Sleep spindle maturity promotes slow oscillation-spindle coupling across child and adolescent development
Figures
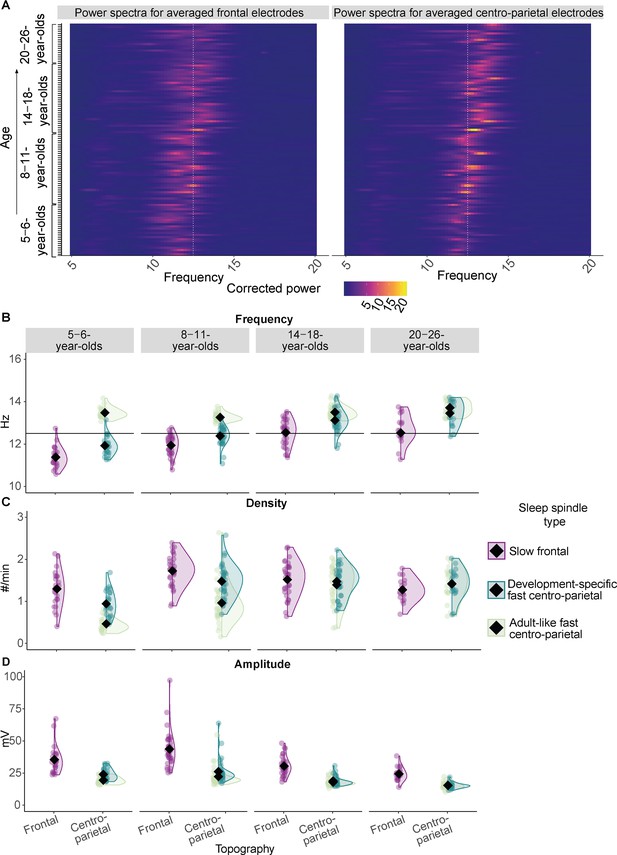
Sleep spindle features across the different age groups.
(A) Background-corrected power spectra in averaged frontal (left) and averaged centro-parietal (right) electrodes for every participant at every test time point (y-axis). Power is coded by color for frequencies between 5–20 Hz (x-axis). The individual peak frequency in the sleep spindle frequency range (x-axis, 9–16 Hz) is reflected by brighter colors for every participant at every age (y-axis). Data are ordered by age from bottom to top (y-axis). The white dotted line at 12.5 Hz illustrates the frequency border for canonical fast sleep spindles (i.e., spindles defined in young adults with a frequency ≈ 12.5–16 Hz and a centro-parietal predominance; Cox et al., 2017). Note: Participants at an older age (top) showed higher peak frequencies. Further, a linear mixed-effects model revealed that peak frequencies in centro-parietal electrodes were significantly faster compared to frontal ones averaged across all age groups (Supplementary file 1b). However, for most of the young children (bottom part of the plot) the peak frequencies and the frequencies of the derived dominant fast spindles (B) were slower than the canonical fast sleep spindle band (i.e., development-specific). (B–D) Results from the comparison of adult-like fast (12.5–16 Hz) and individually identified slow frontal and development-specific fast centro-parietal sleep spindle (B) frequency, (C) density, and (D) amplitude for all four age groups. (B) Horizontal lines at 12.5 Hz illustrate the frequency border for canonical fast sleep spindles (i.e., spindles defined in young adults with a frequency ≈ 12.5–16 Hz and a centro-parietal predominance; Cox et al., 2017). Values are individual raw scores. Diamonds reflect estimated marginal means of the respective linear mixed-effects model. Statistical results can be found in Supplementary files 1a–n.
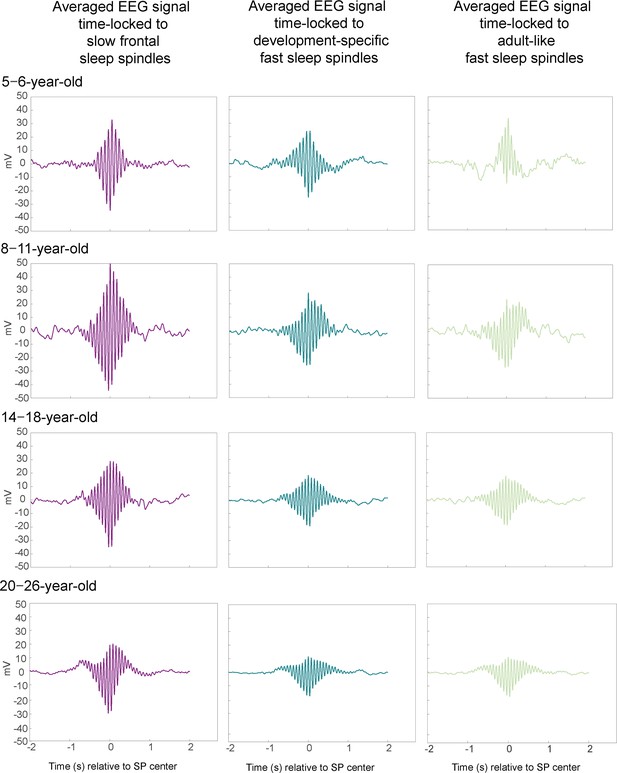
Exemplary averaged electroencephalography (EEG) signals time-locked to slow frontal (electrode F3), development-specific fast (electrode Cz), and adult-like fast (electrode Cz) sleep spindle (SP) centers for one 6-year-old participant as example for the 5- to 6-year-old age group, one participant who underwent EEG at 10 years of age and again at 16 years of age, as example for the 8- to 11- and 14- to 18-year-old age group, and one 21-year-old, as example for the 20- to 26-year-old age group.
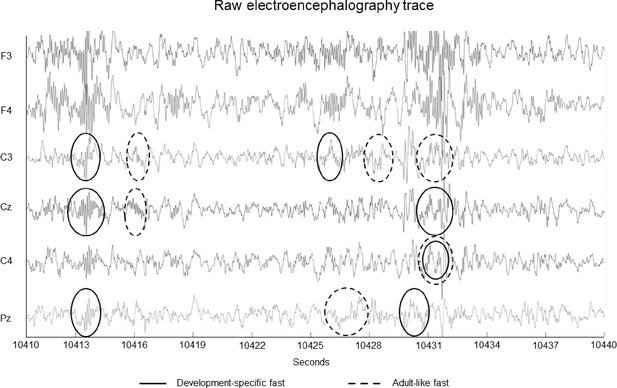
Example 30 second raw electroencephalography (EEG) window from a 6-year-old child with events that were detected as development-specific fast (solid circles) and adult-like fast (dashed circles) sleep spindles.
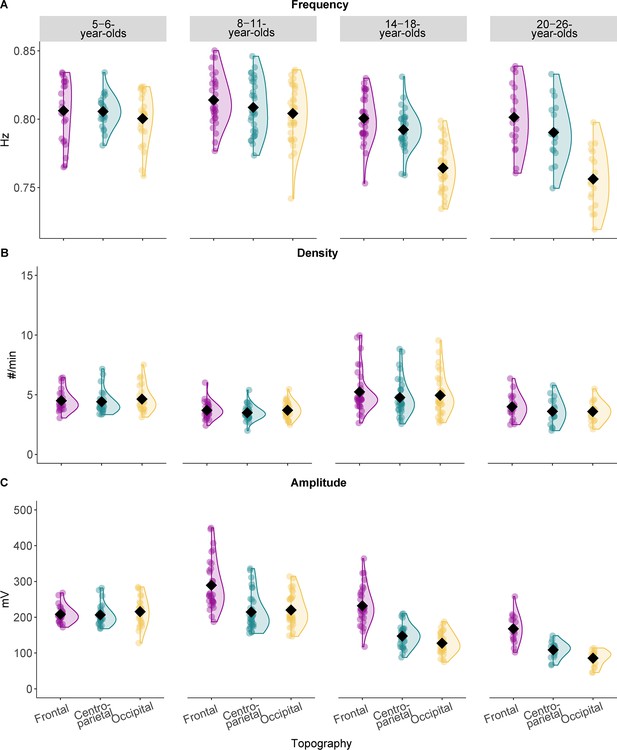
Slow oscillation features across the different age groups.
Results from the comparison of slow oscillation (A) frequency, (B) density, and (C) amplitude for all four age groups. Values are individual raw scores. Diamonds reflect estimated marginal means of the respective linear mixed-effects model. Note that we observed the strongest age-related differences for the slow oscillation amplitude (C) in different topographic locations. Statistical results can be found in Supplementary file 1o–t.
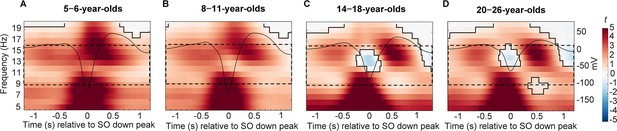
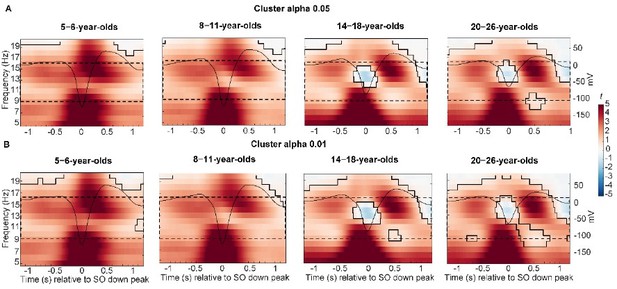
Centro-parietal power differences between trials with and without centro-parietal slow oscillations (in t-score units).
Significant clusters are outlined in black (cluster-based permutation test, cluster α < 0.05, two-sided test). Warmer colors indicate higher power during trials with slow oscillations and colder colors indicate lower power during trials with slow oscillations as compared to trials without slow oscillations. The average centro-parietal slow oscillation for each age group (A–D) is plotted onto the power differences in black to illustrate the relation to slow oscillation phase (scale in mV on the right y-axis of each plot). The sleep spindle frequency range is highlighted by the dashed window. Note that the strongest power increases during slow oscillations were observed in a frequency range reflecting the adult-like fast sleep spindle range (12.5–16 Hz). Results for frontal power and frontal slow oscillations can be found in Figure 3—figure supplement 1.
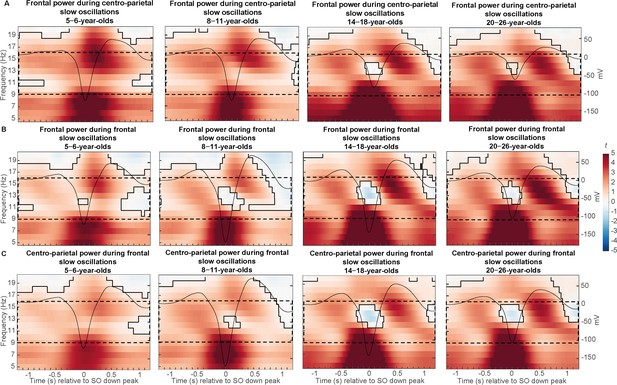
Power differences between trials with and without slow oscillations (in t-score units) in different topographical locations.
(A–B) Frontal and (C) centro-parietal power differences between trials with and without (A) centro-parietal and (B–C) frontal slow oscillations (in t-score units). Significant clusters are outlined in black (cluster-based permutation test, cluster α < 0.05, two-sided test). The average slow oscillation for each age group is plotted onto the power differences in black to illustrate the relation to slow oscillation phase (scale in mV on the right y-axis of each plot). The sleep spindle frequency range is highlighted by the dashed window.
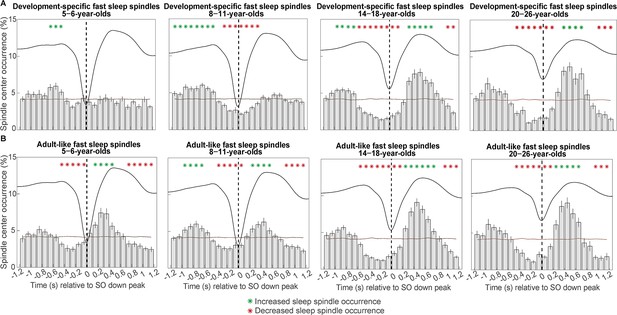
Peri-event time histograms for (A) development-specific fast centro-parietal spindles and (B) adult-like fast centro-parietal spindles showing the proportion of events occurring within 100 ms bins during centro-parietal slow oscillations.
Error bars represent standard errors. Green asterisks mark increased spindle occurrence (positive cluster, cluster-based permutation test, cluster α < 0.05, two-sided test) and red asterisks mark decreased spindle occurrence (negative cluster, cluster-based permutation test, cluster α < 0.05, two-sided test) compared to a surrogate distribution representing random occurrence (horizontal line). The dashed vertical line indicates the slow oscillation down peak. The average centro-parietal slow oscillation of each age group is shown in black to illustrate the relation to the slow oscillation phase. Results for slow oscillations and spindles in different topographies can be found in Figure 4—figure supplement 1.
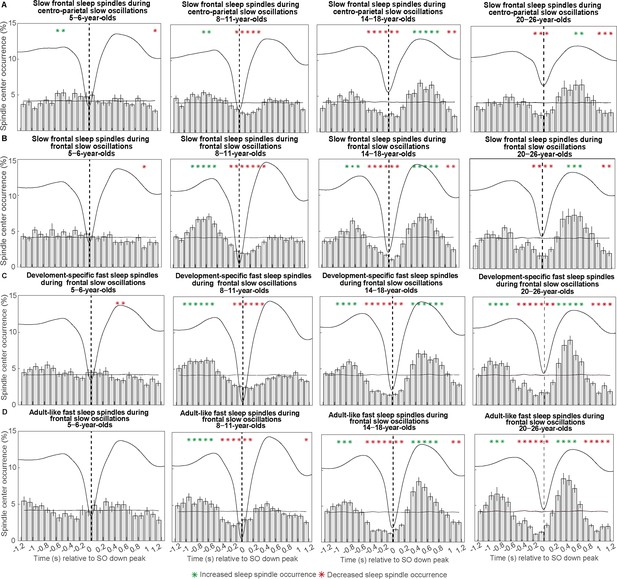
Peri-event time histograms for (A–B) slow frontal, (C) development-specific fast centro-parietal, and (D) adult-like fast spindles showing the proportion of sleep spindles occurring within 100 ms bins during (A) centro-parietal and (B–D) frontal slow oscillations.
Error bars represent standard errors. Green asterisks mark increased spindle occurrence (positive cluster, cluster-based permutation test, cluster α < 0.05, two-sided test) and red asterisks mark decreased spindle occurrence (negative cluster, cluster-based permutation test, cluster α < 0.05, two-sided test) compared to a surrogate distribution representing random occurrence (horizontal line). The dashed vertical line indicates the slow oscillation down peak. The average slow oscillation of each age group is shown in black to illustrate the relation to the slow oscillation phase.
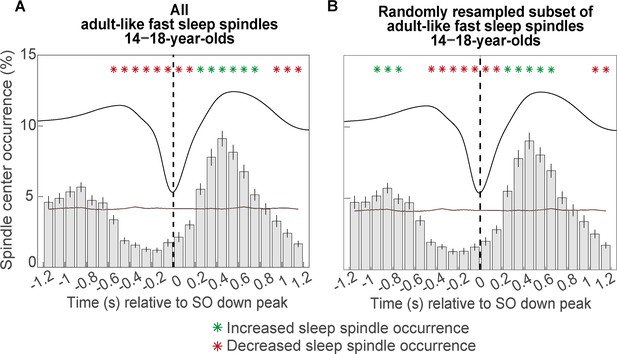
Comparison of the peri-event time histogram results based on (A) all available adult-like fast sleep spindles and (B) when run with a randomly resampled subset of half the number of adult-like fast sleep spindles (100 times randomly resampled and averaged across the 100 resamples).
Peri-event time histogram patterns did look highly similar, indicating that a lower number of sleep spindles does not influence temporal co-occurrence patterns. Error bars represent standard errors. Green asterisks mark increased spindle occurrence (positive cluster, cluster-based permutation test, cluster α < 0.05, two-sided test) and red asterisks mark decreased spindle occurrence (negative cluster, cluster-based permutation test, cluster α < 0.05, two-sided test) compared to a surrogate distribution representing random occurrence (horizontal line). The dashed vertical line indicates the slow oscillation down peak. The average slow oscillation of each age group is shown in black to illustrate the relation to the slow oscillation phase.
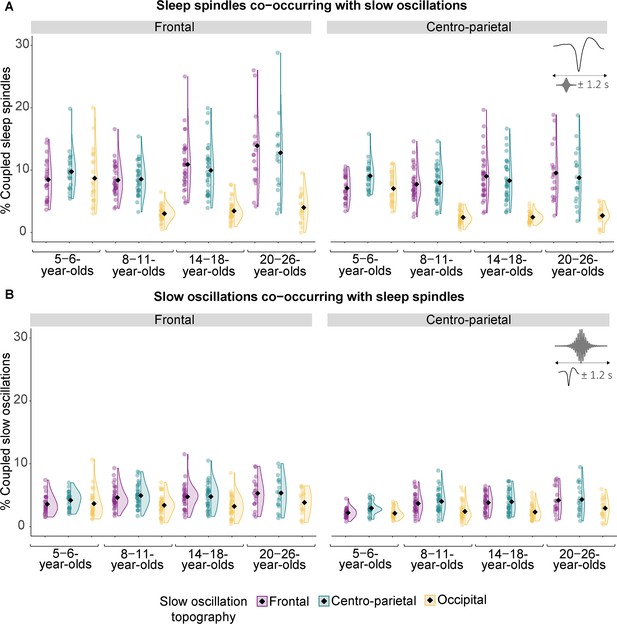
Co-occurrence data of slow frontal and development-specific fast centro-parietal sleep spindles and slow oscillations in different topographical locations.
(A) Percentage of spindle centers occurring within ± 1.2 s around the slow oscillation down peak. (B) Percentage of slow oscillation down peaks occurring within ± 1.2 s around the spindle centers. Diamonds represent estimated marginal means from linear mixed-effects models depicted in Supplementary file 1x–aa.
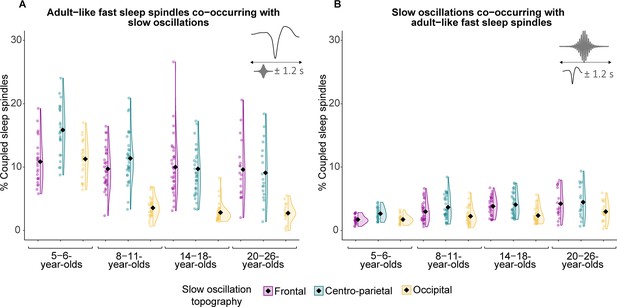
Co-occurrence data of adult-like fast sleep spindles and slow oscillations in different topographical locations.
(A) Percentage of spindle centers occurring within ± 1.2 s around the slow oscillation down peak. (B) Percentage of slow oscillation down peaks occurring within ± 1.2 s around the spindle centers. Diamonds represent estimated marginal means from linear mixed-effects models depicted in Supplementary file 1ab and ac.
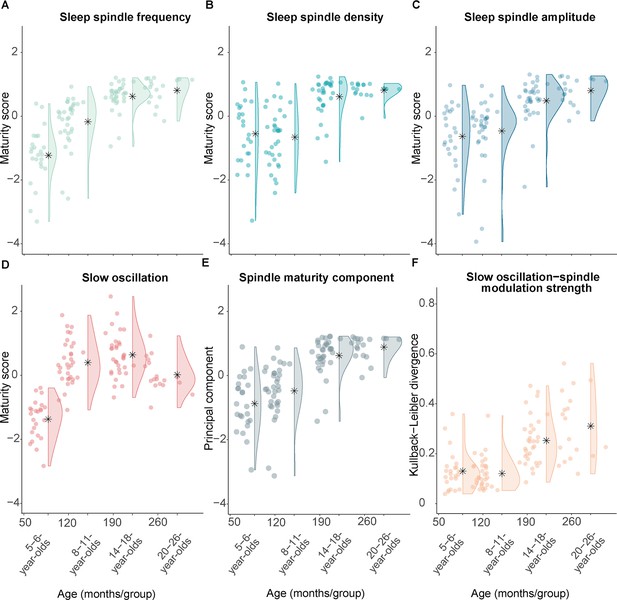
Measures of sleep spindle and slow oscillation maturity and slow oscillation-spindle coupling strength across all age groups.
(A–C) Fast sleep spindle maturity scores for (A) frequency, (B) density, and (C) amplitude. Maturity scores reflect the Z-standardized differences between adult-like and development-specific fast centro-parietal sleep spindles. (D) Slow oscillation maturity scores represent the Z-standardized difference between frontal and centro-parietal amplitudes. (E) First principal component of a principal component analysis on the three fast spindle maturity scores which are shown in (A–C). (F) Kullback-Leibler divergence for development-specific fast centro-parietal sleep spindle modulation during centro-parietal slow oscillations, reflecting slow oscillation-spindle modulation strength. For all measures, higher values are linked to older age. Asterisks illustrate the mean.
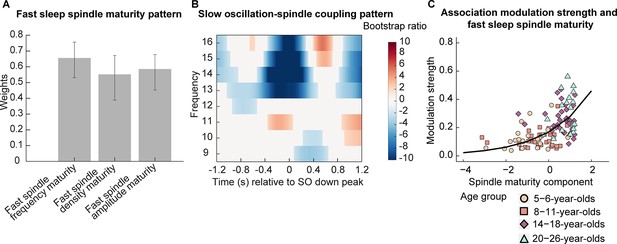
Association between fast spindle maturity and centro-parietal slow oscillation-spindle coupling.
(A–B) Results from a partial least squares correlation revealed one (A) fast spindle maturity profile significantly associated with (B) a centro-parietal slow oscillation-spindle coupling pattern. (A) Weights of the first singular vector dimension of the fast spindle maturity scores. Error bars represent 95% bootstrap confidence intervals. (B) Weights of the first singular vector dimension of the slow oscillation-spindle coupling pattern by means of bootstrap ratios. Only values > 1.96 and < –1.96 are colored. Warmer colors represent higher power and colder colors reflect lower power. (C) Scatterplot of the association between the modulation strength (Kullback-Leibler divergence) of development-specific fast centro-parietal sleep spindles during centro-parietal SOs and the fast spindle maturity component. The curved line represents the prediction from the generalized linear mixed-effects model for the simple effect of the spindle maturity component. For visualization purposes, the four age groups are indicated by different shapes and colors. Results for frontal slow oscillations can be found in Supplementary file 1u and Figure 6—figure supplement 1.
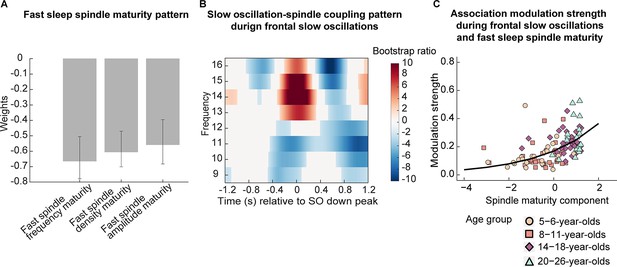
Association between fast spindle maturity and frontal slow oscillation-development-specific fast spindle coupling.
(A–B) Results from a partial least squares correlation revealed one (A) fast spindle maturity profile significantly associated with (B) the frontal slow oscillation-spindle coupling pattern. (A) Weights of the first singular vector dimension of the fast spindle maturity scores. Error bars represent 95% bootstrap confidence intervals. (B) Weights of the first singular vector dimension of the slow oscillation-spindle coupling pattern by means of bootstrap ratios. Only values > 1.96 and < –1.96 are colored. (C) Scatterplot of the association between the modulation strength (Kullback-Leibler divergence) of development-specific fast centro-parietal sleep spindles during frontal SOs and the fast spindle maturity component. The curved line represents the prediction from the generalized linear mixed-effects model for the simple effect of the spindle maturity component (Supplementary file 1u). For visualization purposes, the four age groups are indicated by different shapes and colors.
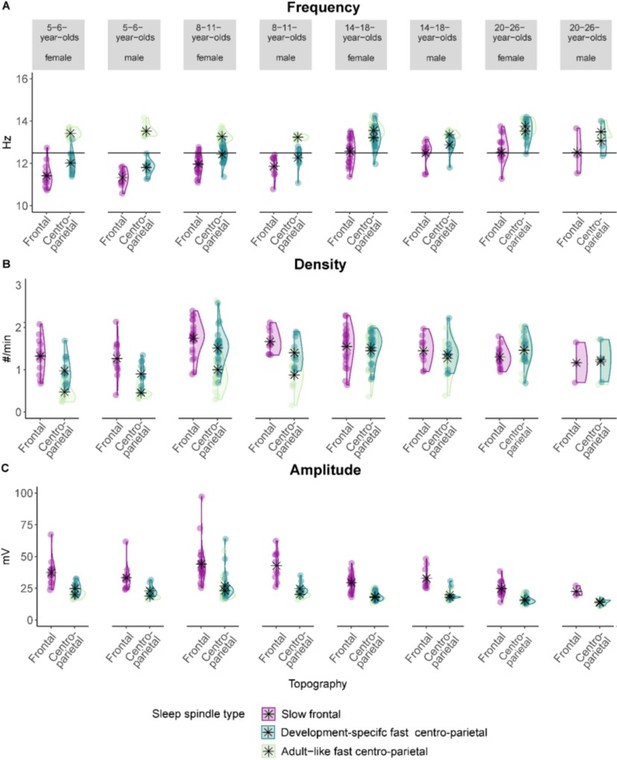
Sleep spindle (A) frequency (B) density, and (C) amplitude for slow frontal, development specific fast centro-parietal, and adult-like fast sleep spindles grouped by age group and sex.
Asterisks represent the mean. Individual data points represent individual participants. Note, specifically for the adolescent and the adult sample, the number of females and males was highly unbalanced.

Slow oscillation (A) frequency (B) density, and (C) amplitude grouped by age group and sex.
Asterisks represent the mean. Individual data points represent individual participants. Note, specifically for the adolescent and the adult sample, the number of females and males was highly unbalanced.
Additional files
-
Supplementary file 1
Complete record of all statistical results and analyses.
(a) Type III analysis of variance table from a linear mixed-effects model on the effects of age group and sleep spindle topography on sleep spindle spectral peak frequency. (b) (A) Post-hoc comparisons for the topography effect on sleep spindle peak frequency based on estimated marginal means (B) Post-hoc comparisons for the age group effect on sleep spindle peak frequency based on estimated marginal means. (c) Type III analysis of variance table from a linear mixed-effects model on the effects of age group and sleep spindle topography on sleep spindle frequency. (d) Post-hoc comparisons for the sleep spindle frequency interaction effect based on estimated marginal means. (e) Type III analysis of variance table from a linear mixed-effects model on the effects of age group and sleep spindle topography on sleep spindle density. (f) Post-hoc comparisons for the sleep spindle density interaction effect based on estimated marginal means. (g) Type III analysis of variance table from a linear mixed-effects model on the effects of age group and sleep spindle topography on sleep spindle amplitude. (h) Post-hoc comparisons for the sleep spindle amplitude interaction effect based on estimated marginal means. (i) Type III analysis of variance table from a linear mixed-effects model on the effects of age group and sleep spindle type (development-specific and adult-like fast sleep spindles) on frequency. (j) Post-hoc comparisons for development-specific and adult-like fast sleep spindle frequency values within the four age groups (interaction effect) based on estimated marginal means. (k) Type III analysis of variance table from a linear mixed-effects model on the effects of age group and sleep spindle type (development-specific and adult-like fast sleep spindles) on density. (l) Post-hoc comparisons for development-specific and adult-like fast sleep spindle density within the four age groups (interaction effect) based on estimated marginal means. (m) Type III analysis of variance table from a linear mixed-effects model on the effects of age group and sleep spindle type (development-specific and adult-like fast sleep spindles) on amplitude. (n) Post-hoc comparisons for development-specific and adult-like fast sleep spindle amplitude within the four age groups (interaction effect) based on estimated marginal means. (o) Type III analysis of variance table from a linear mixed-effects model on the effects of age group and slow oscillation topography on slow oscillation frequency. (p) Post-hoc comparisons for the slow oscillation frequency interaction effect based on estimated marginal means. (q) Type III analysis of variance table from a linear mixed-effects model on the effects of age group and slow oscillation topography on slow oscillation density. (r) Post-hoc comparisons for the effect of age group on slow oscillation density values based on estimated marginal means. (s) Type III analysis of variance table from a linear mixed-effects model on the effects of age group and slow oscillation topography on slow oscillation amplitude. (t) Post-hoc comparisons for the slow oscillation amplitude interaction effect based on estimated marginal means. (u) Results of the generalized linear mixed-effects model on the association between the fast sleep spindle maturity component and frontal slow oscillation-development-specific fast spindle modulation strength. (v) Results of the generalized linear mixed-effects model on the association between the fast sleep spindle maturity component and (A) centro-parietal and (B) frontal slow oscillation-development-specific fast spindle modulation strength while also controlling for age. (w) Sleep architecture. (x) Type III analysis of variance table from a linear mixed-effects model on the effects of age group and slow oscillation topography on the co-occurrence of slow frontal sleep spindles with slow oscillations. (y) Type III analysis of variance table from a linear mixed-effects model on the effects of age group and slow oscillation topography on the co-occurrence of development-specific fast centro-parietal sleep spindles with slow oscillations. (z) Type III analysis of variance table from a linear mixed-effects model on the effects of age group and slow oscillation topography on the co-occurrence of slow oscillations with slow frontal sleep spindles. (aa) Type III analysis of variance table from a linear mixed-effects model on the effects of age group and slow oscillation topography on the co-occurrence of slow oscillations with development-specific fast centro-parietal sleep spindles. (ab) Type III analysis of variance table from a linear mixed-effects model on the effects of age group and slow oscillation topography on the co-occurrence of adult-like fast centro-parietal sleep spindles with slow oscillations. (ac) Type III analysis of variance table from a linear mixed-effects model on the effects of age group and slow oscillation topography on the co-occurrence of slow oscillations with adult-like fast centro-parietal sleep spindles.
- https://cdn.elifesciences.org/articles/83565/elife-83565-supp1-v1.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/83565/elife-83565-mdarchecklist1-v1.pdf