Catalytic activity and autoprocessing of murine caspase-11 mediate noncanonical inflammasome assembly in response to cytosolic LPS
Figures

Caspase-11 catalytic activity and autoprocessing are required for cytosolic lipopolysaccharide (LPS)-induced speck formation.
(A) Schematic representation of Casp11 fluorescent reporter constructs used in this study indicating mCherry fused to the C-terminus of wild-type (WT), catalytically inactive (C254A), or non-cleavable (D285A) Casp11. (B–E) C57BL/6 (B6) or Casp11-/- primary bone marrow-derived macrophages (BMDMs) with the indicated transgenes or empty-vector control were primed with Pam3CSK4 for 4 hr, followed by transfection with LPS (2 μg/mL) from S. enterica serovar Minnesota. (B) Caspase-11-mediated pore formation was assayed and quantified 8 hr later as percentage of B6 cells or Casp11-mCherry-expressing cells that took up the Live/Dead Green Fluorescent dead cell dye (Invitrogen). Nuclei are stained with Hoechst. (C) Casp11-mCherry expression and gasdermin D (GSDMD) processing in response to LPS (2 μg/mL) transfection were assessed (8 hr post-transfection) by western blotting for mCherry and GSDMD as indicated. β-actin was used as a loading control. (D, F) Cells expressing indicated Casp11-mCherry constructs were fixed 6 hr post-LPS transfection (D) or Legionella pneumophila infection (F, MOI (multiplicity of infection) of 50) and prepared for confocal microscopy. Nuclei are stained with Hoechst. White arrows indicate Casp11-mCherry specks. Scale bar, 10 μm. (E, G) Speck formation from cells in (D) and (F), respectively, was quantified as percentage of Casp11-mCherry-expressing cells containing a speck. Each data point represents four image frames (100–150 cells) per well and three wells per condition for a total of 300–450 cells. All error bars represent mean ± SEM of triplicate wells; representative of three independent experiments. ***p<0.001, ****p<0.0001, ns, not significant. Two-way ANOVA with Sidak’s multiple-comparison test.
-
Figure 1—source data 1
Source data for Figure 1B.
Caspase-11-mediated membrane pore formation was assessed by imaging primary bone marrow-derived macrophages (BMDMs) after uptake of Live/Dead green fluorescent dye. Nuclei are stained with Hoechst.
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig1-data1-v1.zip
-
Figure 1—source data 2
Source data for Figure 1C.
Casp11-mCherry expression and gasdermin D (GSDMD) processing in response to lipopolysaccharide (LPS) transfection was assessed by western blotting for mCherry and GSDMD as indicated. β-actin was used as a loading control.
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig1-data2-v1.zip
-
Figure 1—source data 3
Source data for Figure 1D.
Bone marrow-derived macrophages (BMDMs) expressing indicated Casp11-mCherry constructs were fixed post-lipopolysaccharide (LPS) transfection and prepared for confocal microscopy. Nuclei are stained with Hoechst.
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig1-data3-v1.zip
-
Figure 1—source data 4
Source data for Figure 1F.
Bone marrow-derived macrophages (BMDMs) expressing indicated Casp11-mCherry constructs were fixed post-infection with Legionella pneumophila (MOI = 50) and prepared for confocal microscopy. Nuclei are stained with Hoechst.
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig1-data4-v1.zip
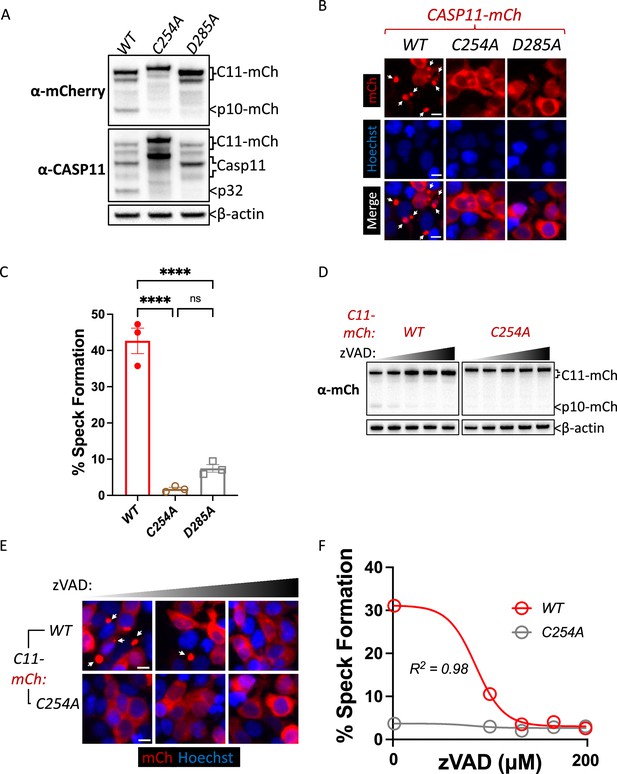
Caspase-11 catalytic activity and autoprocessing at the interdomain linker are required for spontaneous caspase-11 oligomerization in HEK293T cells.
(A) Wild-type (WT), catalytically inactive (C254A), and non-cleavable (D285A) Casp11-mCherry expression plasmids were transfected (0.25 μg) into HEK293T cells. Cell lysates were immunoblotted for mCherry, Casp11, and β-actin (loading control) 10 hr post-transfection. (B) HEK293T cells were transfected with wild-type (WT), catalytically inactive (C254A), or non-cleavable (D285A) Casp11-mCherry as described in ‘Materials and methods’ and imaged by fluorescence microscopy 18 hr post-transfection. Nuclei (blue) were stained with Hoechst, white arrows denote Casp11-mCherry specks. Scale bar = 10 μm. (C) Speck formation in (B) was quantified as percentage of Casp11-mCherry-expressing cells containing at least one speck. (D) HEK293T cells were transfected with Casp11-mCherry constructs as in (B) and 6 hr post-transfection, and cells were incubated with increasing amounts of pan-caspase inhibitor zVAD (0–200 μM; twofold increments). Whole-cell lysates were isolated 12 hr post-transfection and immunoblotted for mCherry or β-actin loading control as indicated. Cleaved p10-mCherry is denoted. (E) Casp11-mCherry speck formation was assayed in zVAD-treated cells by fluorescence microscopy as in (B). (F) Speck formation in (E) was quantified as percentage of Casp11-mCherry-expressing cells containing at least one speck. Dose–response curves in (F) were plotted by least-squares nonlinear regression ([Log2(inhibitor) vs. response (three parameters)]; Y = Bottom + (Top-Bottom)/(1 + 10(X-LogIC50)); R2 indicated). Error bars represent mean ± SEM of triplicate wells (800–900 cells per well); representative of 2–3 independent experiments. Bar graphs in (C) were analyzed by two-way ANOVA with Sidak’s multiple-comparison test, ***p<0.001.
-
Figure 2—source data 1
Source data for Figure 2A.
Indicated Casp11-mCherry expression plasmids were transfected into HEK293T cells. Cell lysates were immunoblotted for mCherry, Casp11, and β-actin (loading control) 10 hr post-transfection.
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig2-data1-v1.zip
-
Figure 2—source data 2
Source data for Figure 2B.
HEK293T cells were transfected with wild-type (WT), catalytically inactive (C254A), or non-cleavable (D285A) Casp11-mCherry and imaged by fluorescence microscopy 18 hr post-transfection. Nuclei (blue) were stained with Hoechst.
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig2-data2-v1.zip
-
Figure 2—source data 3
Source data for Figure 2D.
HEK293T cells were transfected with Casp11-mCherry constructs and 6 hr post-transfection, cells were incubated with increasing amounts of pan-caspase inhibitor zVAD (0–200 μM; twofold increments). Whole-cell lysates were isolated 12 hr post-transfection and immunoblotted for mCherry or β-actin as loading control as indicated. Cleaved p10-mCherry is denoted.
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig2-data3-v1.zip
-
Figure 2—source data 4
Source data for Figure 2E.
Casp11-mCherry speck formation was assayed in zVAD-treated cells by fluorescence microscopy. Nuclei are stained with Hoechst.
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig2-data4-v1.zip
-
Figure 2—source data 5
Source data for Figure 2F.
Speck formation in Figure 2E was quantified as percentage of Casp11-mCherry-expressing cells containing at least one speck. Dose–response curves were plotted by least-squares nonlinear regression ([Log2(inhibitor) vs. response (three parameters)]; Y = Bottom + (Top-Bottom)/(1 + 10(X-LogIC50)); R2 indicated).
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig2-data5-v1.zip
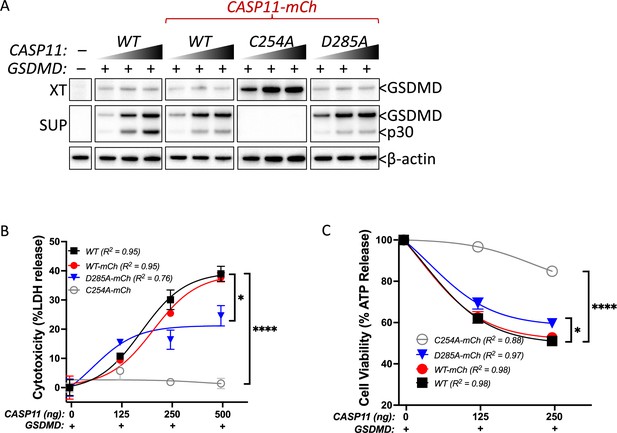
Casp11-mCherry maintains enzymatic function in HEK293T cells.
Gasdermin D (GSDMD) expression plasmid was co-transfected with increasing doses of indicated Casp11 constructs in HEK293T cells. WT untagged Casp11 was included as positive control. After 12 hr (A) or 16 hr (B, C), Casp11 activity was determined by (A) immunoblotting for GSDMD processing in supernatants (sup) and whole-cell lysates (XT), (B) cytotoxicity, and (C) cell viability as indicated in ‘Materials and methods.’ Error bars indicate mean ± SEM of triplicate wells; representative of 2–3 independent experiments. Dose–response curves in (B) were plotted by least-squares nonlinear regression ([Log2(agonist) vs. response (three parameters)]; Y = Bottom + (Top-Bottom)/(1 + 10(LogEC50-X)); R2 indicated). Dose–response curves in (C) were plotted by least-squares nonlinear regression ([Log2(inhibitor) vs. response (three parameters)]; Y = Bottom + (Top-Bottom)/(1 + 10(X-LogIC50)); R2 indicated). Two-way ANOVA with Sidak’s multiple-comparison test (highest doses), *p<0.05, ****p<0.0001.
-
Figure 2—figure supplement 1—source data 1
Source data for Figure 2—figure supplement 1A.
Gasdermin D (GSDMD) expression plasmid was co-transfected with increasing doses of indicated Casp11 construct in HEK293T cells. WT untagged Casp11 was included as positive control.
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig2-figsupp1-data1-v1.zip
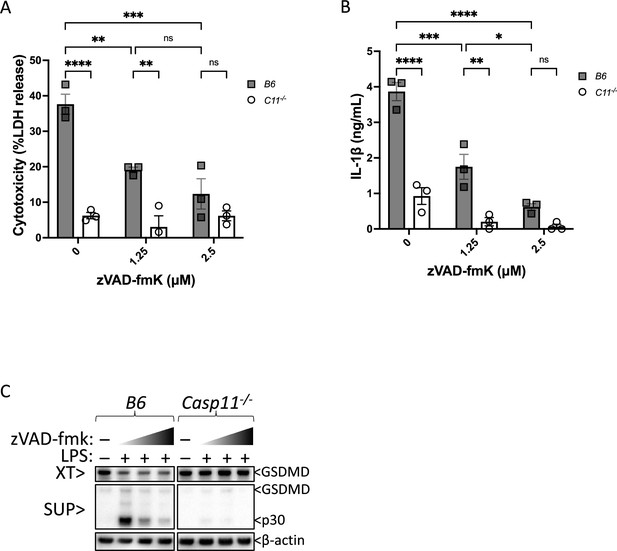
Casp11 catalytic activity mediates gasdermin D (GSDMD) cleavage, pyroptosis, and IL-1β release in response to intracellular lipopolysaccharide (LPS) in primary bone marrow-derived macrophages (BMDMs).
Wild-type (B6) or Casp11-/- primary BMDMs were primed with Pam3CSK4 for 4 hr, followed by transfection with the indicated concentrations of LPS from S. enterica serotype Minnesota. To inhibit Casp11 activity, cells were incubated with indicated concentrations of zVAD beginning 30 min before (and lasting through) LPS transfection. After 16 hr, pyroptosis was measured by (A) assessing supernatants for lactate dehydrogenase (LDH) release (normalized to maximum cell lysis by TritonX-100), (B) analyzing IL-1β release in supernatants by ELISA, and (C) immunoblotting for GSDMD cleavage in supernatants (sup) and whole-cell lysates (XT). β-actin is indicated as loading control. Error bars = mean ± SEM of triplicate wells; representative of 2–3 independent experiments. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ns, not significant. Two-way ANOVA with Sidak’s multiple-comparison test.
-
Figure 2—figure supplement 2—source data 1
Source data for Figure 2—figure supplement 2C.
Immunoblot for gasdermin D (GSDMD) cleavage in supernatants (sup) and whole-cell lysates (XT). β-actin is indicated as loading control.
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig2-figsupp2-data1-v1.zip
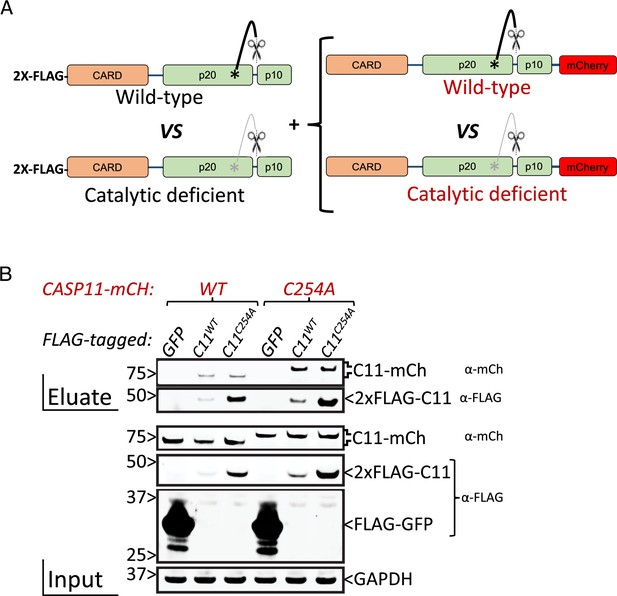
Catalytic activity is not required for caspase-11 intermolecular interactions.
(A) Tagged caspase-11 expression used for FLAG-based co-immunoprecipitation. (B) HEK293T cells were transiently transfected with 2X-FLAG-tagged wild-type (WT) or catalytically inactive (C254A) Casp11 expression plasmids alongside WT or C254A mCherry-tagged Casp11 (5 μg). 48 hr post-transfection, whole-cell lysates were immunoprecipitated by anti-FLAG antibodies as described in ‘Materials and methods,’ and immunoblotted for mCherry, FLAG, or GAPDH as a loading control, as indicated.
-
Figure 2—figure supplement 3—source data 1
Source data for Figure 2—figure supplement 3B.
HEK293T cells were transiently transfected with 2X-FLAG-tagged wild-type (WT) or catalytically inactive Casp11 expression plasmids alongside WT or C254A mCherry-tagged Casp11 (5 μg). 48 hr post-transfection, whole-cell lysates were immunoprecipitated by anti-FLAG antibodies as described in ‘Materials and methods,’ and immunoblotted for mCherry, FLAG, or GAPDH as a loading control, as indicated.
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig2-figsupp3-data1-v1.zip
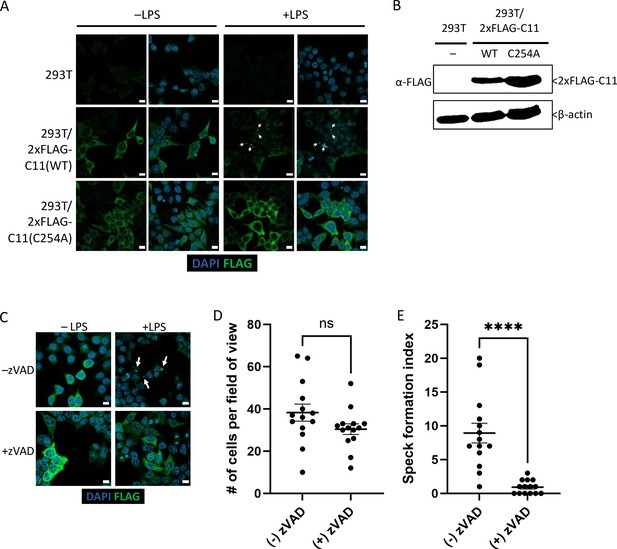
Caspase-11 catalytic activity is required for lipopolysaccharide (LPS)-induced oligomerization in HEK293T cells.
(A) HEK293T cells stably expressing 2xFLAG-Casp11 (WT) or 2xFLAG-Casp11 (C254A) were transfected with LPS (1 μg/mL) from S. enterica serovar Minnesota and imaged by fluorescence microscopy 24 hr post-transfection. Casp11 was stained with anti-FLAG-FITC (green), and nuclei (blue) were stained with DAPI. White arrows denote Casp11 specks. Scale bar = 10 μm. (B) Casp11 protein levels in each stable HEK293T cell line were assayed by immunoblotting for FLAG or β-actin (loading control). (C) HEK293T cells stably expressing WT 2xFLAG-Casp11 were transfected with LPS as in (A), in the presence of pan-caspase inhibitor zVAD (200 μM). Casp11 was stained by immunofluorescence using anti-FLAG-FITC (green) and imaged by confocal microscopy (×63 objective). Nuclei (blue) were stained with DAPI. White arrows denote Casp11 specks. Scale bar = 10 μm. Speck formation in (C) was quantified by (D) number of cells per field of view, and (E) number of cells per field of view with at least one speck, denoted as ‘speck formation index.’ Error bars represent mean ± SEM of 14 fields of view (500 cells) from three independent experiments. Data were analyzed by Student’s t-test, ns, not significant, ****p<0.0001.
-
Figure 3—source data 1
Source data for Figure 3B.
2xFLAG-Casp11 protein levels in each stable HEK293T cell line were determined by immunoblotting for FLAG. β-actin was used as loading control.
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig3-data1-v1.zip
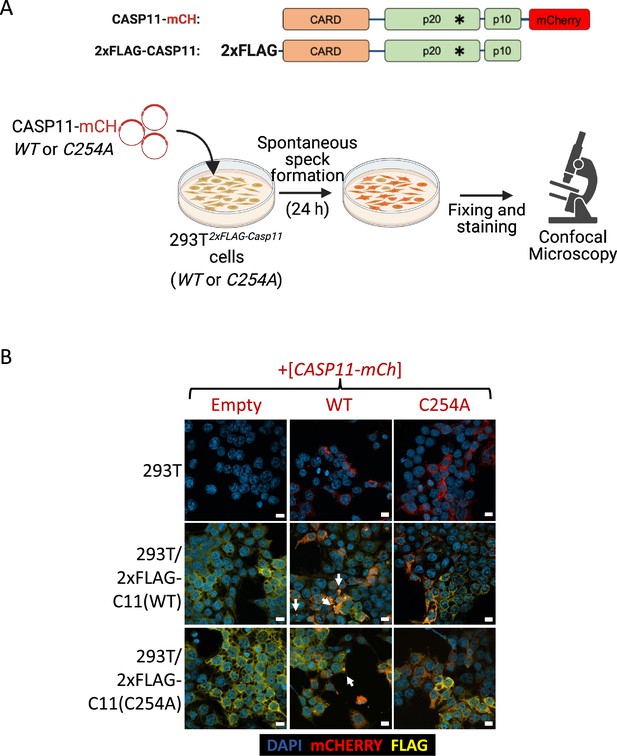
N- and C-terminal caspase-11 subunits co-localize in caspase-11 specks.
(A) Schematic representing experimental design of colocalization experiment (created with Biorender.com). (B) HEK293T cells stably expressing wild-type (WT) or catalytically inactive (C254A) 2xFLAG-Casp11 were transiently transfected with mCherry-tagged WT or C254A Casp11 DNA constructs for 24 hr. Stably expressed 2xFLAG-Casp11 was stained by immunofluorescence using anti-FLAG (yellow) and cells were imaged by confocal microscopy (×63 objective). Nuclei (blue) are stained with DAPI. White arrows denote Casp11 specks. Scale bars = 10 µM. Images are representative of ≥3 fields of view per condition (150–200 cells) and ≥3 independent biological replicates.
-
Figure 3—figure supplement 1—source data 1
Source data for Figure 3—figure supplement 1B.
HEK293T cells stably expressing wild-type (WT) or catalytically inactive (C254A) 2xFLAG-Casp11 were transiently transfected with mCherry-tagged WT or C254A Casp11 constructs for 24 hr. Stably expressed 2xFLAG-Casp11 was stained by immunofluorescence using anti-FLAG (yellow) and cells were imaged by confocal microscopy (×63 objective). Nuclei (blue) are stained with DAPI.
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig3-figsupp1-data1-v1.zip
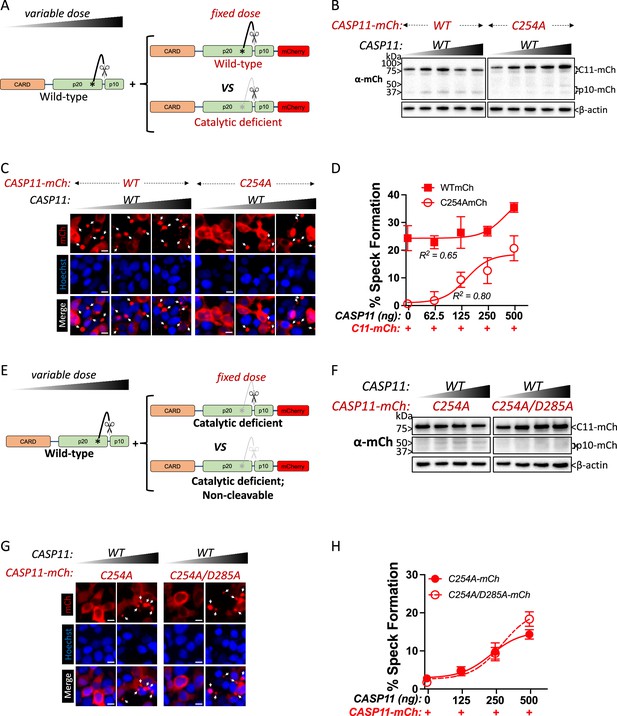
Wild-type caspase-11 recruits catalytically inactive caspase-11 to speck complexes independently of trans-processing.
(A, E) Schematic diagram indicating co-transfection combinations used in (B–H). Untagged full-length wild-type (WT) caspase-11 gene constructs were transfected at increasing doses, together with a fixed amount of indicated mCherry-tagged Casp11. (B) 12 hr post-transfection, whole-cell lysates were harvested and immunoblotted for mCherry or β-actin as a loading control. (C) 18 hr following transfection, the cells were imaged by fluorescence microscopy. Nuclei (blue) are stained with Hoechst, white arrows represent Casp11 oligomers (specks), scale bar = 10 μm. (D) Speck formation was quantified as percentage of mCherry-expressing cells containing at least one speck. (F) Whole-cell lysates of indicated transfected cells was assayed as in (B). (G) Cells transfected with indicated constructs were imaged as in (C). (H) Speck formation with respect to increasing levels of WT Casp11 was plotted as in (D). Error bars indicate mean ± SEM of triplicate wells (800–900 cells per well); representative of 2–3 independent experiments. Dose–response curves were plotted by least-squares nonlinear regression ([Log2(agonist) vs. response (three parameters)]; Y = Bottom + (Top-Bottom)/(1 + 10(LogEC50-X)); R2 indicated).
-
Figure 4—source data 1
Source data for Figure 4B.
12 hr post-transfection of indicated plasmids, whole-cell lysates were harvested and immunoblotted for mCherry or β-actin as a loading control.
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig4-data1-v1.zip
-
Figure 4—source data 2
Source data for Figure 4C.
Untagged full-length wild-type (WT) caspase-11 gene constructs were transfected at increasing doses, together with a fixed amount of indicated mCherry-tagged Casp11. 18 hr following transfection, the cells were imaged by fluorescence microscopy. Nuclei (blue) were stained with Hoechst.
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig4-data2-v1.zip
-
Figure 4—source data 3
Source data for Figure 4D.
Speck formation in Figure 4C was quantified as percentage of Casp11-mCherry-expressing cells containing at least one speck. Dose–response curves were plotted by least-squares nonlinear regression ([Log2(agonist) vs. response (three parameters)]; Y = Bottom + (Top-Bottom)/(1 + 10(LogEC50-X))).
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig4-data3-v1.zip
-
Figure 4—source data 4
Source data for Figure 4F.
Whole-cell lysates of indicated transfected cells were harvested and immunoblotted for mCherry. β-actin was used as loading control.
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig4-data4-v1.zip
-
Figure 4—source data 5
Source data for Figure 4G.
HEK293T cells transfected with indicated Casp11 constructs were imaged by fluorescence microscopy. Nuclei (blue) were stained with Hoechst.
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig4-data5-v1.zip
-
Figure 4—source data 6
Source data for Figure 4H.
Speck formation in Figure 4G was quantified as percentage of Casp11-mCherry-expressing cells containing at least one speck. Dose–response curves were plotted by least-squares nonlinear regression ([Log2(agonist) vs. response (three parameters)]; Y=Bottom + (Top-Bottom)/(1+10(LogEC50-X))).
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig4-data6-v1.zip
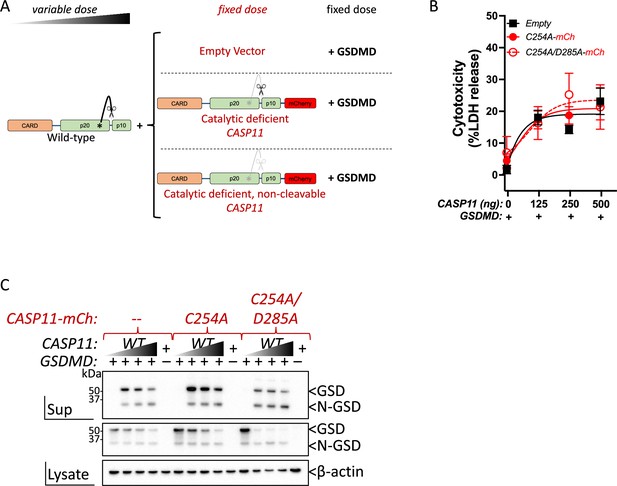
Caspase-11 activity remains intact despite co-transfection with catalytically inactive caspase-11.
(A) Schematic indicating plasmids used in co-transfection studies. (B, C) Unlabeled wild-type (WT) Casp11 was transfected into HEK293T cells at increasing doses, together with a fixed dose (0.25 μg) of indicated mCherry-tagged Casp11 construct, and a fixed dose of murine gasdermin D (GSDMD, 0.05 μg). 14 hr post-transfection, Casp11-mediated cytotoxicity was measured by (B) determining percent lactate dehydrogenase (LDH) release, and (C) immunoblotting for GSDMD cleavage in supernatants (sup) and whole-cell lysates. β-actin is indicated as loading control. Error bars = mean ± SEM of triplicate wells; representative of two independent experiments. Dose–response curves in (B) were plotted by least-squares nonlinear regression ([Log2(agonist) vs. response (three parameters)]; Y = Bottom + (Top-Bottom)/(1 + 10(LogEC50-X))).
-
Figure 4—figure supplement 1—source data 1
Source data for Figure 4—figure supplement 1B.
Unlabeled wild-type (WT) Casp11 was transfected into HEK293T cells at increasing doses, together with a fixed dose (0.25 μg) of indicated mCherry-tagged Casp11 construct and a fixed dose of murine gasdermin D (GSDMD, 0.05 μg). 14 hr post-transfection, Casp11-mediated cytotoxicity was measured by determining percent lactate dehydrogenase (LDH) release. Dose–response curves in (B) were plotted by least-squares nonlinear regression ([Log2(agonist) vs. response (three parameters)]; Y = Bottom + (Top-Bottom)/(1 + 10(LogEC50-X))).
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig4-figsupp1-data1-v1.zip
-
Figure 4—figure supplement 1—source data 2
Source data for Figure 4—figure supplement 1C.
HEK293T whole-cell lysates and supernatants (sup) from Figure 4—figure supplement 1B were immunoblotted for gasdermin D (GSDMD). β-actin is indicated as loading control.
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig4-figsupp1-data2-v1.zip
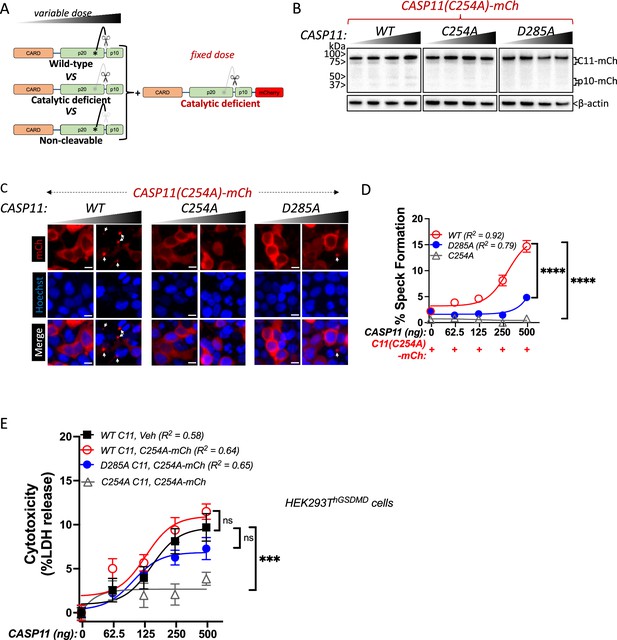
Caspase-11 autoprocessing mediates noncanonical inflammasome assembly.
(A) Schematic diagram indicating co-transfection combinations used in (B–D). Unlabeled wild-type (WT), catalytically inactive (C254A), or non-cleavable (D285A) caspase-11 constructs were transfected into HEK293T cells at increasing doses, together with a fixed dose of catalytically inactive (C254A) mCherry-tagged caspase-11. (B) 12 hr post-transfection, whole-cell lysates were harvested and immunoblotted for mCherry or β-actin as loading control. (C) 18 hr following transfection, cells were imaged by fluorescence microscopy. Nuclei (blue) are stained with Hoechst, white arrows denote caspase-11 specks, scale bar = 10 μm. (D) Speck formation was quantified as percentage of mCherry-expressing cells containing at least one speck. (E) HEK293T cells stably expressing human gasdermin D (hGSDMD) were transiently transfected with a fixed dose of empty plasmid (Veh) OR mCherry-tagged C254A caspase-11, plus increasing doses of unlabeled WT, C254A, or D285A caspase-11 constructs. Cytotoxicity was measured as percent lactate dehydrogenase (LDH) release 18 hr post-plasmid transfection. All error bars = mean ± SEM of triplicate wells (800–900 cells per well); representative of three independent experiments. Dose–response curves were plotted by least-squares nonlinear regression ([Log2(agonist) vs. response (three parameters)]; Y = Bottom + (Top-Bottom)/(1 + 10(LogEC50-X)); R2 indicated). Two-way ANOVA (highest doses) with Sidak’s multiple-comparison test, ns, not significant, ***p<0.001, ****p<0.0001.
-
Figure 5—source data 1
Source data for Figure 5B.
Unlabeled wild-type (WT), catalytically inactive (C254A), or non-cleavable (D285A) caspase-11 constructs were transfected into HEK293T cells at increasing doses, together with a fixed dose of catalytically inactive (C254A) mCherry-tagged caspase-11. 12 hr post-transfection, whole-cell lysates were harvested and immunoblotted for mCherry or β-actin as loading control.
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig5-data1-v1.zip
-
Figure 5—source data 2
Source data for Figure 5C.
Unlabeled wild-type (WT), catalytically inactive (C254A), or non-cleavable (D285A) caspase-11 constructs were transfected into HEK293T cells at increasing doses, together with a fixed dose of catalytically inactive (C254A) mCherry-tagged caspase-11. 18 hr following transfection, cells were imaged by fluorescence microscopy. Nuclei (blue) are stained with Hoechst.
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig5-data2-v1.zip
-
Figure 5—source data 3
Source data for Figure 5D.
Speck formation in (C) was quantified as percentage of mCherry-expressing cells containing at least one speck. Dose–response curves were plotted by least-squares nonlinear regression ([Log2(agonist) vs. response (three parameters)]; Y = Bottom + (Top-Bottom)/(1 + 10(LogEC50-X))).
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig5-data3-v1.zip
-
Figure 5—source data 4
Source data for Figure 5E.
HEK293T cells stably expressing human gasdermin D were transiently transfected with a fixed dose of empty plasmid (Veh) OR mCherry-tagged C254A caspase-11, plus increasing doses of unlabeled wild-type (WT), catalytically inactive (C254A), or non-cleavable (D285A) caspase-11 constructs. Cytotoxicity was measured as percent lactate dehydrogenase (LDH) release 18 hr post-plasmid transfection (with respect to 1% Triton X-100-induced cytotoxicity). Dose–response curves were plotted by least-squares nonlinear regression ([Log2(agonist) vs. response (three parameters)]; Y = Bottom + (Top-Bottom)/(1 + 10(LogEC50-X)); R2 indicated).
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig5-data4-v1.zip

Catalytic activity is required for caspase-11 speck formation downstream of homodimerization.
(A) Schematic representation of fluorescent Casp11 constructs that allow for inducible dimerization by the chemical dimerizer AP20187 (created with BioRender.com). (B) HEK293T cells were transfected with WT or catalytically inactive (C254A) DmrB-(ΔCARD)-Casp11-FLAG-mCherry constructs. 24 hr post-transfection, cells were incubated with AP20187 (1 μM) for 6 hr and imaged by confocal microscopy. Nuclei (blue) are stained with Hoechst. White arrows indicate Casp11-mCherry specks. Scale bar, 15 μm. (C) Speck formation in (B) was quantified as percentage of mCherry-expressing cells containing at least one speck. (D) The same constructs in (A–C) were transiently transfected into HEK293T cells stably expressing human gasdermin D (HEK293ThGSDMD) and incubated in AP20187 (1 μM) for 6 hr. Lysates were immunoblotted for GSDMD and mCherry, with GAPDH as loading control. Error bars represent mean ± SEM of triplicate wells (800–900 cells per well); representative of three independent experiments. Data were analyzed by two-way ANOVA with Sidak’s multiple-comparison test, ***p<0.001, ****p<0.0001.
-
Figure 6—source data 1
Source data for Figure 6B.
HEK293T cells were transfected with WT or catalytically inactive (C254A) DmrB-(ΔCARD)-Casp11-FLAG-mCherry constructs. 24 hr post-transfection, cells were incubated with AP20187 (1 μM) for 6 hr and imaged by confocal microscopy. Nuclei (blue) are stained with Hoechst.
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig6-data1-v1.zip
-
Figure 6—source data 2
Source data for Figure 6D.
HEK293T cells stably expressing human gasdermin D (HEK293ThGSDMD) were transfected with WT or catalytically inactive (C254A) DmrB-(ΔCARD)-Casp11-FLAG-mCherry constructs, and incubated in AP20187 (1 μM) for 6 hr. Lysates were harvested and immunoblotted for GSDMD and mCherry, with GAPDH as loading control.
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig6-data2-v1.zip
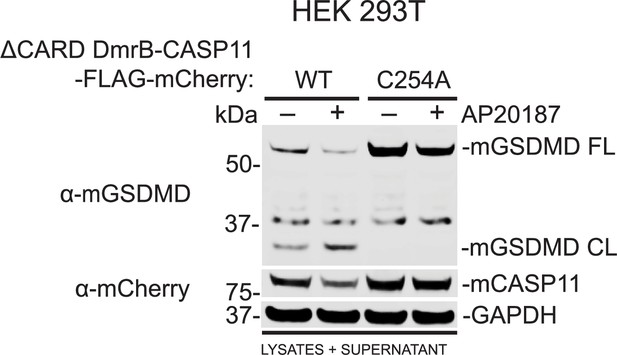
Inducible dimerization promotes Casp11 enzymatic activity.
HEK293T cells were transiently co-transfected with ΔCARD DmrB-Casp11-FLAG-mCherry constructs (0.1 μg; Figure 4A) and murine gasdermin D (GSDMD, 0.01 μg) for 24 hr before the addition of AP20187 (1 μM) for 6 hr. Casp11 protein expression and cytotoxicity were determined by immunoblotting for mCherry and GSDMD in pooled lysates and supernatants. GAPDH is indicated as loading control. FL = full-length; CL = cleaved.
-
Figure 6—figure supplement 1—source data 1
Source data for Figure 6—figure supplement 1.
HEK293T cells were transiently co-transfected with ΔCARD DmrB-Casp11-FLAG-mCherry constructs (0.1 μg; Figure 4A) and murine gasdermin D (GSDMD, 0.01 μg) for 24 hr before the addition of AP20187 (1 μM) for 6 hr. Casp11 protein expression and cytotoxicity were determined by immunoblotting for mCherry and GSDMD in pooled lysates and supernatants. GAPDH is indicated as loading control. FL = full-length; CL = cleaved.
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig6-figsupp1-data1-v1.zip
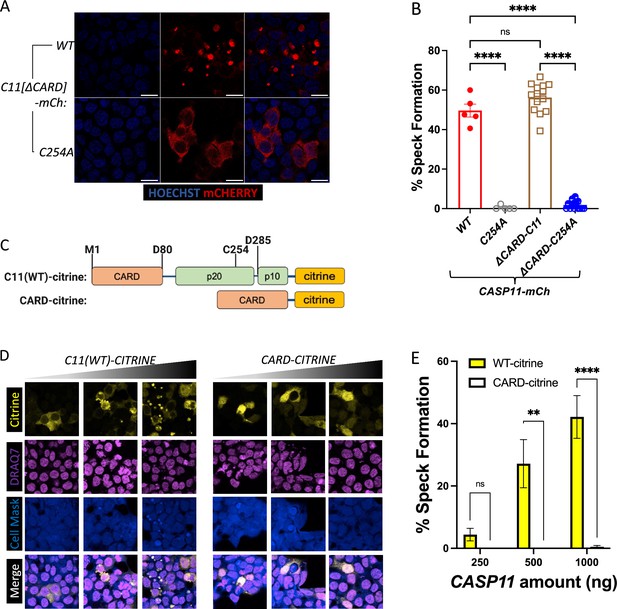
Casp11 CARD is neither necessary nor sufficient to mediate spontaneous Casp11 oligomerization in HEK293T cells.
(A) HEK293T cells were transfected with indicated Casp11-mCherry constructs. 18 hr post-transfection, cells were fixed and prepared for microscopy. Nuclei are stained with Hoechst. White arrows indicate Casp11 mCherry specks. Scale bar, 15 μm. (B) Speck formation in (A) was quantified as percentage of overall mCherry-expressing cells containing at least one speck. (C) Schematic diagram describing fluorescent reporter constructs used in (D, E). (D) The fluorescent reporter citrine was fused to the C-terminus of wild-type (WT) or CARD-only Casp11, transfected into HEK293T cells as indicated, and imaged by confocal microscopy 18 hr post-transfection. Nuclei (magenta) were stained with DRAQ7, cytosolic content (blue) was stained with cell tracer violet. (E) Casp11-citrine speck formation (yellow) was quantified as percentage of overall Casp11-citrine-expressing cells that contained at least one speck. Error bars represent mean ± SEM of triplicate wells (800–900 cells per well); a single experiment was performed. Two-way ANOVA with Sidak’s multiple-comparison test, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ns, not significant.
-
Figure 6—figure supplement 2—source data 1
Source data for Figure 6—figure supplement 2A.
HEK293T cells were transfected with indicated Casp11-mCherry constructs. 18 hr post-transfection, cells were fixed and prepared for microscopy. Nuclei were stained with Hoechst.
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig6-figsupp2-data1-v1.zip
-
Figure 6—figure supplement 2—source data 2
Source data for Figure 6—figure supplement 2D.
The fluorescent reporter citrine was fused to the C-terminus of wild-type (WT) or CARD-only Casp11, transfected into HEK293T cells as indicated, and imaged by confocal microscopy 18 hr post-transfection. Nuclei (magenta) were stained with DRAQ7, cytosolic content (blue) was stained with cell tracer violet.
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig6-figsupp2-data2-v1.zip
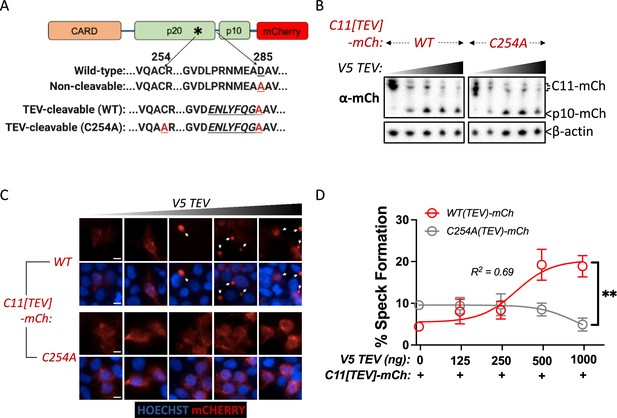
Caspase-11 processing at the interdomain linker functions together with catalytic activity to mediate higher-order caspase-11 oligomerization.
(A) Schematic indicating Casp11-mCherry constructs that allow for inducible processing by tobacco etch virus (TEV) protease. The TEV protease consensus cleavage sequence (ENLYFQ/G) replaced the endogenous cleavage site in Casp11-mCherry constructs with preserved (WT) or mutated (C254A) catalytic activity. (B) HEK293T cells were transfected with the indicated TEV-cleavable Casp11-mCherry constructs, together with increasing doses of V5-TEV protease (0–500 ng). Whole-cell lysates were harvested 12 hr post-transfection and immunoblotted for mCherry or β-actin (loading control). (C, D) 18 hr post-transfection, cells were imaged by fluorescence microscopy and speck formation was quantified as percentage of Casp11-mCherry-expressing cells containing at least one speck. White arrows indicate Casp11-mCherry specks. Scale bar, 10 μm. Error bars represent mean ± SEM of triplicate wells (800–900 cells per well); representative of three independent experiments. Dose–response curves were plotted by least-squares nonlinear regression ([Log2(agonist) vs. response (three parameters)]; Y = Bottom + (Top-Bottom)/(1 + 10(LogEC50-X)); R2 indicated). Data were analyzed by two-way ANOVA with Sidak’s multiple-comparison test (highest doses), **p<0.01.
-
Figure 7—source data 1
Source data for Figure 7B.
HEK293T cells were transfected with the indicated TEV-cleavable Casp11-mCherry constructs, together with increasing doses of V5-TEV protease (0–500 ng/well). Whole-cell lysates were harvested 12 hr post-transfection and immunoblotted for mCherry or β-actin (loading control).
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig7-data1-v1.zip
-
Figure 7—source data 2
Source data for Figure 7D.
HEK293T cells from (B) were imaged by fluorescence microscopy and speck formation was quantified as percentage of Casp11-mCherry-expressing cells containing at least one speck. Dose–response curves were plotted by least-squares nonlinear regression ([Log2(agonist) vs. response (three parameters)]; Y = Bottom + (Top-Bottom)/(1 + 10(LogEC50-X)); R2 indicated).
- https://cdn.elifesciences.org/articles/83725/elife-83725-fig7-data2-v1.zip

Proposed mechanism of caspase-11 inflammasome assembly (created with BioRender.com).
Additional files
-
Supplementary file 1
Tables of plasmids, cell lines and oligonucleotides used in this study.
- https://cdn.elifesciences.org/articles/83725/elife-83725-supp1-v1.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/83725/elife-83725-transrepform1-v1.pdf





