The evolution and structure of snake venom phosphodiesterase (svPDE) highlight its importance in venom actions
Figures
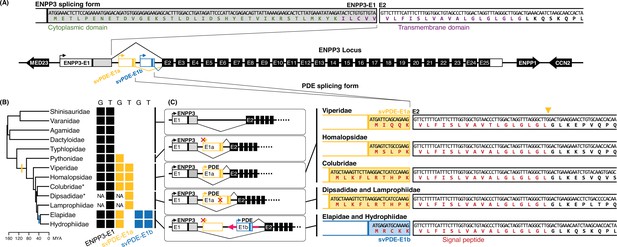
The alternative splicing and evolutionary features of ENPP3 and svPDE.
(A) N-terminal of peptide sequences of ENPP3 and PDE encoded by alternative splicing. The scheme of exons and genomic synteny is presented with flanking coding genes. The 5′ untranslated regions (5′ UTR) and coding regions of first exons are separated by colored vertical lines, which are the translation start codons. Only the lengths of E1, E1a, and E1b are drawn in scale. Putative promoters identified in the proximal upstream are indicated with arrows attached to the first exons. The same elements are expressed by the same colors throughout the figure. The sequences around splicing junctions of E1 and E2 are zoomed in with translated peptides of functional domains highlighted in corresponding colors. (B) Presences and absences of ENPP3-specific and svPDE-specific E1s in different clades. Color-filled squares denote the exons identified in the genomes ‘G’ and transcriptomes ‘T,’ where ‘NA’ indicates the clades without available genome assemblies. Clades with asterisks use Duvernoy’s glands as their venom delivery system. The colored vertical lines on the phylogenetic tree indicate the inferred branches that svPDE-E1a (yellow) and svPDE-E1b (blue) emerged. (C) The scheme of alternative splicing of ENPP3 and svPDE transcripts in different clades. The possible malfunctioned elements, including svPDE-E1a promoters in Pythonidae, Elapidae, and Hydrophiidae, and ancestral translation start sites in Dipsadidae and Lamprophiidae, are crossed out in red. The long pink arrow indicates the mobile element. The yellow triangle indicates the putative cleavage site of the signal peptidase.

The sequence alignment of the translated peptides of ENPP3 inferred from the genomic exons (N. naja and N. atra) and the snake venom phosphodiesterase (svPDE) peptides revealed from proteomic approaches.
Regions of each exon are underlined with solid and dotted lines alternately. The cytoplasmic and transmembrane domains are colored in green and plum, respectively. The codons with non-synonymous substitutions and the corresponding amino acids are highlighted in red and black, respectively. The codons with synonymous substitutions are highlighted in blue. Identical sequences are collapsed into one sequence for representation. The yellow arrow indicates the putative cleavage site of the signal peptide, which is in line with the N-terminal end of the purified svPDE protein.

Presences and absences of ENPP family members across eukaryotes.
Seven members of ENPP families are represented in pentagons with corresponding colors. Their genomic synteny are shown with the conserved flanking genes (blacked pentagons). ENPP genes with gradient colors are genes diverged before the divergence of two named ENPP family members (e.g., a gene phylogenetically clustered with the common ancestor of ENPP3 and ENPP1 is drawn in a gradient yellow). Genes with only a few conserved exons regarded as evolutionary relics are indicated with dotted borders. One human ENPP7 denoted by slashes is a confirmed pseudogene in the latest genome annotation release. Genes on a single continuous horizontal line are located on the same contig or chromosome. Vertical lines indicate the assembled ends of contigs or chromosomes. Genes fragmentedly located on different contigs that could infer the coordinates by genomic synteny are drawn by order. The branch lengths of the cladogram are not drawn proportional to the evolutionary time.

The multiple sequence alignment of the translated peptides around the E1-E2 junction region.
Peptides started from the start codon (ATG, methionine) of exon 1 to the 5′ partial region of exon 2 are shown. The cytoplasmic domains, the transmembrane domains, signal peptides, and cleavage sites are colored in green, plum, blue, and yellow. Abbreviations are listed in the figure. Only a few species are selected as representatives of Elapidae and Hydrophiidae, whose svPDE-E1a is conserved in genomes but not expressed in the transcriptomes. The ENPP3 transcript of T. nigriceps was partially assembled and does not cover the 5′ end of the coding sequence.

The multiple sequence alignment of (A) the putative core promoters located on the upstream of svPDE-E1a, (B) the alternative translation start sites of svPDE-E1a in Dipsadidae and Lamprophiidae, and (C) the putative core promoters located on the upstream of svPDE-E1b.
Putative functional motifs are highlighted with corresponding colors. Abbreviations of clades and species common names are the same as Figure 1—figure supplement 3 with an additional one, CSME, as the consensus sequence of mobile element (DR0148352).

The multiple sequence alignment of the coding regions of svPDE-E1a and 5′ partial E2.
Conserved start codons of svPDE-E1a are highlighted in black and gray, corresponding to the expressed and nonexpressed transcripts, respectively. For all species with available genomes, the interval sequences between svPDE-E1a and conserved E2 are canonical GT-AG introns, which are shown in the alignments with splicing sites highlighted in blue. Other regions of intron sequences are omitted for a clear view. For species that only have transcriptomic data, the sequences shown in the alignment were obtained from the assembled transcripts. In Dipsadidae and Lamprophiidae, the alternative start codons located on the elongated svPDE-1a due to using alternative 3′ splicing sites are highlighted in red.
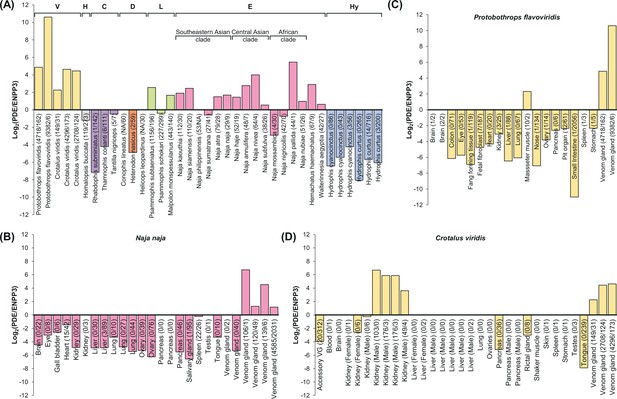
Species-specific and tissue-specific expression of snake venom phosphodiesterase (svPDE) over ENPP3 splicing forms.
The expression of each splicing form was calculated by the number of reads spanning the specific E1s and the conserved E2. The log2 transformed ratios are shown in the figure and a count of zero was adjusted to a pseudo count of one for valid log transformation. The unadjusted counts are presented in the axis labels as the format of svPDE/ENPP3. The alternative splicing forms not identified in the transcriptomes and no available genomes to infer their presence are denoted as NA. (A) Species-specific expressions in venom glands and, for Colubridae and Dipsadidae, Duvernoy’s gland, an anatomic gland structure similar to venom glands, were compared. Samples with less than five svPDE transcripts and less than five ENPP3 transcripts are regarded as having no expression for both transcripts and not shown. Clades are colored in yellow, gray, purple, orange, green, pink, and blue with the clade prefixes of Viperidae, Homalopsidae, Colubridae, Dipsadidae, Lamprophiidae, Elapidae, and Hydrophiidae, respectively. (B–D) Tissue-specific expression of svPDE over ENPP3 in selected species: (B) Naja naja (Elapidae) and (C) Protobothrops flavoviridis (Viperidae) and (D) Crotalus viridis (Viperidae).
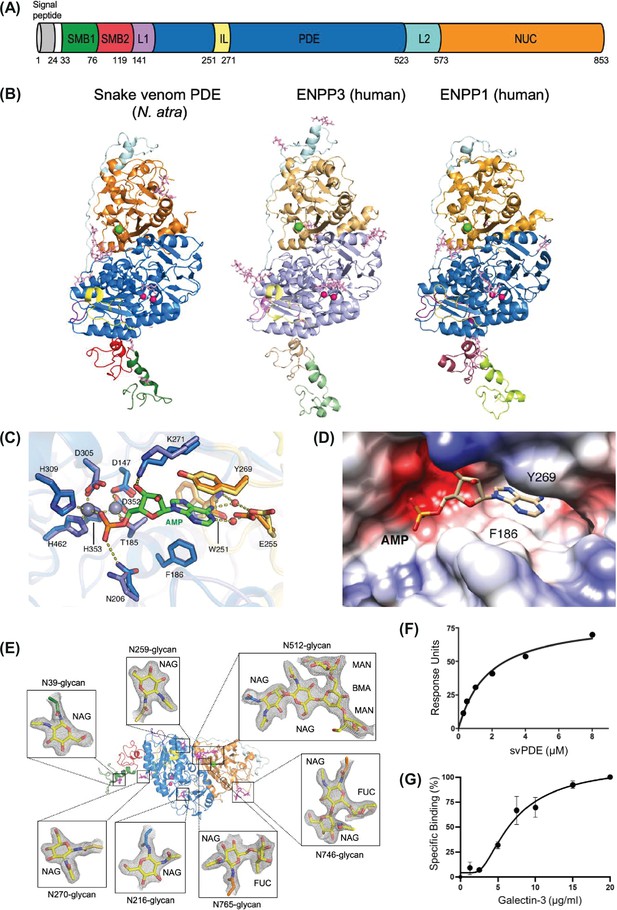
SvPDE sharing similar structural folding and binding partners with human ENPP1 and ENPP3.
(A) Domain organizations of Naja atra snake venom phosphodiesterase (svPDE). SMB1, somatomedin-B-like 1 domain; SMB2, somatomedin-B-like 2 domain; L1, loop 1; PDE, catalytic phosphodiesterase domain; IL, insertion loop; L2, loop 2; NUC, nuclease-like domain. (B) Crystal structures of svPDE from N. atra (PDB code: 5GZ4), human ENPP3 (PDB code: 6C01), and human ENPP1 (PDB code: 6WET) in cartoon representation. Zinc and calcium ions are shown as hot pink and green spheres, respectively. N-glycans are shown as light pink sticks. (C) Superposition of active sites of svPDE from N. atra in the apo and AMP-complexed forms. Zinc atoms are shown as gray spheres and water atoms are shown as red ones. Residues involving AMP binding in the apo and AMP-complexed forms are shown in slate/orange and marine/yellow, respectively. Hydrogen bonds and coordinate bonds are shown as dashed yellow lines. (D) Electrostatic potential surface of the nucleotide-binding pocket of svPDE from N. atra in the AMP-complexed form. (E) N-glycans at Asn39, Asn216, Asn259, Asn270, Asn512, and Asn746 of svPDE from N. atra are shown as sticks with electron densities. 2Fo-Fc electron density maps contoured at 1.0σ. (F) Surface plasmon resonance (SPR) investigation of the binding between svPDE (0.25, 0.5, 1.0, 2.0, 4.0, and 8.0 μM) and the immobilized insulin receptor. KD of svPDE binding to the insulin receptor were obtained by steady-state affinity model. (G) Binding affinity measurement of Gal-3 with svPDE from N. atra. Standard deviations of three replicates were indicated.
-
Figure 3—source data 1
The original uncropped image of SDS-PAGE.
- https://cdn.elifesciences.org/articles/83966/elife-83966-fig3-data1-v2.zip

Purification of snake venom phosphodiesterase (svPDE) from the crude venom of Naja atra habitated in Taiwan.
SvPDE purification was performed using (A) Sephadex G-75 followed by (B) MonoQ, (C) HiTrap Heparin, (D) MonoS, and (E) Superdex 200 columns. Red-highlighted fractions contained svPDE. (A) At first, size-exclusion chromatography separated the crude venom into four fractions: high (HW), medium (MW), and low molecular weight proteins (LW), and small peptides (SP), in which svPDE with a molecular weight of ~100k Da appeared in the HW, the first fraction of the elution. (B) Secondly, anion-exchange chromatography with a MonoQ column captured negatively charged proteins, leaving svPDE in P1, the flow-through fraction. (C) The next affinity chromatography (HiTrap Heparin) and (D) cation-exchange chromatography (MonoS) further separated svPDE from other high molecular weight venom components. (E) Lastly, the ultra-pure svPDE was acquired after the Superdex 200 column. The purity of svPDE analyzed by SDS-PAGE under reducing conditions is shown in the inset of the figure (see Figure 3—source data 1 for original uncropped image). The svPDE purified from N. atra venom was estimated to be about 0.1–0.2% of crude venom.

The structural overlay of AMP-complexed snake venom phosphodiesterase (svPDE), ENPP3, and ENPP1.
(A) The crystal structure of AMP-complexed svPDE is superimposed with AMP-complexed ENPP1 (PDB accession: 6WFJ) (Dennis et al., 2020). (B) The crystal structure of AMP-complexed svPDE is superimposed with AMP-complexed ENPP3 (PDB accession: 6F2V) (Döhler et al., 2018).

Identification of N-glycosylated sites and N-glycan patterns of snake venom phosphodiesterase (svPDE) from Naja atra based on electron density distributions from X-ray diffraction data and mass spectrometric methods.
The most complete composition of the major form at each site is depicted in the table. Glycan residues observed in crystal structures are highlighted in dark colors, and those depicted as light colors were only observed according to the mass spectrometric data. Four glycans (N39, N270, N746, and N765) were fucosylated and three glycans (N216, N259, and N512) adopted high mannose structures. The raw data can be retrieved from the following links: here, here and here.

Putty (sausage) representation of the crystal structures of snake venom phosphodiesterase (svPDE) from Naja atra.
The radii of the backboned structures are proportional to the averaged B-factors of the individual residues (or the Cα atoms), which are color-ramped from blue to red for low to high B-factors.

Nucleotide and NAD-hydrolysis activities of snake venom phosphodiesterase (svPDE) from Naja atra.
(A) HPLC analysis of 40 nM svPDE incubated with 1 mM ATP for 0, 1, 3, 5, 15, and 30 min (AU: absorbance unit). (B) Michaelis–Menten plot of the hydrolysis of ATP by 40 nM svPDE. (C) HPLC analysis of 40 nM svPDE incubated with 1 mM ADP for 0, 1, 3, 5, 15, 60, and 90 min. (D) Michaelis–Menten plot of the hydrolysis of ADP by 40 nM svPDE. (E) HPLC analysis of 80 nM svPDE incubated with 1 mM NAD for 0, 1, 2, 4, and 6 min. (F) Michaelis–Menten plot of the hydrolysis of NAD by 80 nM svPDE (NMN: nicotinamide mononucleotide).
Additional files
-
Supplementary file 1
Summary of genomic and transcriptomic data used in this study.
- https://cdn.elifesciences.org/articles/83966/elife-83966-supp1-v2.docx
-
Supplementary file 2
The summaries of structure and biochemical analyses.
(A) Crystallographic and refinement statistics. (B) Direct biochemical measurement of the substrate specificity.
- https://cdn.elifesciences.org/articles/83966/elife-83966-supp2-v2.docx
-
Supplementary file 3
The summaries of the sequencing and assembly results.
(A) Statistics of PacBio CCS reads. (B) Statistics of Illumina paired-end reads (C) Statistics of draft assembly.
- https://cdn.elifesciences.org/articles/83966/elife-83966-supp3-v2.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/83966/elife-83966-mdarchecklist1-v2.pdf





