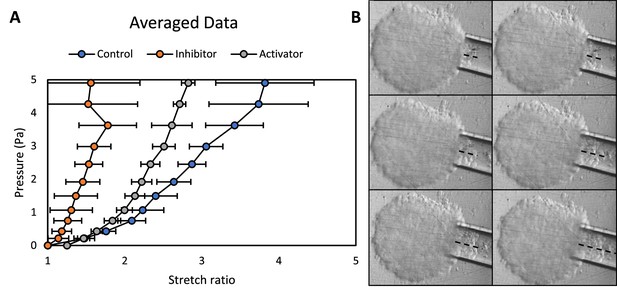
Stiffness of cushion explants.
(A). Pressure-stretch curves of cushion explants treated with YAP inhibitor and activator, as well as the control. n≥10 explants. (B). Micropipette aspiration measurements, dash lines indicate tissue displacements within the tips.
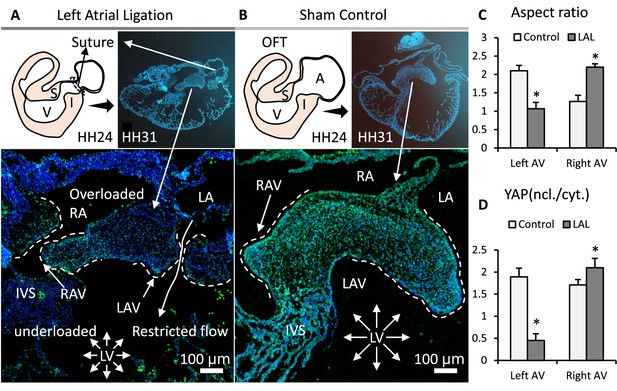
Interrupted biomechanics altered YAP activation and led to valve defects in-vivo.
(A). LAL performed at HH24 led to an underdeveloped left AV septal valve and overdeveloped right AV septal valve at HH31. (B). Normally developed sham control hearts. (C). Aspect ratios of AV septal valves, which evaluate valve elongation. (D). D. Intensity ratios of nuclear vs. cytoplasmic YAP expressions in the septal AV valves of LAL and sham control hearts. Data are presented by mean ± SEM, n=6 sections from three embryos, *p<0.05, two-tail student t-tests. A, atrium; V, ventricle; I, inferior cushion; S, superior cushion; LA, left atrium; RA, right atrium; LV, left ventricle; RV, right ventricle; IVS, interventricular septum; LAV, left atrioventricular septal valve; RAV, right atrioventricular septal valve.
-
Figure 5—source data 1
Data used to generate Figure 5C and D.
- https://cdn.elifesciences.org/articles/83209/elife-83209-fig5-data1-v2.xlsx
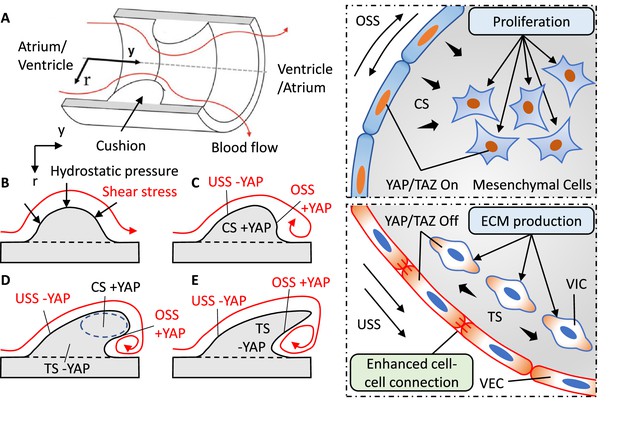
Local collaboration of shear and hydrostatic stress can guide complex morphogenesis via YAP signaling.
(A). Model of a symmetric endocardial cushion in the AV canal or OFT. (B). Cross-section of the model shows the flowing blood generates hydrostatic stress and shear stress on cushions. (C). Oscillatory Shear Stress (OSS) and Compressive Stress (CS) promoted YAP nuclear translocation in the VEC and VIC, respectively. Unidirectional Shear Stress (USS) inhibited YAP nuclear translocation. CS promoted cushion growth by stimulating the proliferation of mesenchymal cells. (D). Shear stress drives cushion remodeling into leaflet structure by modulating the cell-cell adhesions between VECs. (E). Tensile Stress (TS) inhibited YAP nuclear translocation worked together with USS for a compact and elongated morphology.





