Kazrin promotes dynein/dynactin-dependent traffic from early to recycling endosomes
Figures
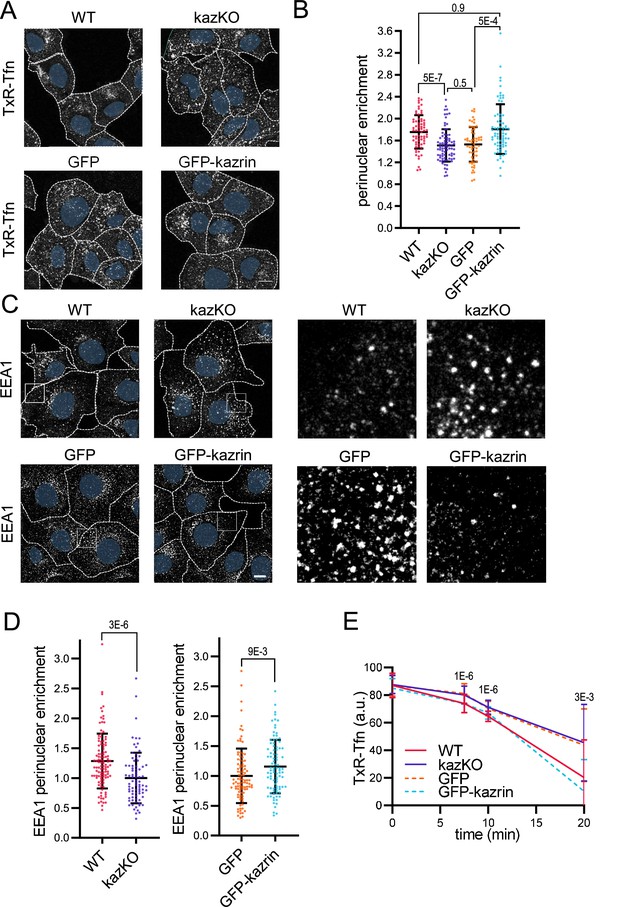
Kazrin depletion impairs endosomal traffic.
(A) Confocal images of wild-type (WT) and kazKO MEF or kazKO MEF expressing low levels (See Materials and methods (M & M)) of GFP or GFP-kazrin C, incubated with Texas Red-Tfn (TxR-Tfn) at 16 °C and chased at 37 °C for 10 min. Scale bar, 10 μm. Cell borders are indicated by dashed lines and nuclei in blue. (B) Scattered plot of the mean ± SD (Standard deviation) TxR-Tfn perinuclear enrichment (See M & M) for the cells described in A, after 10 min incubation at 37 °C. p-values of the two-tailed Mann-Whitney tests are shown. n>58 cells for each sample. Refer also to Figure 1—figure supplement 1 for the effects of kazrin depletion in Cos7 cells, Figure 1—figure supplement 2 for the strategy of kazKO MEF generation, Figure 1—figure supplement 3A for pericentriolar localization of internalized transferrin (Tfn) in WT cells, and Figure 1—figure supplement 4 for the effects of kazrin depletion on TxR-Tfn uptake in MEF. (C) Confocal images of the WT and kazKO MEF, or kazKO MEF expressing low levels of GFP or GFP-kazrin C, fixed and stained with anti-EEA1 and A568-conjugated secondary antibodies. A 17 μm2 magnified insets showing endosomes in the peripheral areas are shown on the right. Scale bar, 10 μm. Cell borders are indicated with dashed lines and nuclei in blue. (D) Scattered plots of the mean ± SD early endosome autoantigen 1 (EEA1) perinuclear enrichment (See M & M) in the cells described in C. The values were normalized to the corresponding kazKO cells (either kazKO or kazKO GFP). p-values of the two-tailed Mann-Whitney tests are shown. n>80 cells for each sample. Refer to Figure 1—figure supplement 3B for pericentriolar localization of EEA1 in WT cells and Figure 1—figure supplement 4 for the effects of kazrin depletion on the RAB11 perinuclear enrichment. (E) Line plot of the mean ± SD TxR-Tfn fluorescence intensity per cell in WT and kazKO MEFs, or kazKO MEFs expressing low levels of GFP and GFP-kazrin C, at the indicated time points after loading early endosomes (EEs) with TxR-Tfn at 16 °C and release at 37 °C to allow recycling (See M & M for further details). Data were normalized to the average intensity at time 0. p-values of the two-tailed Student t-tests are shown. n>16 cells per sample and time point.
-
Figure 1—source data 1
Data for graphs presented in Figure 1B, D and E.
- https://cdn.elifesciences.org/articles/83793/elife-83793-fig1-data1-v2.zip
Kazrin depletion in Cos7 cells alters endosomal trafficking.
-
Figure 1—figure supplement 1—source data 1
Un-cropped blots for Figure 1—figure supplement 1A.
- https://cdn.elifesciences.org/articles/83793/elife-83793-fig1-figsupp1-data1-v2.zip
-
Figure 1—figure supplement 1—source data 2
Data for graphs presented in Figure 1—figure supplement 1C.
- https://cdn.elifesciences.org/articles/83793/elife-83793-fig1-figsupp1-data2-v2.zip
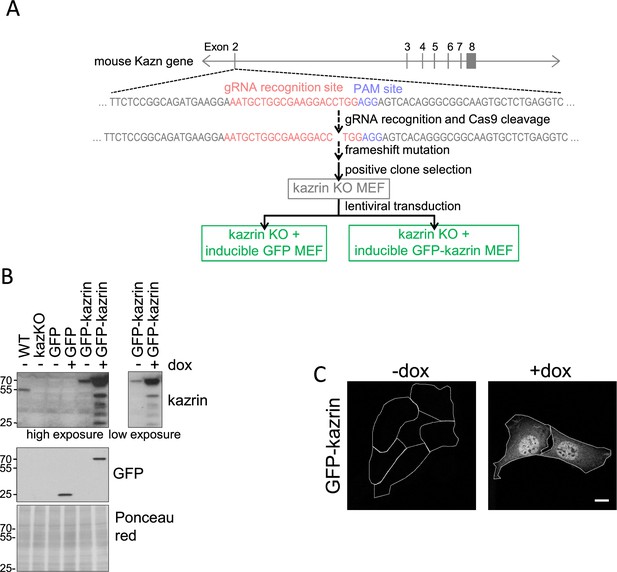
Generation of kazKO MEF and kazKO MEF expressing GFP or GFP-kazrin C.
(A) Strategy for the establishment of kazKO MEFs created with the CRISPR-Cas9 technology. The gRNA was designed to recognize a sequence at the beginning of exon 2 of the mouse Kazn gene, after the initiation codon of kazrin C, and followed by a PAM site. The Cas9 nuclease gene was transfected in a plasmid into the cells, together with the gRNA. Cas9 cleavage often leads to a frameshift mutation that impedes the expression of the gene. The plasmid encoding the gRNA and the Cas9 also encodes GFP, which allows sorting and isolation of transfected cells by FACS. The resulting clones were analyzed by immunoblot, and those with no kazrin expression were selected. One of them was used as the base for another three cell lines in which genes encoding GFP or GFP-kazrin C were inserted in the genome. The inserted constructs were preceded by a tetracycline-response element. This was achieved by lentiviral transduction and selection by FACS. Thus, none of these cell lines have endogenous kazrin expression but express GFP or GFP-kazrin C upon doxycycline addition. (B) Immunoblots of cell lysates from wild-type (WT) and kazKO MEF or kazKO MEFs expressing GFP and GFP-kazrin C, in the presence (+) or absence (−) of 5 µg/ml doxycycline for 24 hr (dox). The membranes were probed with a polyclonal rabbit serum against the N-terminus of kazrin C, anti-GFP, or stained with Ponceau red (as a loading control). A high and a low exposure for the kazrin signal are shown. (C) Confocal images of GFP-kazrin C kazKO MEFs in the presence (+) or absence (−) of 5 µg/ml doxycycline for 24 hr (dox). Scale bar, 10 µm.
-
Figure 1—figure supplement 2—source data 1
Un-cropped blots for Figure 1—figure supplement 2B.
- https://cdn.elifesciences.org/articles/83793/elife-83793-fig1-figsupp2-data1-v2.zip
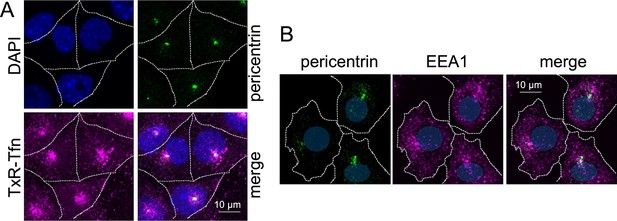
Texas Red-Tfn (TxR-Tfn) and early Endosomes endosomes (EEs) concentrate in a pericentriolar juxtanuclear region in WT MEF.
(A) MIP of confocal fluorescence images of WT MEF loaded with TxR-Tfn for 10 min, fixed and stained with DAPI and with rabbit anti-pericentrin and secondary A488-conjugated antibodies. The individual channels and the merge are shown. (B) MIP of confocal fluorescence images of WT MEF, fixed and stained with rabbit anti-pericentrin and mouse anti-early endosome Autoantigen 1 (EEA1) andtibodies and secondary A488 and A568-conjugated antibodies, respectively. The individual channels and the merge are shown. Dashed lines indicate the cell periphery and nuclei are in blue.
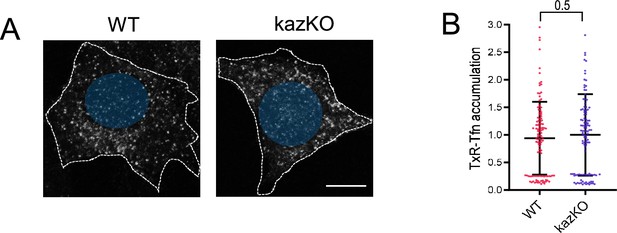
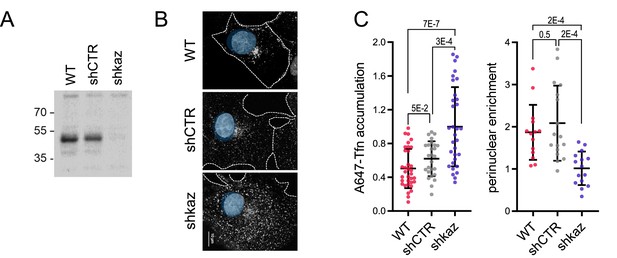
Depletion of kazrin does not impair Texas Red-Tfn (TxR-Tfn) uptake.
(A) Confocal images of wild-type (WT) and kazKO MEF incubated for 10 min with 20 µg/ml TxR-Tfn at 37 °C. Scale bar, 10 µm. (B) Scattered plot of the mean ± SD TxR-Tfn fluorescence intensity per cell for WT and kazKO cells. The fluorescence intensity in WT and kazKO cells was normalized to the mean value of the kazKO cells. The p-value of a Mann-Whitney test is shown. n>100 cells for each sample.
-
Figure 1—figure supplement 4—source data 1
Data for graph presented in Figure 1—figure supplement 4B.
- https://cdn.elifesciences.org/articles/83793/elife-83793-fig1-figsupp4-data1-v2.zip
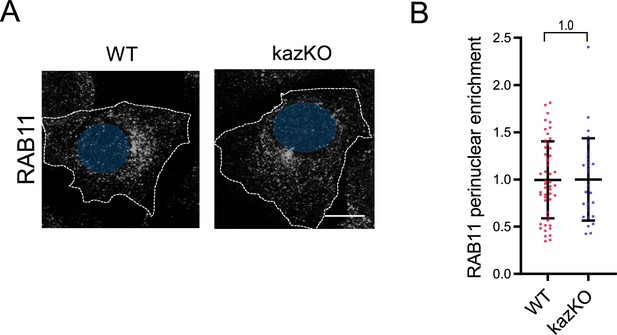
Depletion of kazrin does not significantly alter the distribution of Ras-Related in Brain 11 (RAB11) compartments.
(A) Confocal images of wild-type (WT) and kazKO MEFs, fixed and stained with anti-RAB11 and A488-conjugated secondary antibodies. The dashed lines indicate the cell periphery and nuclei are in blue. Scale bar, 10 µm. (B) Scattered plot of the mean ± SD RAB11 perinuclear enrichment (See M & M for further details) for WT and kazKO MEF. The data were normalized to the mean of the kazKO cells. p-values of two-tailed Student t-tests are shown. n>29 cells for each sample.
-
Figure 1—figure supplement 5—source data 1
Data for graph presented in Figure 1—figure supplement 5B.
- https://cdn.elifesciences.org/articles/83793/elife-83793-fig1-figsupp5-data1-v2.zip
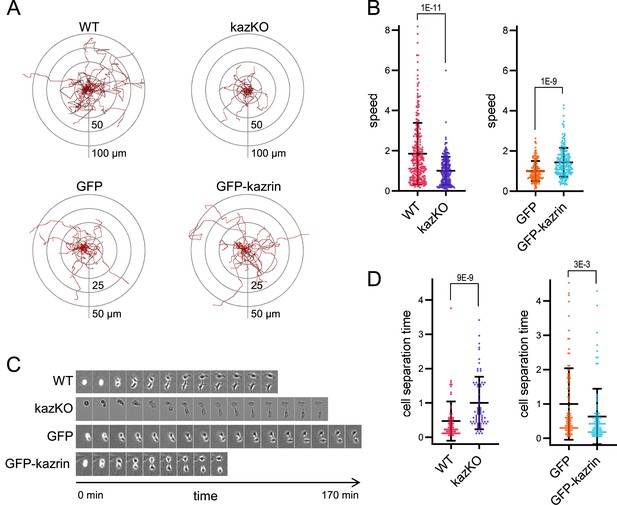
Kazrin depletion impairs cell migration and division.
(A) Paths described by individually migrating wild-type (WT) and kazKO MEF or kazKO MEFs expressing GFP or GFP-kazrin C at low levels (See M & M). The cells were embedded in Matrigel and tracked for 9 hr with a 10 min time lapse. All tracks start at the (0,0) coordinate in the graph. See also Video 1 for examples of individual migrating cells. (B) Scattered plot of the mean ± SD speed of cells described in (A). The data wre normalized to the mean of the corresponding KO cells (either kazKO or kazKO expressing GFP). p-values of the two-tailed Mann-Whitney tests are shown. n>100 cells per condition. See also Figure 2—figure supplement 1 for the effects of kazrin depletion on directionality. (C) Time-lapse epifluorescence images of WT and kazKO MEFs or kazKO MEFs expressing GFP or GFP-kazrin C at low levels, as they divide. Cells were recorded every 10 min. See also Video 2 for examples of individual dividing cells. Windows are 55 x 74 μm2 for WT MEF, 38 x 50 μm2 for kazKO MEF and 60 x 80 μm2 for GFP and GFP-kazrin C expressing MEF. (D) Mean time ± SD between substrate attachment and complete cell separation of the cells described in C. The data were normalized to the mean of the corresponding KO (kazKO or kazKO expressing GFP). p-values of the two-tailed Mann-Whitney tests are shown. n>68 dividing cells per condition.
-
Figure 2—source data 1
Data for graphs presented in Figure 2B and D.
- https://cdn.elifesciences.org/articles/83793/elife-83793-fig2-data1-v2.zip
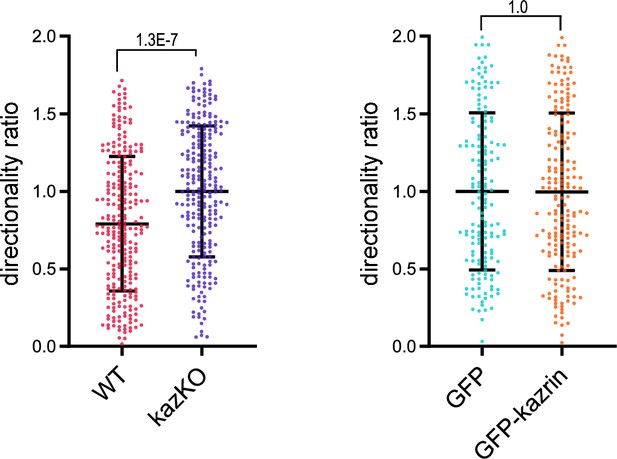
Effect of kazrin depletion on the directionality ratio.
Scattered plots of the mean ± SD directionality ratio of individually migrating wild-type (WT) and kazKO cells or kazKO MEF expressing low levels (See M & M) of GFP or GFP-kazrin C. Cells were embedded in Matrigel and tracked for 9 hr. The data were normalized to the corresponding kazKO cells. p-values of the two-tailed Mann-Whitney tests are shown. n>155 cells recorded per condition.
-
Figure 2—figure supplement 1—source data 1
Data for graphs presented in Figure 2—figure supplement 1.
- https://cdn.elifesciences.org/articles/83793/elife-83793-fig2-figsupp1-data1-v2.zip
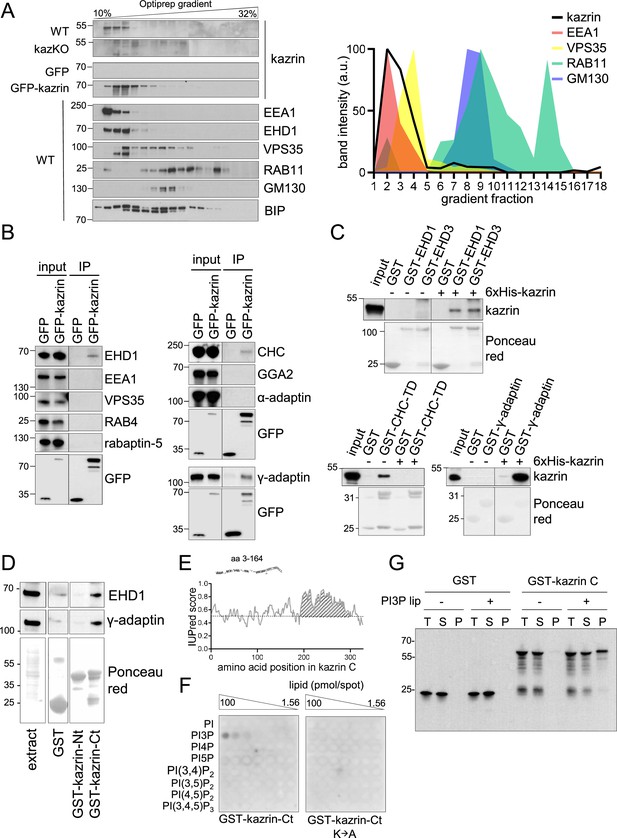
Kazrin is an endosomal protein.
(A) Left, immunoblots of Optiprep density gradient fractionations of membrane lysates of wild-type (WT) and kazKO MEF or kazKO MEF moderately expressing (See M & M) GFP or GFP-kazrin C. The membranes were probed with antibodies against the kazrin C N-terminus, EEA1, and EHD1 (EE markers), VPS35 (RAB5/RAB7 transition endosomal marker), RAB11 (RE/Golgi marker), GM130 (cis-Golgi marker) and BIP (Binding immunoglobulin protein) (ER marker). The antibody against EHD1 is likely to recognize other Eps15 homology domain (EHD) proteins. Band intensity plots per fraction for kazrin or the indicated intracellular membrane markers are shown on the right. The signal intensities of each fraction were normalized to the maximum for each antibody. All gradients were loaded with the same amount of total protein. Refer also to Figure 3—figure supplement 1 for co-fractionation of kazrin and early endosome autoantigen 1 (EEA1) in the lightest gradient fractions in IMCD3 cells. (B) Immunoblots of anti-GFP-agarose precipitates from lysates of kazKO MEF moderately expressing GFP or GFP-kazrin C, probed with antibodies against the indicated proteins. 10 µg of total protein were loaded as input. (C) Immunoblots of pull-downs from glutathione-Sepharose beads coated with GST, or GST fused to full-length EHD1 or EHD3, the clathrin heavy chain terminal domain (CHC-TD) or the γ-adaptin ear domain, incubated with purified 6xHis-kazrin C. The membranes were probed with an anti-kazrin antibody (ab74114, from Abcam) and stained with Ponceau red to visualize the GST fusion constructs. Refer also to Figure 3—figure supplement 2 for evidence indicating co-immunoprecipitation of endogenous kazrin with γ-adaptin and clathrin. (D) Immunoblots of pull-downs from glutathione-Sepharose beads coated with GST, or GST fused to the N- (amino acids 1–174) or C- (amino acids 161–327) terminal portions of kazrin C, incubated with non-denaturing extracts from MEFs. 10 µg of total protein were loaded as input. Ponceau red staining of the same membrane (lower panels) is shown to visualize the protein extract or the GST fusion constructs. (E) Prediction of IDRs in kazrin C. The graph shows the probability of each residue of being part of an intrinsically disordered region (IDR), according to the IUPred2A software. Residues in the shaded area have a consistent probability over 0.5 to form part of an IDR. (F) Immunoblots of a lipid-binding assay performed with either the purified GST-kazrin C C-terminal portion (amino acids 161–327) (GST-kaz-Ct) or an equivalent construct in which the poly-K region has been mutated to poly-A. The membranes used in this assay contain a concentration gradient of the indicated phosphoinositides. Membranes were probed with an anti-GST antibody. (G) Immunoblot of a liposome pelleting assay probed with an anti-GST antibody. GST or GST-kazrin C were incubated in the presence (+) or absence (−) of liposomes containing 5% phosphatidylinositol 3-phosphate (PI3P). Liposomes were recovered at 100.000 g for 1.5 hr. One equivalent of the input (T), one equivalent of the supernatant (S), and ten equivalents of the pellet (P) were loaded per sample.
-
Figure 3—source data 1
Un-cropped blots for Figure 3A, B, C, D and G.
- https://cdn.elifesciences.org/articles/83793/elife-83793-fig3-data1-v2.zip
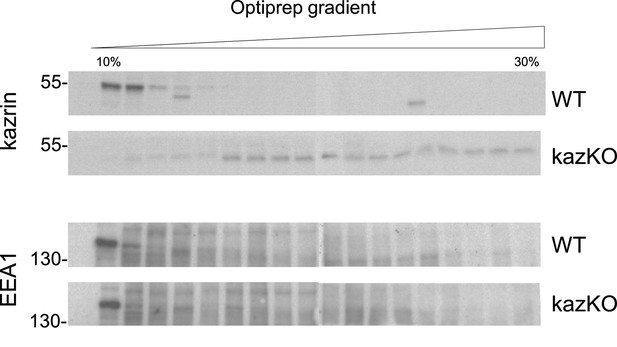
Endogenous kazrin co-fractionates with early endosome autoantigen 1 (EEA1) in the lightest fractions in IMCD3 cells.
Immunoblots of Optiprep density gradient fractions of membrane lysates of IMCD3 WT and kazKO cells, were probed with a rabbit serum raised against the N-terminus of kazrin C or with an anti-EEA1 antibody.
-
Figure 3—figure supplement 1—source data 1
Un-cropped blots for Figure 3—figure supplement 1.
- https://cdn.elifesciences.org/articles/83793/elife-83793-fig3-figsupp1-data1-v2.zip
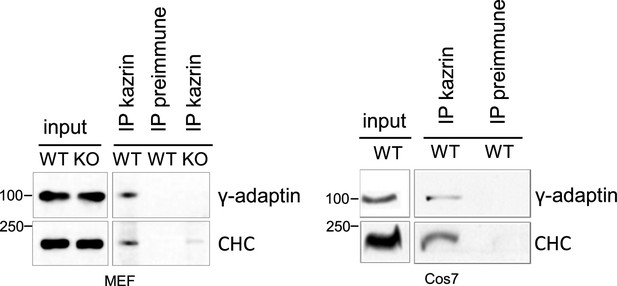
Antibodies raised against kazrin C co-immunoprecipitate γ-adaptin and clathrin.
Immunoblot of protein A-Sepharose precipitates from wild-type (WT) or kazKO MEFs or Cos7 cells using a mixed serum raised against the N and the C-terminal portions of kazrin C or a pre-immunization serum, probed with anti-γ-adaptin or anti-clathrin heavy chain (CHC) antibodies. Endogenous kazrin could not be detected with any of the tested antibodies in the immunoprecipitates because the antibody chain has a molecular weight similar to that of kazrin (approx. 50 Kda). kazKO MEF was used as specificity control instead.
-
Figure 3—figure supplement 2—source data 1
Un-cropped blots for Figure 3—figure supplement 2.
- https://cdn.elifesciences.org/articles/83793/elife-83793-fig3-figsupp2-data1-v2.zip
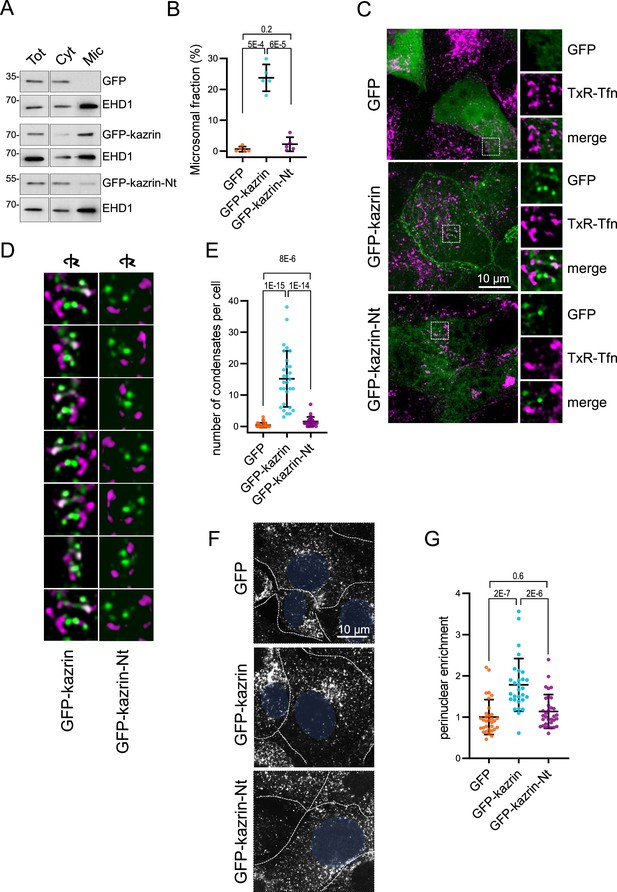
The predicted intrinsically disordered region (IDR) of kazrin C is required for its endocytic function.
(A) Immunoblots of subcellular fractionations from kazKO cells expressing moderate amounts (See M & M) of GFP, GFP-kazrin C or a GFP-kazrin C construct lacking the C-terminal predicted IDR (GFP-kazrin C-Nt). Cells were lysed in a non-denaturing buffer and centrifuged at 186,000 g for 1 hr to separate membranes (Mic) from the cytosol (Cyt). 15 µg of the total extract (Tot), and 1 and 5 equivalents of the cytosolic and membrane fractions were loaded per lane, respectively. (B) Scattered plot of the mean ± SD percentage of the GFP-signal associated with the microsomal fraction (Mic) in kazKO MEF expressing moderate amounts of GFP, GFP-kazrin C or GFP-kazrin C-Nt. Student´s t-tests p-values are shown. n=5 independent experiments for each sample. See M & M for experimental details. (C) MIP of confocal images of kazKO MEF expressing moderate amounts of GFP, GFP-kazrin C, or GFP-kazrin-Nt, loaded with 20 µg/ml of Texas Red-Tfn (TxR-Tfn) at 16 °C to accumulate endocytic cargo on early Endosomes endosomes (EEs). The images from the GFP and TxR channels and the merge from 5 × 5 µm2 fields are shown on the right. (D) Frames showing consecutive 60o turn snapshots of the 5 × 5 µm2 3D reconstruction animations of the insets shown in C for GFP-kazrin C and GFP-kazrin-C-Nt, showing the association of GFP-kazrin C foci, but not GFP-kazrin C-Nt, with TxR-Tfn-loaded EEs. (E) Scattered plot of the mean ± SD of the number of condensates per cell, visible with the GFP filter channel in the kazKO cells described in (B). p-values of the two-tailed Mann-Whitney test are shown. n=29 cells for each sample. Refer also to Video 3 for four 3D reconstruction animations of TxR-Tfn loaded endosomes associated with GFP-kazrin C, and Video 5 for GFP-kazrin C-Nt; Figure 4—figure supplement 2 for co-localization of GFP-kazrin C with adhesion molecules; Figure 4—figure supplement 3 for analysis of the association of GFP-kazrin C and GFP-kazrin C-Nt foci with TxR-Tfn loaded endosomes; and Figure 4—figure supplement 4 and Video 4 for co-localization of GFP-kazrin C with Eps15 homology domain (EHD) proteins. (F) Confocal micrographs of kazKO cells expressing low amounts (see M & M) of GFP, GFP-kazrin C, or GFP-kazrin C-Nt loaded with 20 µg/ml of TxR-Tfn at 16 °C and chased for 10 min at 37 °C. Cell borders are indicated by dashed lines and the nuclei in blue. (G) Scattered plots of the mean ± SD TxR-Tfn perinuclear enrichment for the cells and experimental conditions described in D. See M & M for experimental details. The data is normalized to the mean value of kazKO cells expressing GFP. p-values of the two-tailed Mann-Whitney test are shown. n>25 cells for each sample.
-
Figure 4—source data 1
Un-cropped blots for Figure 4A.
- https://cdn.elifesciences.org/articles/83793/elife-83793-fig4-data1-v2.zip
-
Figure 4—source data 2
Data for graphs presented in Figure 4B, E and G.
- https://cdn.elifesciences.org/articles/83793/elife-83793-fig4-data2-v2.zip
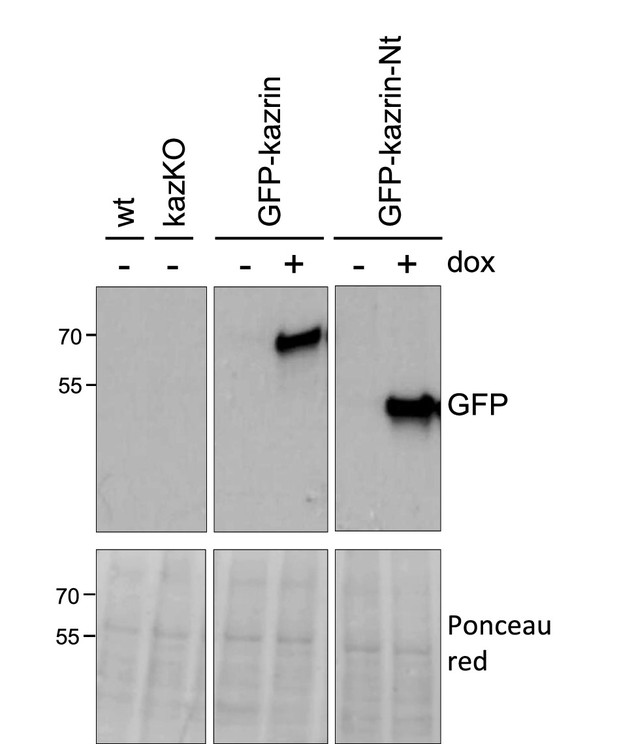
Expression of GFP-kazrin C-Nt in kazKO MEF.
Ponceau red staining (lower panels) and immunoblots against GFP of wild-type (WT) and kazKO MEFs, or kazKO cells expressing GFP-Kazrin C or a GFP-Kazrin C construct lacking the predicted intrinsically disordered region (IDR) (GFP-kazrin-Nt) in the absence (−) or presence (+) of 5 µg/ml doxycycline for 24 hr (dox).
-
Figure 4—figure supplement 1—source data 1
Un-cropped blots for Figure 4—figure supplement 1.
- https://cdn.elifesciences.org/articles/83793/elife-83793-fig4-figsupp1-data1-v2.zip
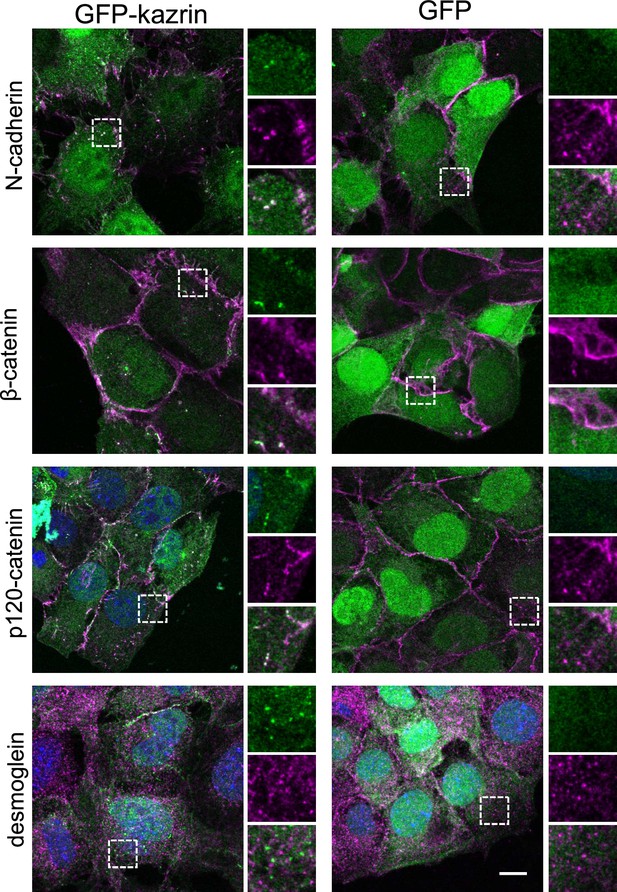
Kazrin C co-localizes with p120 and β-catenins and N-cadherin at the plasma membrane (PM) and internal structures.
Merged confocal images of kazKO MEFs expressing moderate levels (See M & M) of GFP or GFP-kazrin C, fixed and stained with anti-N-cadherin, β-catenin, p120-catenin, or desmoglein antibodies and A568-conjugated secondary antibodies. Merged images and individual channels of 10 × 10 µm2 are shown. Scale bar, 10 μm.
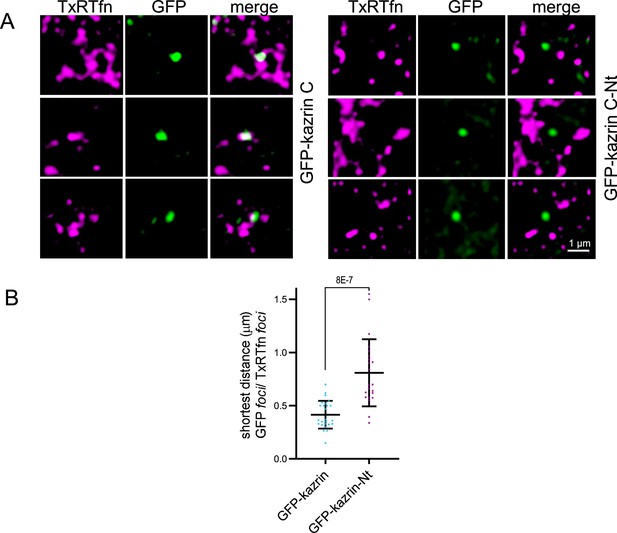
Deletion of the C-terminal predicted intrinsically disordered region (IDR) reduces association of kazrin C with endosomes.
(A) Snapshots of 3D reconstruction animations (see also Video 3 (left) and 5 (right)) of 5 × 5 µm2 crops from confocal fluorescence microscopy Z-stacks of kazKO MEFs expressing moderate levels of GFP-kazrin C or GFP-kazrin C-Nt, loaded with Texas Red-Tfn (TxR-Tfn) at 16 °C. Individual channels and merged images are shown (B) Scattered plot showing the mean ± SD of the maximal distance between the centroids of the closest GFP and TxR foci, as a measure of their association. 3D reconstructions of at least 20 GFP-kazrin C and GFP-kazrin C-Nt foci from different cells were generated and manually analyzed using the line toolbar of Fiji (See M & M for further details). Student´s t-tests p-values are shown.
-
Figure 4—figure supplement 3—source data 1
Data for graph presented in Figure 4—figure supplement 3B.
- https://cdn.elifesciences.org/articles/83793/elife-83793-fig4-figsupp3-data1-v2.zip
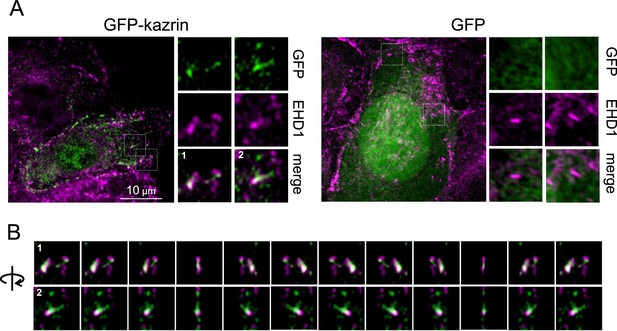
GFP-kazrin C forms condensates associated with endosomal Eps15 Homology Domain (EHD)-protein-enriched subdomains.
(A) MIP of merged confocal images of kazKO MEFs expressing moderate levels (See M & M) of GFP or GFP-kazrin C (GFP-kaz), fixed and stained with anti-EHD1 and A568-conjugated secondary antibodies. Individual channels and merged images of 5 × 5 µm2 magnified crops are shown on the right. See also Video 4 for 3D reconstruction animations of the GFP-kazrin C EHD1 crops. (B) Frames showing consecutive 30o turn snapshots of the 5 × 5 µm2 insets in Video 4, corresponding to crop 1 and crop 2 in A.
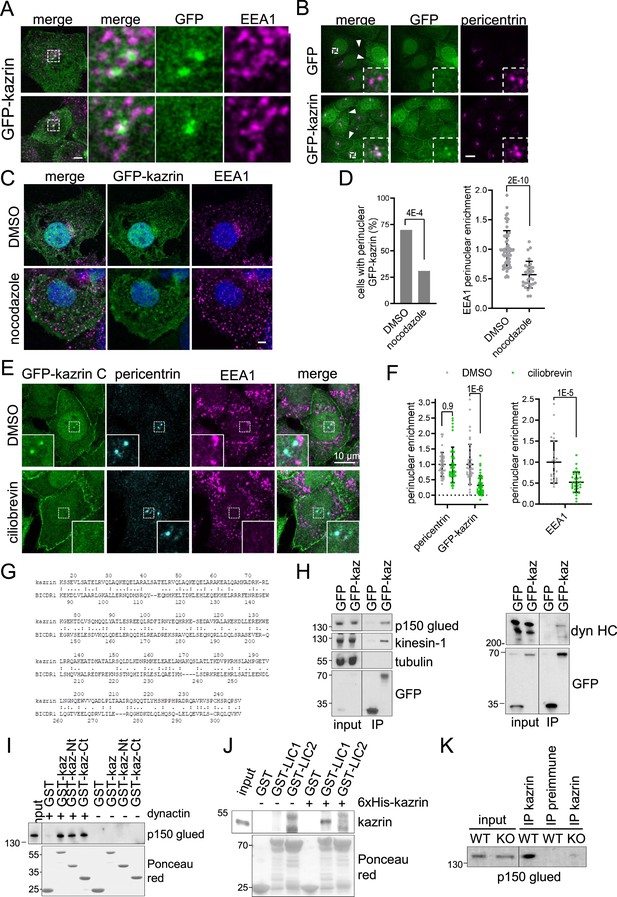
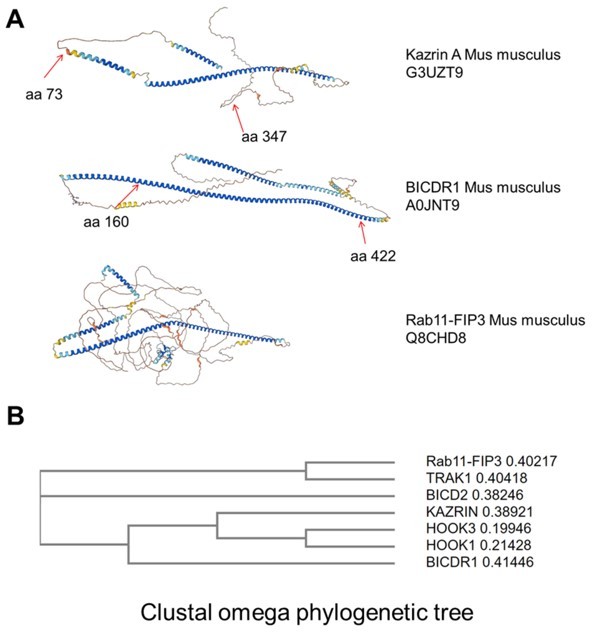
Karin C concentrates in the pericentriolar region and interacts with dynactin and dynein.
(A) Merged confocal fluorescence micrographs of kazKO MEF moderately expressing (See M & M) GFP-kazrin C, fixed and stained with anti-EEA1 and A568-conjugated secondary antibodies. Individual channels and merged images of 6 x magnifications are shown. Scale bar, 10 μm. (B) Merged and individual channels of confocal fluorescence micrographs of kazKO MEF moderately expressing GFP or GFP-kazrin C, fixed and stained with anti-pericentrin and A568-conjugated secondary antibodies. 3.5 x magnifications are shown. Arrowheads indicate cell-cell borders. Scale bar, 10 μm. Refer also to Figure 5—figure supplement 1 and Videos 6 and 7 for live imaging. (C) Confocal fluorescence micrographs of kazKO MEF moderately expressing GFP-kazrin C, treated with DMSO or 100 ng/ml nocodazole, fixed, and stained with anti-EEA1 and A568-conjugated secondary antibodies and DAPI. Scale bar, 5 μm. (D) Bar plot showing the percentage of cells with a perinuclear localization of GFP-kazrin C (left) and scattered plot of the mean ± SD EEA1 perinuclear enrichment (right) in the cells and the experimental conditions described in C. See M & M for further details. The data was normalized to the mean of the mock-treated cells. p-values of a two-sided Fisher’s exact test (left) and a two-tailed Student t-test (right) are shown. n>32 cells for each sample. (E) Confocal fluorescence micrographs of kazKO MEF moderately expressing GFP-kazrin C, treated with DMSO or 40 nM ciliobrevin, fixed, and stained with mouse anti-EEA1 and rabbit anti-pericentrin antibodies and A488 and A568-conjugated secondary antibodies, respectively, and DAPI. 5.4 × 5.4 µm2 magnified areas where the pericentrin foci accumulate are shown. (F) Scattered plot of the mean ± SD intensity signal of pericentrin and GFP-kazrin C in the pericentriolar dots (left), for the cells and experimental conditions described in E, normalized to the mean signal of pericentrin or GFP-kazrin C, in cells treated with DMSO. Scattered plot of the mean ± SD EEA1 perinuclear enrichment (right) in cells treated as described in E. See M & M for further details. The data was normalized to the mean of the mocktreated cells. p-values of the two-tailed Student t-test are shown. n>44 cells for each sample. (G) Sequence comparison between kazrin C and human BICDR1 (LALIGN). (H) Immunoblots of anti-GFP agarose immunoprecipitates (IP) from cell lysates of kazKO MEF moderately expressing GFP or GFP-kazrin C, probed for the indicated proteins. (I) Immunoblots of pull-downs with of glutathione-Sepharose beads coated with purified GST or GST fused to kazrin C (GST-kazrin) or its N- (amino acids 1–176) (GST-kazrin-Nt) or C-terminal (amino acids 161–327) (GST-kazrin-Ct) portions, incubated with (+) or without (−) dynactin complex, purified from pig. The membranes were probed with an anti-p150 glued antibody or stained with Ponceau red to detect the GST constructs. (J) Immunoblots of pull-downs with glutathione-Sepharose beads coated with GST, GST-LIC1, or GST-LIC2, incubated with purified 6xHis-kazrin C. The membranes were probed with a mouse anti-kazrin antibody or stained with Ponceau red to detect the GST constructs. (K) Immunoblot of protein A-Sepharose immunoprecipitates (IP) from WT or kazKO MEFs using a mix of rabbit polyclonal serums against the N- and C-terminal domains of kazrin C or a pre-immunization serum, probed with an anti-p150 glued (dynactin) antibody. The low amounts of endogenous kazrin could not be detected in the immunoprecipitates with any of the antibodies tested because the antibody had a molecular weight similar to endogenous kazrin (about 50 Kda) and interfered with the detection. The kazKO MEF was used as a specificity control instead.
-
Figure 5—source data 1
Un-cropped blots for Figure 5H,I, J and K.
- https://cdn.elifesciences.org/articles/83793/elife-83793-fig5-data1-v2.zip
-
Figure 5—source data 2
Data for graphs presented in Figure 5D and F.
- https://cdn.elifesciences.org/articles/83793/elife-83793-fig5-data2-v2.zip
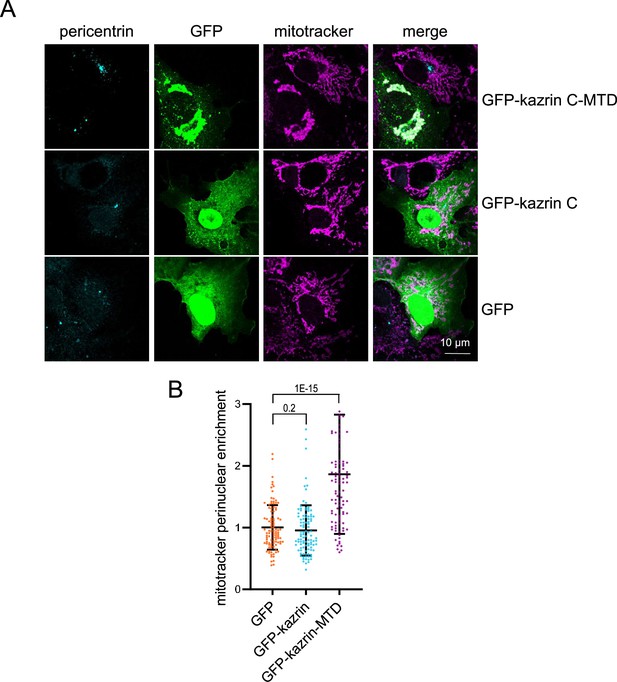
GFP-Kazrin C fused to the mitochondrial targeting domain (MTD) of centrosomin conveys mitochondria to the pericentriolar region.
(A) Confocal micrographs of Cos7 cells transiently expressing GFP, GFP-kazrin C, or a chimera of GFP-kazrin C fused to the mitochondrial targeting domain (MTD) of D. melanogaster centrosomin fixed and stained with anti-TOMM20 and A568 secondary antibodies to visualize mitochondria and anti-pericentrin and A648-conjugated secondary antibodies. (B) Scattered plot of the mean ± SD mitochondria perinuclear enrichment in cells treated as described in A. See M & M for further details. The data were normalized to the mean of the cells mock-treated. The p-values of the two-tailed Mann-Whitney tests are shown. n=102 cells for each sample.
-
Figure 5—figure supplement 2—source data 1
Data for graphs presented in Figure 5—figure supplement 2B.
- https://cdn.elifesciences.org/articles/83793/elife-83793-fig5-figsupp2-data1-v2.zip
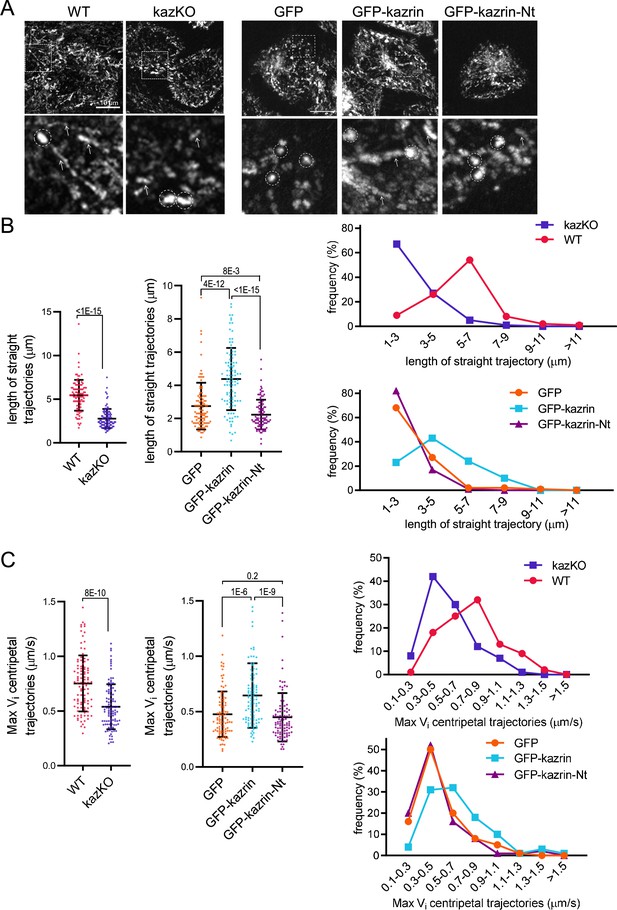
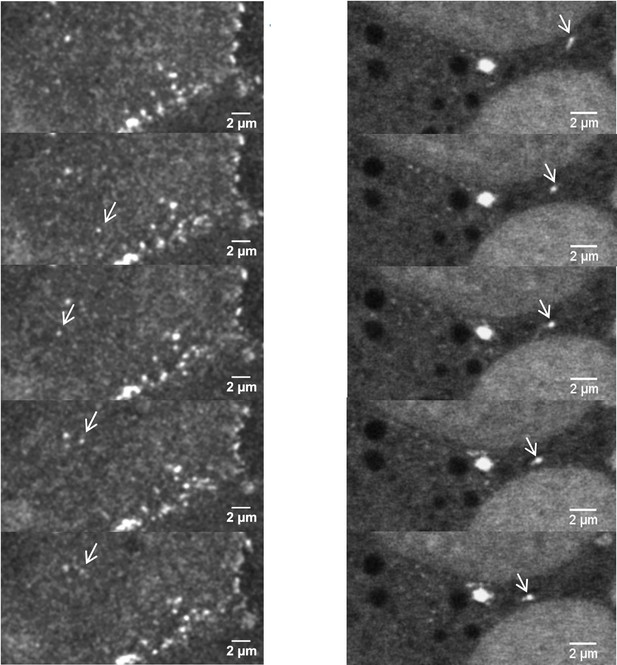
Depletion of kazrin impairs endosome motility.
(A) Time projections of MIP of confocal fluorescence microscopy videos taken for 90 s with a 3 s time-lapse, of wild-type (WT) and kazKO MEF or kazKO MEF expressing low levels of GFP, GFP-kazrin C of a GFP-kazrin C construct lacking the C-terminal predicted intrinsically disordered region (IDR) (GFP-kazrin-Nt) (See M & M), showing trajectories of early endosome (EE) loaded with Texas Red-Tfn (TxR-Tfn) at 16℃. Cells were shifted to 37℃ and immediately imaged. Scale bar = 10 µm. A magnified 10 × 10 µm2 inset is shown below. Arrows point to straight trajectories and dashed circles indicate constrained endosome movements. (B) Scattered plots of the mean ± SD lengths of endosome trajectories (longer than 1 µm) (left graphs) for the cells and experimental conditions described in A. p-values of the two-tailed Mann-Whitney tests are shown. n=100 endosomes for each sample recorded in more than 20 cells. Line plots for the frequencies of the trajectory length in each cell type are shown on the right. (C). Scattered plots of the mean ± SD maximum instantaneous velocities (Vi) (left graphs) of retrograde endosome trajectories (longer than 1 µm) for the cells and experimental conditions described in A. p-values of the two-tailed Mann-Whitney tests are shown. n=100 endosomes for each sample recorded in more than 20 cells. Line plots for the frequencies of the maximum Vi for each cell type are shown on the right. See also Video 8 for life imaging of an example of WT and kazKO cells loaded with TxR-Tfn, and Videos 9 and 10 for four different magnified fields showing endosome motility in different WT and kazKO cells, respectively. See also Video 11 for an example of life imaging of kazKO cells expressing low levels of either GFP, GFP-kazrin C, or GFP-kazrin C-Nt loaded with TxR-Tfn, and Videos 12–14 for four different magnified fields showing endosome motility in these cell types.
-
Figure 6—source data 1
Data for graphs are presented in Figure 6B and C.
- https://cdn.elifesciences.org/articles/83793/elife-83793-fig6-data1-v2.zip
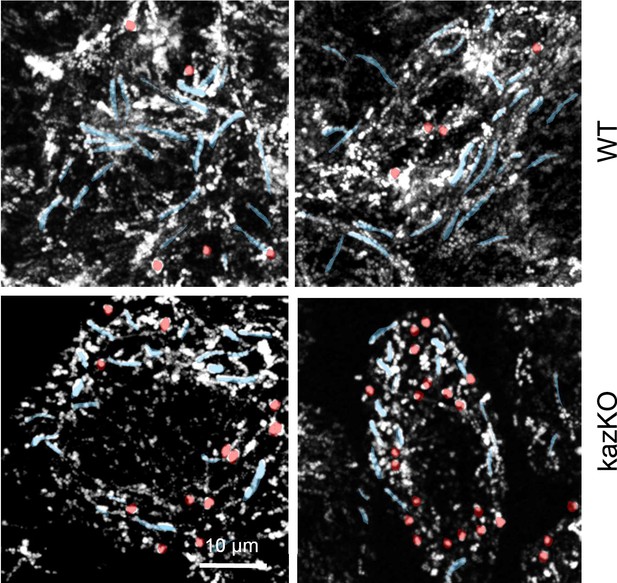
Depletion of kazrin impairs endosome motility.
(A) Time projections of MIP of confocal fluorescence microscopy videos taken for 90 s with a 3 s time-lapse, of wild-type (WT) and kazKO MEF, showing trajectories (highlighted in blue) and confined movements (highlighted in red) of early endosome (EE) loaded with Texas Red-Tfn (TxR-Tfn) at 16℃. Cells were shifted to 37℃ and immediately imaged.
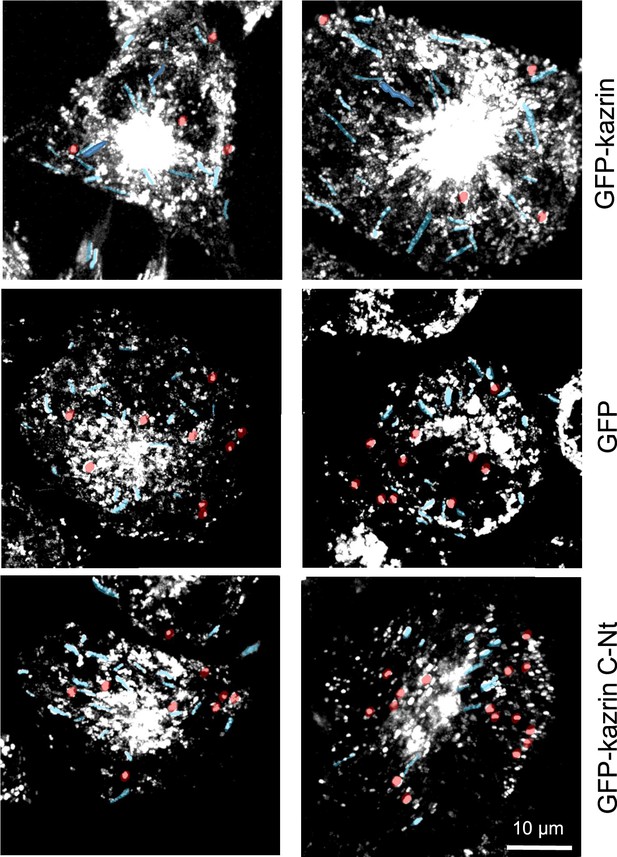
Depletion of kazrin impairs endosome motility.
(A) Time projections of MIP of confocal fluorescence microscopy videos taken for 90 s with a 3 s time-lapse, of kazKO MEF expressing low levels (See M & M) of GFP, GFP-kazrin C or a GFP-kazrin C construct lacking the C-terminal predicted intrinsically disordered region (IDR) (GFP-kazrin-Nt), showing elongated trajectories (highlighted in blue) and confined movements (highlighted in red) of early endosome (EE) loaded with Texas Red-Tfn (TxR-Tfn) at 16℃. Cells were shifted to 37℃ and immediately imaged.
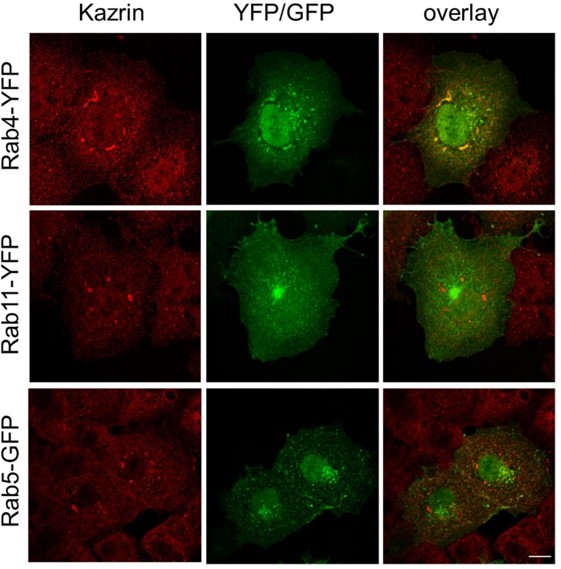
Endogenous kazrin C might interact with overexpressed RAB4 in Cos7 cells.
Fluorescence micrographs of Cos7 cells transiently expressing RAB4-YFP, RAB11YFP or RAB5-GFP, fixed and stained with a rabbit serum against kazrin C and a A568conjugated secondary antibody. The single red and green channels and the merge of the same field are shown. Bar = 10 µm.

Kazrin does not seem to interact with the RAB4, RAB5 or RAB11 GTPases in vitro.
A. Immunoblot of GFP-TRAP immunoprecipitations from nondenaturing extracts from Cos7 cells transiently expressing GFP, GFP-kazrin C or N and Cterminal truncations of kazrin C (amino acids 1 to 174, and amino acids 161 to 327, respectively), probed for the RAB4 GTPase (upper panel) or stained with Ponceau red (lower panel) for detection of GFP or GFP-kazrin C constructs in the immunoprecipitates. B. Immunoblot of pull down assays of glutathione-Sepharose beads coated with increasing concentrations of GTP loaded RAB4, RAB5 and RAB11 fused to GST, incubated in the presence of non-denaturing protein extracts from Cos7 cells, The membranes were probed for endogenous kazrin and stained with Ponceau red to detect GST or the GST-RAB constructs.
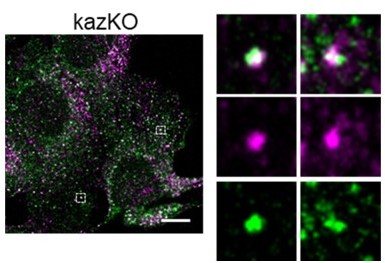
Colocalization of internalized TxRTfn and EEA1 in kazKO MEF.
Merged confocal fluorescence microscopy of kazKO MEF loaded with TxR-Tfn (magenta) for 20 min, fixed an stained with anti-EEA1 and A641-conjugated secondary antibodies (Green). Scale bar, 10 µm.
Videos
Videos of individually migrating wild-type (WT) and kazKO MEF, and kazKO MEF expressing low levels of GFP and GFP-kazrin C.
The cells were embedded in Matrigel and imaged with an epifluorescence microscope.
Videos of dividing wild-type (WT) and kazKO MEF, and kazKO MEF expressing low levels of GFP and GFP-kazrin C, from the moment the mother cell attached to the substrate until the daughter cells were completely separated.
Scale bar = 10 μm. The cells were embedded in Matrigel and imaged with an epifluorescence microscope.
Four 3D reconstructions of Z stacks of kazKO MEF expressing moderate amounts of GFP-kazrin C loaded with Texas Red-Tfn (TxR-Tfn) at 16℃ to accumulate endocytic cargo in early endosome s (EEs).
Cells were shifted to 37℃ and fixed after 10 min. The windows are 5 × 5 µm2.
Two 3D reconstructions of Z stacks of kazKO MEF expressing moderate amounts of GFP-kazrin C, fixed, and stained with α-EHD1 and A568-conjugated secondary antibodies.
The windows are 5 × 5 µm2.
Four 3D reconstructions of Z stacks of kazKO cells expressing moderate amounts of a GFP-kazrin C lacking the C-terminal predicted intrinsically disordered region (IDR) (GFP-kazrin C-Nt) loaded with TxR-Tfn at 16℃ to accumulate endocytic cargo in early endosome (EE).
Cells were shifted to 37℃ and fixed after 10 min. The windows are 5 × 5 µm2.
2.65 s time-lapse video of the juxtanuclear region of a kazKO MEF moderately expressing GFP-kazrin C (GFP-kaz) with a confocal microscopy.
A 51.2 x 51.2 μm2 window is shown.
2.65 seconds time-lapse video of the juxtanuclear region of a kazKO MEF moderately expressing GFP-kazrin C (GFP-kaz) with a confocal microscopy.
Scale bar, 2 μm.
3 s time-lapse live-cell videos showing Texas Red-Tfn (TxR-Tfn) loaded endosomal dynamics in WT and kazKO MEF.
The windows are 42.5 × 42.5 µm2. Cells were loaded with TxR-Tfn at 16℃ to accumulate endocytic cargo in early endosomes (EEs) and imaged immediately after the shift to 37℃. The images correspond to the maximum intensity Z projection.
Four 3s time-lapse live-cell videos showing Texas Red-Tfn (TxR-Tfn) loaded endosomal dynamics in WT MEF.
The windows are 12.8 × 12.5 µm2. Cells were loaded with TxR-Tfn at 16℃ to accumulate endocytic cargo in early endosomes (EEs) and imaged immediately after the shift to 37℃. The images correspond to the maximum intensity Z projection.
Four 3 s time-lapse live-cell videos showing Texas Red-Tfn (TxR-Tfn) loaded endosomal dynamics in kazKO MEF.
The windows are 12.8 × 12.5 µm2. Cells were loaded with TxR-Tfn at 16℃ to accumulate endocytic cargo in early endosomes (EEs) and imaged immediately after the shift to 37℃. The images correspond to the maximum intensity Z projection.
3 s time-lapse live-cell videos showing Texas Red-Tfn (TxR-Tfn) loaded endosomal dynamics in kazKO MEF expressing low levels (See M&M) of GFP, GFP-kazrin C, or GFP-kazrin Nt.
The windows are 42.5 × 42.5 µm2. Cells were loaded with TxR-Tfn at 16℃ to accumulate endocytic cargo in early endosomes (EEs) and imaged immediately after the shift to 37℃. The images correspond to the maximum intensity Z projection.
Four 3 s time-lapse live-cell videos showing Texas Red-Tfn (TxR-Tfn) loaded endosomal dynamics in kazKO MEF expressing low levels (See M & M) of GFP-kazrin C.
The windows are 12.8 × 12.5 µm2. Cells were loaded with TxR-Tfn at 16℃ to accumulate endocytic cargo in early endosomes (EEs) and imaged immediately after the shift to 37℃. The images correspond to the maximum intensity Z projection.
Four 3 time-lapse live-cell videos showing Texas Red-Tfn (TxR-Tfn) loaded endosomal dynamics in kazKO MEF expressing low levels (See M & M) of GFP.
The windows are 12.8 × 12.5 µm2. Cells were loaded with TxR-Tfn at 16℃ to accumulate endocytic cargo in early endosomes (EEs) and imaged immediately after the shift to 37℃. The images correspond to the maximum intensity Z projection.
Four 3 s time-lapse live-cell videos showing Texas Red-Tfn (TxR-Tfn) loaded endosomal dynamics in kazKO MEF expressing low levels (See M & M) of GFP-kazrin C-Nt.
The windows are 12.8 × 12.5 µm2. Cells were loaded with TxR-Tfn at 16℃ to accumulate endocytic cargo in early endosomes (EEs) and imaged immediately after the shift to 37℃. The images correspond to the maximum intensity Z projection.
Tables
Plasmids.
| Plasmid | Insert | Backbone |
|---|---|---|
| pGEX-5X-3 | GST | pGEX-5X-3 |
| pGST-hB24 | GST + kazrin C, human gene KIAA1026 | pGEX-4T-2 |
| pGST-kaz-Ct (161-327) | GST + kazrin C Ct (aa 161–327) | pGEX-5X-3 |
| pGST-kaz-Nt (1-176) | GST + kazrin C Nt (aa 1–176) | pGEX-5X-3 |
| pGST-kaz-Ct-KA | GST + kazrin C Ct (aa 161–327) -(281-KRKKKK-286, AAAAAA) | pGEX-5X-3 |
| pQE11-kazrin | 6xHis + kazrin C | pQE11 |
| pGST-EHD1 | GST + EHD1 | pGEX-5X-3 |
| pGST-EHD3 | GST + EHD3 | pGEX-5X-3 |
| pGST-γ-Adaptin-ear | GST + human AP1 Adaptin G1 ear (aa 702–925) | pGEX-5X-3 |
| pGST-CHC17-TD | GST + human CHC17-aa1-483 (CHC TD +linker) | pGEX-5X-3 |
| pGST-LIC1 | GST + dynein light intermediate chain 1 | pGEX-5X-3 |
| pGST-LIC2 | GST + dynein light intermediate chain 2 | pGEX-5X-3 |
| pX458-kaz KO 1 | Cas9 and Cas9 target sequence 1 | pSpCas9(BB)–2A-GFP (pX458) |
| pX458-kaz KO 2 | Cas9 and Cas9 target sequence 2 | pSpCas9(BB)–2A-GFP (pX458) |
| pVSV-G | Lentivirus envelope protein | pLenti-CMV |
| pAX8 | Lentivirus packaging protein | pLenti-CMV |
| pINDUCER-EGFP | EGFP | pINDUCER20 |
| pINDUCER-EGFP-kazrin C | EGFP + kazrin C | pINDUCER20 |
| pINDUCER-EGFP-kazrin C-Nt | EGFP + kazrin C (aminoacids 1–176) | |
| pKLO.1_shKzrn | cloneID TRCN00001 82832 | pLK0.1 |
| pLK0.1 | SHC002 | pLK0.1 |
| pCMV-dR8.2dvpr | ||
| pCMV-VSG-G | ||
| pEGFP-C2 | ||
| pEGFP-kazrin C | pEGFP-C2 + kazrin C | pEGFP-C2 |
| pEGFP-kazrin C-MTD | pEGFP-C2 + kazrin C fused to the Mitochondrial Targeting domain of D. melanogaster centrosomin CnnT splice variant (AT9084) (aa 212–480) | pEGFP-C2 |