Two forms of asynchronous release with distinctive spatiotemporal dynamics in central synapses
Figures
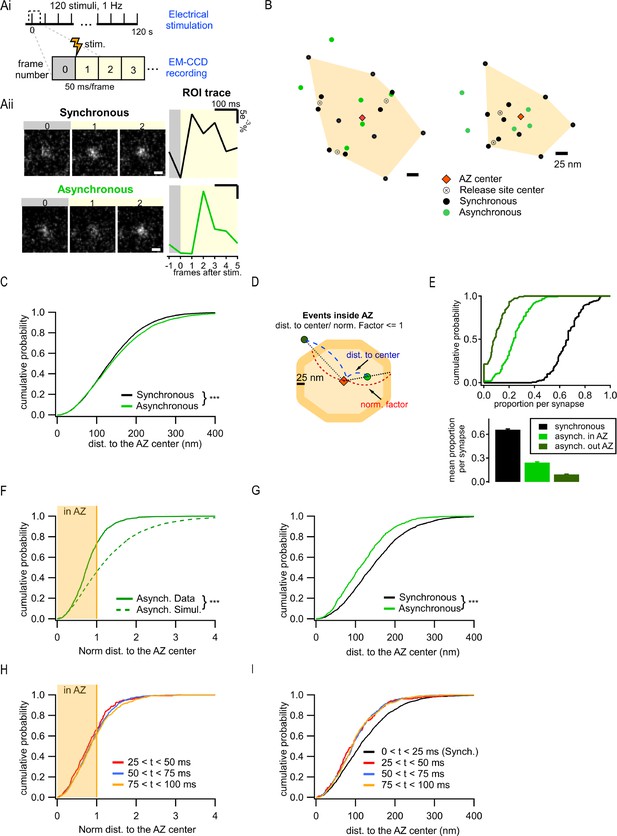
Two spatially distinct populations of asynchronous release events at individual synaptic boutons.
(Ai) Stimulation and recording protocol. Each square represents a single frame of 50 ms duration. (Aii) Left: examples of raw fluorescence images of a single vesicle release event before (frame 0) and after (frames 1,2) a single action potential (AP) stimulation, for one synchronous (top) and one asynchronous (bottom) event. Scale Bar is 500 nm. Right: the corresponding event time course was determined with a circular ROI of 10 pixels and expressed as dF/F0. Note the frame number in which an increase in fluorescence is detected: #1 for synchronous and #2 for asynchronous. (B) Two examples of active zones (AZs) with the localization of synchronous (black) and asynchronous (green) events. The AZ areas are delimited by the convex hull of the synchronous events (orange), whose centers are depicted by an orange diamond. The locations of release sites (crossed circles) were determined by hierarchical clustering analysis of synchronous events. (C) Cumulative distribution of the distances to the AZ center for synchronous (black) and asynchronous (green) events (***p<0.0001, K-S test). (D) Cartoon illustrating the normalization of the distance to the AZ center for release events. The orange area represents an AZ, with its center (red diamond) and two examples of release events, one localized inside the AZ area (light green) and one outside (dark green). The 25 nm rim (approximating vesicle radius) was added to the AZ border to account for the uncertainty in the localization of the events that define the AZ area. Distance normalization was performed using the line connecting the AZ center and the event’s location, such that the distance from the AZ center to the event (delimited in blue) was normalized to the distance from the center to the border of the AZ (delimited in red) along the same line. Events with normalized distance to the AZ center ≤1 were classified as being inside the AZ; events with normalized distance >1 were classified as being outside the AZ. (E) Cumulative distributions (top) and mean proportions per synapse (bottom) of synchronous events, and asynchronous events inside (light green) or outside (dark green) the AZ (as defined in D) across the synapse population. (F) Cumulative distributions of the normalized distances to the AZ center for all asynchronous events (continuous green line, N = 1089) and for simulated asynchronous events whose localization was randomly assigned (see ‘Materials and methods’) (dotted green line, N = 10,890) (***p<0.0001, K-S test). The shaded area represents the fraction of events localized inside the AZ (normalized distance to the center ≤1). (G) Cumulative distributions of the distances to the AZ center for a subpopulation of asynchronous events localized inside the AZ (green line, N = 799) and for synchronous events (black line, N = 1956) (***p<0.0001, K-S test). (H) Cumulative distributions of the normalized distances to the AZ center for asynchronous events recorded with 25 ms/frame acquisition rate using near-TIRF imaging and detected in three temporal windows: 25–50 ms (red), 50–75 ms (blue), and 75–100 ms (orange). The shaded area represents the fraction of events localized inside the AZ (normalized distance to the center ≤1). (I) Cumulative distributions of the distances to the AZ center for events recorded with 25 ms/frame acquisition rate using near-TIRF imaging, comparing synchronous events (black, 0–25 ms) and asynchronous events detected in three temporal windows: 25–50 ms (red), 50–75 ms (blue), and 75–100 ms (orange).
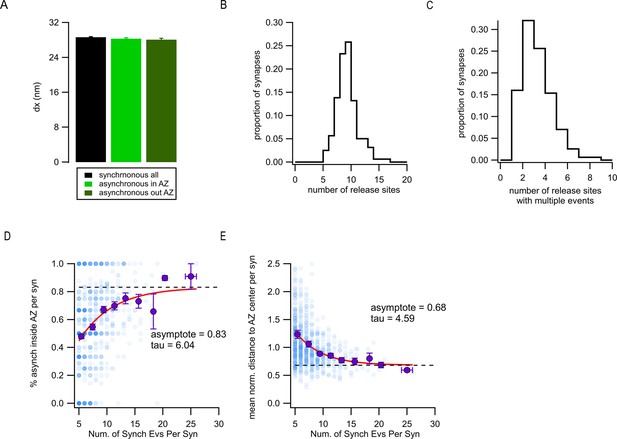
Ectopic asynchronous events are not an artifact of the variable localization precision or insufficient number of detected events.
(A) Error of event localization (localization precision, dx) for synchronous events, asynchronous events inside the active zone (AZ), and ectopic asynchronous events. (B) Distribution of the number of release sites per AZ in synapse population. (C) Same as (B), for repeatedly reused release sites (i.e. with two or more events per release site detected). (D) Proportion of asynchronous events located inside the AZ vs. the total number of synchronous events detected for every synapse (blue, N = 944 synapses). The intensity of the color increases proportionally with the number of synapses represented in each point. The binned values (purple) were fitted by a single exponential (red). (E) Mean normalized distance to the AZ center of asynchronous events vs. the total number of synchronous events detected for every synapse (blue, N = 944 synapses). The intensity of the color increases as several points overlap. The binned values (purple) were fitted by a single exponential.
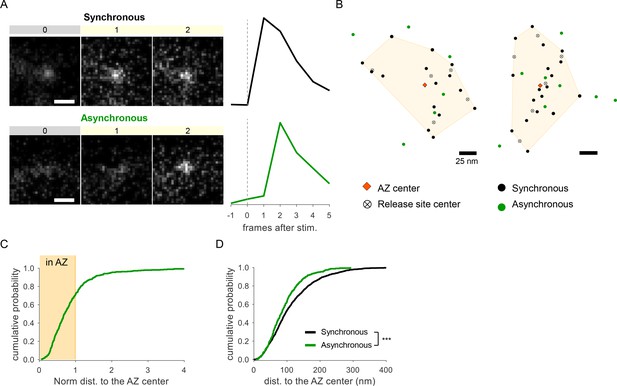
Detection of two spatially distinct populations of asynchronous events at individual synaptic boutons using glutamate sensor iGluSnFR.
(A) Examples of raw fluorescence images of single glutamate release events detected using SF-iGluSnFR(A184S) before (frame 0) and after (frames 1,2) a single action potential (AP) stimulation, for one synchronous (top) and one asynchronous (bottom) event. Scale Bar is 1 µm. Each square represents a single frame of 50 ms duration. Right: the corresponding event time course was determined with a circular ROI of 10 pixels and expressed as dF/F0. (B) Two examples of active zones (AZs) with the localization of synchronous (black) and asynchronous (green) events detected by imaging of SF-iGluSnFR(A184S). The AZ areas are delimited by the convex hull of the synchronous events (yellow), whose centers are depicted by an orange diamond. The locations of release sites (crossed circles) were determined by hierarchical clustering analysis of synchronous events. (C) Cumulative distribution of the normalized distances to the AZ center for all asynchronous events detected by near-TIRF imaging of SF-iGluSnFR(A184S). The shaded area represents the fraction of events localized inside the functionally defined AZ (normalized distance to the center ≤1). 1370 asynchronous events from 105 synapses, 10 coverslips, 3 independent cultures. (D) Cumulative distributions of the distances to the AZ center for a subpopulation of asynchronous events localized inside the AZ (green line) and for synchronous events (black line) as detected by imaging of SF-iGluSnFR(A184S).
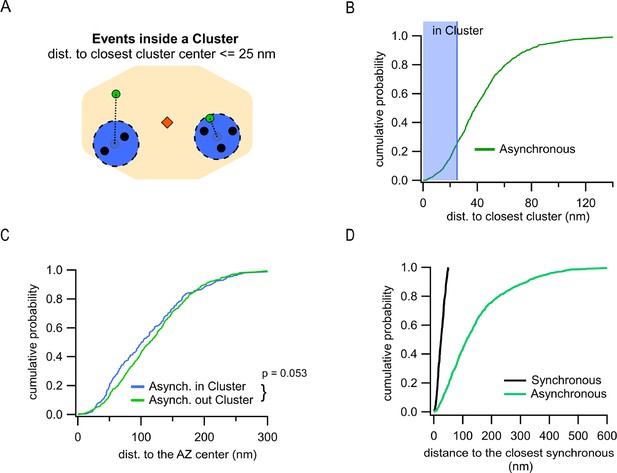
Differential utilization of release sites by synchronous and asynchronous release events.
(A) Cartoon showing a single active zone (AZ) (orange) with two sample clusters/release sites (blue circles) illustrating the assignment of asynchronous events (green dots) as being inside or outside a cluster. Black dots represent synchronous events inside a cluster. Asynchronous events localized ≤25 nm from any cluster center were considered to be a part of that cluster (right example), while asynchronous events at longer distances to any cluster center were considered as being outside of a cluster (left example). (B) Cumulative distribution of the distances to the closest cluster for the subpopulation of asynchronous events inside the AZ (N = 799). The shaded area highlights the proportion of events that were considered as part of a cluster. (C) Cumulative distributions of the distances to the AZ center for asynchronous events within the AZ, which are classified as being either inside (blue, N = 203) or outside (green, N = 596) a cluster (n.s., p=0.053, K-S test). (D) Cumulative distributions of the distances from synchronous or asynchronous events to the nearest synchronous event. Only synchronous events corresponding to release sites undergoing repeated utilization (with at least two release events detected) were used in this analysis.
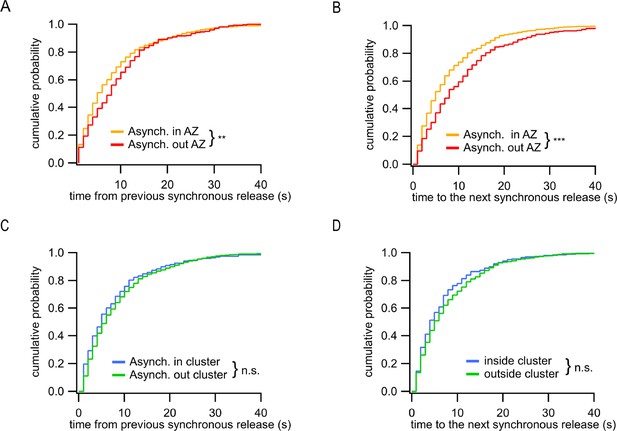
Distinct temporal features of ectopic asynchronous release events.
(A) Cumulative distributions of the time period between each asynchronous event and the immediately preceding synchronous event for asynchronous events inside the active zone (AZ) (yellow, N = 799) or outside the AZ (red, N = 290) (**p=0.0017, K-S test). (B) Same as (A) for the time period from the two types of asynchronous events to the next synchronous event (***p<0.0001, K-S test). (C) Same as (A) for asynchronous events inside a cluster (blue, N = 203) or outside a cluster (green, N = 596) (n.s., p=0.18, K-S test). (D) Same as (B) for asynchronous events inside a cluster (blue, N = 203) or outside a cluster (green, N = 596) (n.s., p=0.31, K-S test).
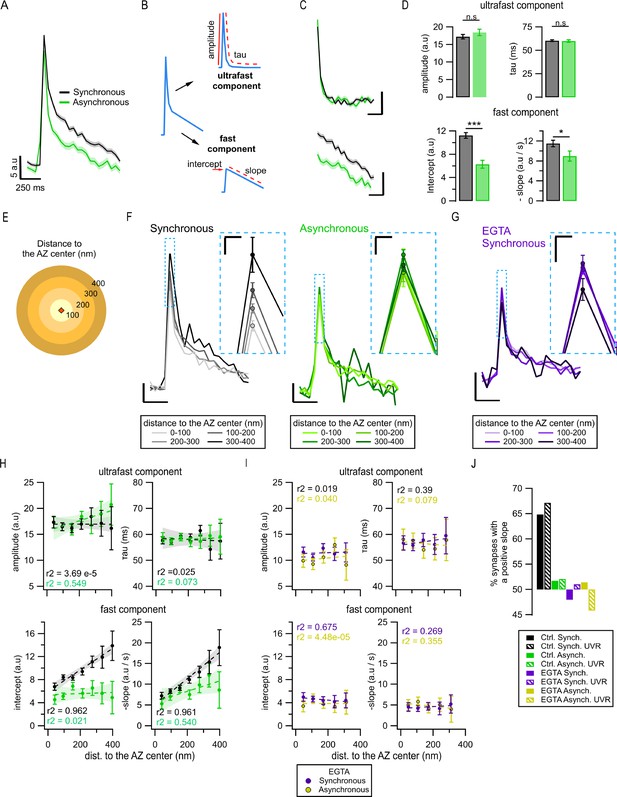
Exo-endocytosis coupling of synchronous and asynchronous release events.
(A) Mean pHluorin signals of synchronous (black, N = 1774) and asynchronous (green, N = 975) release events; SEM displayed as a shading. (B) Cartoon illustrating how the decay of the pHluorin signal was dissected in two components for each individual event (see ‘Materials and methods’): the first, known as ultrafast component, was characterized by the tau and amplitude of an exponential decay; the second, known as fast, was defined by the intercept and slope of a linear fit. (C) Average ultrafast and fast components from the traces in (A). Each component was isolated from the traces of individual events and then averaged. Calibration bars: 5 a.u., 250 ms. (D) Quantification of ultrafast (top) and fast (bottom) components from the individual synchronous (black) and asynchronous (green) events (*p=0.028; ***p<0.0001, n.s., not significant, K-S test or two-sample t-test). (E) Cartoon representing the concentric spatial bins (100 nm width) around the active zone (AZ) center (red diamond), used to average the pHluorin signals at the individual boutons. (F) Mean pHluorin signals per bin (as in E), for synchronous (black) and asynchronous (green) release events. The inset is a zoom-in on the peak of the signal showing increase in amplitude with the distance to the AZ center for synchronous, but not for asynchronous events. Calibration bars: 5 a.u., 250 ms. Inset: 2.5 a.u., 25 ms. (G) Mean pHluorin signals per bin for synchronous events in the presence of EGTA-AM. The inset is a zoom-in of the peak showing that the signal’s amplitude no longer increases with the distance to the AZ center. Calibration bars: 5 a.u., 250 ms. Inset: 2.5 a.u., 25 ms. (H) Quantification of the ultrafast (top) and fast (bottom) components of the pHluorin signal decay as a function of the distance to the AZ center for synchronous (black) and asynchronous (green) events. The circles represent the binned data with SEM bars. The lines are linear fits of the data, and the shadows are the confidence intervals for the linear regression of the data. (I) Same as in (H) but for the events recorded in the presence of EGTA-AM. Note the lack of correlation between the distance to the AZ center and any of the features measured. (J) Proportion of synapses that show a positive correlation between the amplitude of the pHluorin signal and the distance to the AZ center (see ‘Materials and methods’), for the two forms of release and their different treatments. Bars labeled as univesicular (UVR) correspond to the analyses in which we removed multivesicular (MVR) events (i.e. UVR-only conditions). The values are plotted relative to 50%, which represents the probability of observing a positive or negative correlation by chance. Note that EGTA-AM strongly reduces the proportion of synapses with a positive correlation for synchronous release, while removing MVR events does the opposite.
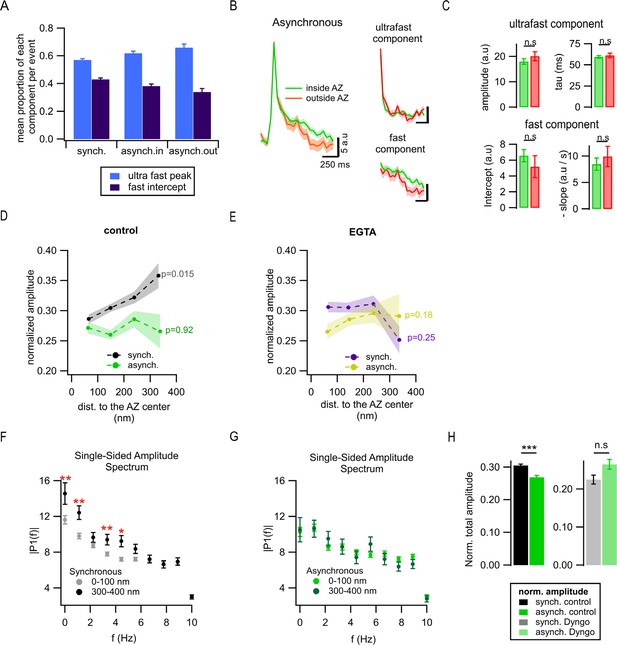
Exo-/endocytosis coupling of different forms of release.
(A) Mean proportion of two components of endocytosis (ultrafast and fast) among synchronous events, asynchronous events inside the active zone (AZ), and ectopic asynchronous events. (B) Mean pHluorin signals from asynchronous events located inside (green, N = 682) and outside (red, N = 253) the AZ (left), and their ultrafast and fast components of decay are shown separately (right). SEM is displayed as shading. (C) Quantification of ultrafast (upper bar graphs) and fast (bottom bar graphs) components of pHluorin decay of the individual asynchronous events located inside (green) or outside (red) the AZ. n.s., not significant, K-S test. (D) Mean normalized (x − min / max − min) event amplitude per 100 nm concentric spatial bin (same data as shown in Insets in Figure 5F), plotted as a function of distance to AZ center for synchronous (black) and asynchronous (green) release events. Pearson’s linear correlation algorithm was used to determine correlation and significance. The shadows represent the SEM. The p-values were obtained after a linear correlation analysis. (E) Same as in (D) in the presence of EGTA-AM. (F, G) Mean single-sided amplitude spectrums calculated using the discrete Fourier transform from pHluorin signals of events located at 0–100 nm and 300–400 nm from the AZ center, for synchronous (F) and asynchronous (G) events. Each frequency was compared statistically by two-sample K-S test, only the statistically different frequencies are labeled. Note that the populations of synchronous events differ in a distance-dependent manner only at low to medium frequencies, but the asynchronous events do not. *p<0.05; **p<0.01, K-S test. (H) Mean normalized (x − min / max − min) amplitude of individual synchronous vs. asynchronous release events in basal conditions (two left bars) or in the presence of dynamin inhibitor dyngo-4a (50 µM) (two right bars). Note that the alterations in synchronous event amplitude across the two conditions (basal vs. dyngo-4a) cannot be interpreted to represent changes in endocytosis since multiple independent factors that influence event amplitude (i.e. prevalence of multivesicular [MVR], background, etc.) are also altered by the presence of dyngo-4a. ***p<0.001; n.s., not significant, two-sample t-test.
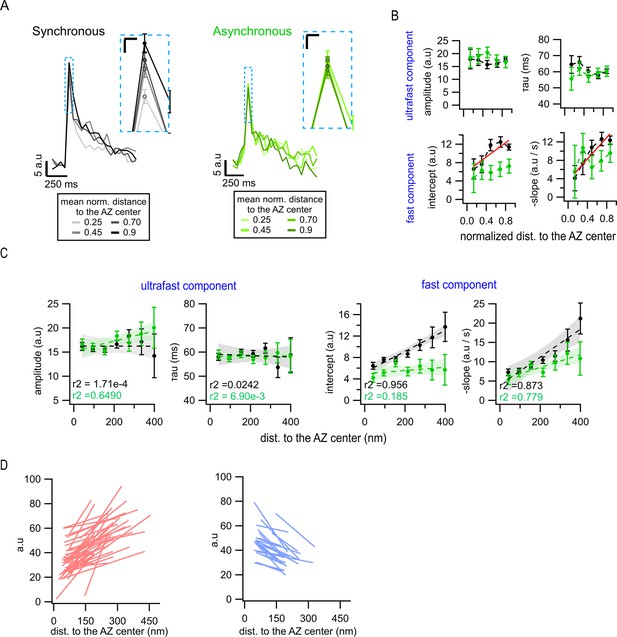
Spatial dependence of endocytosis kinetics of different forms of release.
(A) Spatial analysis of the pHluorin signal using normalized distances to the active zone (AZ) center. Mean pHluorin signals are shown for bins at four different normalized distances (0.25, 0.45, 0.70, and 0.90) for synchronous (left, black) and asynchronous (right, green) events. The inset highlights the peak area. Calibration bars: 5 a.u., 250 ms. Inset: 2.5 a.u., 25 ms. (B) Quantification of the ultrafast and fast components of endocytosis as a function of the normalized distance to the AZ center for synchronous (black) and asynchronous (green) events in the population of univesicular (UVR)-only events (i.e. after multivesicular [MVR] events were removed). The filled circles represent the binned data with SEM bars. The red lines represent linear fits. (C) Same as (B) for non-normalized distances to the AZ center in the population of UVR-only events (i.e. after MVR events were removed). (D) Measurements of a correlation between event’s amplitude and distance to the AZ center at individual boutons. Synchronous event amplitudes were binned (bin of 80 nm), plotted as a function of distance to the AZ center and linearly fitted for each bouton. These linear fits representing spatial dependence of the event amplitude at individual synapses displayed a positive slope (left, red lines) or a negative slope (right, blue lines) slope. Only the 100 synapses with the largest number of events were included in this analysis. The threshold to accept a positive or negative correlation was set to R2 > ±0.1 to mitigate the effects of noise. Note that the proportion of synapses showing a positive slope is visibly larger than the ones with a negative slope, which is quantified in Figures 5J and 6I.

Differential exo-/endocytosis coupling of synchronous and asynchronous release is not caused by differences in localization precision or lateral diffusion.
(A) Error of event localization (localization precision, dx) for all synchronous events, synchronous events in the first concentric spatial bin (bin 1; 0–100 nm from the active zone [AZ] center), or in the fourth concentric spatial bin (bin 4; 300–400 nm from the AZ center). Note that the small (~7%) difference in localization error between the two locales at the AZ is opposite in the direction of change from the differences in event amplitude and cannot account for it. (B) Average spatial profile of synchronous events in the first concentric spatial bin (0–100 nm from the AZ center, gray), and in the fourth concentric spatial bin (300–400 nm from the AZ center, black). For each event, the x and y signals were averaged to reduce the noise, obtaining a single profile. Calibration bars x = 200 nm, y = 25 a.u. (C–F) Average spatial profiles of synchronous events at 37°C (red) or room temperature (blue), compared separately for the first three frames (C), and quantified in terms of their peak amplitudes (D), width (E), and ultrafast component of decay (F). The traces were normalized to the mean peak amplitude in frame 1. The mean traces in (C) were fitted by a single Gaussian with the amplitude and width as free parameters. The error bars represent standard deviations of the fit coefficients. Mean ultrafast component was measured from individual synchronous events (n.s., not significant; p=0.064, K-S test).
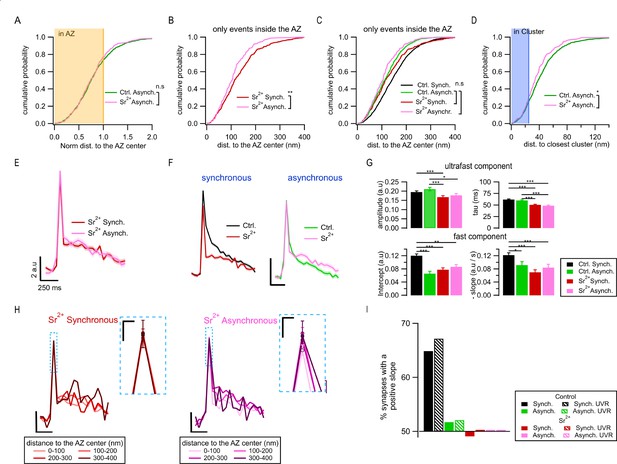
Spatiotemporal properties of Sr2+-evoked asynchronous release events.
(A) Cumulative distributions of the normalized distances to the active zone (AZ) center for asynchronous events recorded under control conditions (green, N = 1089) and those recorded in 4 mM Sr2+ (see ‘Materials and methods’; pink, N = 232; n.s., p=0.79, K-S test). The shaded area represents the fraction of events localized inside the AZ (normalized distance to the center ≤ 1). (B) Cumulative distributions of the distances to the AZ center for synchronous events (red line, N = 392) and asynchronous events localized inside the AZ (pink, N = 177), both recorded in Sr2+ (**p=3.3e-4, K-S test). (C) Same data as in (B), plotted together with their equivalents in control conditions: synchronous (black) and asynchronous (green) (same data as in Figure 1G). Only the nonsignificant comparisons are shown (all the other comparisons are given in Supplementary file 1). (D) Cumulative distributions of the distances to the closest cluster/release site for asynchronous events in control conditions (green, N = 799) and in Sr2+ (pink, N = 177). (*p=0.023, K-S test). The shaded area highlights the proportion of events that were considered to be in a cluster. (E) Mean pHluorin signals of synchronous (red, N = 1413) and asynchronous (pink, N = 917) events recorded in Sr2+ with SEM displayed as a shading. (F) Comparison of the mean normalized pHluorin signals (Min-max normalization of each individual trace: trace – min(trace)/max(trace) – min(trace)) of synchronous (left) and asynchronous (right) events under control and Sr2+ conditions. Calibration bar: 0.1 a.u., 250 ms. (G) Quantification of the ultrafast component (upper bar graphs: amplitude and tau) and fast component (bottom bar graphs: intercept and slope) of the pHluorin signal decay for synchronous and asynchronous events in control and Sr2+ conditions, as indicated (***p<0.0001, Kruskal–Wallis test, all statistical comparisons are given in Supplementary file 1). (H) Spatial analysis of the pHluorin signal in Sr2+ conditions using the same approach and bin size as in Figure 5E. Mean pHluorin signals per spatial bin are shown for synchronous (red) and asynchronous (pink) events in the presence of Sr2+. Insets show zoom-in of the peaks. Calibration bars: 2 a.u., 250 ms. Inset: 1 a.u., 25 ms. (I) Proportion of synapses that show a positive correlation between the amplitude of the pHluorin signal and the distance to the AZ center (see ‘Materials and methods’) for the two forms of release in control and Sr2+ conditions. Bars labeled as univesicular (UVR) correspond to the analyses in which we removed multivesicular (MVR) events (i.e. UVR-only conditions). The values are plotted relative to 50%, which represents the probability of observing a positive or negative correlation by chance. Sr2+ (red and pink) eliminated the positive correlation between the amplitude of synchronous events and distance to the AZ center.
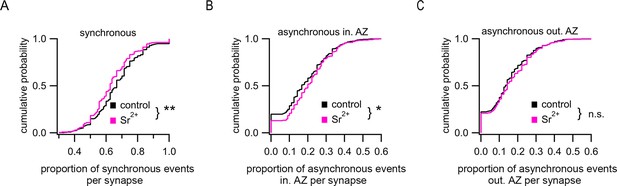
Application of Sr2+ causes desynchronization of release.
(A–C) Proportions of synchronous events (A), asynchronous events inside the active zone (AZ) (B), and ectopic asynchronous events (C) per synapse in control conditions or in the presence of 4 mM Sr2+. Synapses with any number of detected events were included in this analysis. *p<0.05, **p<0.01, n.s., not significant, two-sample K-S test.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Transfected construct (synthetic) | pFU-vGluT1- pHGFP-W | Viral Vectors Core at Washington University | n/a | |
| Transfected construct (synthetic) | pAAV.hSynapsin.SF-iGluSnFR.A184S | Addgene | 106174-AAV1 | |
| Biological sample (rat, Long-Evans) | Hippocampus of rat pups | Charles River | 006 | |
| Chemical compound, drug | APV | Sigma-Aldrich | 79055-68-8 | |
| Chemical compound, drug | CNQX disodium salt hydrate | Sigma-Aldrich | 115066-14-3 | |
| Chemical compound, drug | Minimum Essential Media (MEM) – no phenol red | Gibco | 51200038 | |
| Chemical compound, drug | Characterized fetal bovine serum | Gibco | 10437028 | |
| Chemical compound, drug | Penicillin-streptomycin (5000 U/ml) | Gibco | 15070063 | |
| Chemical compound, drug | N-2 Supplement (100×) | Gibco | 17502048 | |
| Chemical compound, drug | Neurobasal-A Medium | Gibco | 12349015 | |
| Chemical compound, drug | B-27 supplement (50×), serum free | Gibco | 17504044 | |
| Chemical compound, drug | GlutaMAX Supplement | Gibco | 35050061 | |
| Chemical compound, drug | PDL(poly-D-lysine) | BD Biosciences | 40210 | |
| Chemical compound, drug | Trypsin-EDTA (0.05%), phenol red | Gibco | 25300054 | |
| Software, algorithm | MATLAB | MathWorks | RRID:SCR_001622 | |
| Software, algorithm | u-track 2.0 | https://www.utsouthwestern.edu/labs/danuser/software/#utrack_anc; (Aguet et al., 2013; Jaqaman et al., 2008) |
Additional files
-
Supplementary file 1
Table containing the statistical information for all figures.
- https://cdn.elifesciences.org/articles/84041/elife-84041-supp1-v1.xlsx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/84041/elife-84041-mdarchecklist1-v1.docx





