Cortico-thalamo-cortical interactions modulate electrically evoked EEG responses in mice
Figures
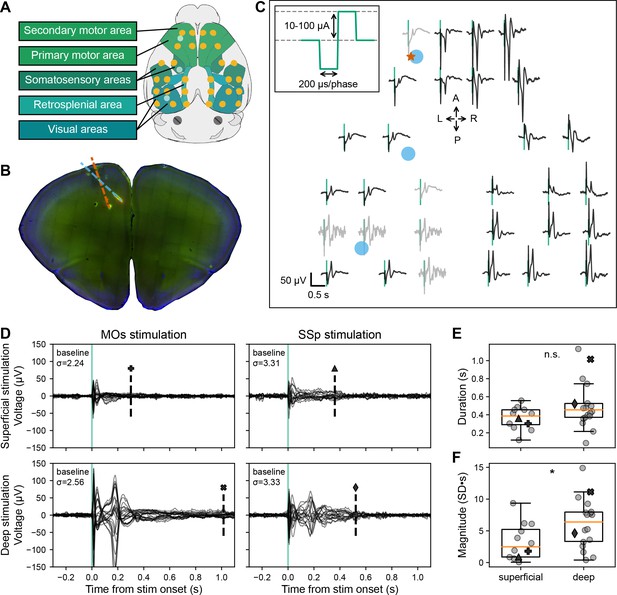
Evoked EEG responses to single pulse electrical stimulation in awake, head-fixed mice.
(A) Schematic of the 30-channel surface array (yellow circles) implanted on top of the skull over major brain areas: motor, somatosensory, retrosplenial, and visual areas (schematic created using brainrender; Claudi et al., 2021). The circular, platinum EEG electrodes are 500 μm in diameter. The three light blue circles correspond to the locations of the three acute craniotomies to place up to three Neuropixels probes and the bipolar stimulating electrode. The schematic also shows two skull screws over the cerebellum that serve as the reference and the ground for the EEG signals. (B) Histological image of a coronal brain slice showing the location of the bipolar stimulating electrode (with tips in secondary motor area [MOs], layer 5; red dashed line) and one of the Neuropixels probes (spanning layers of motor and anterior cingulate areas; blue dashed line) with fluorescent dyes (that appear red and green in the image). (C) Evoked responses from each of the 30 EEG electrodes from the awake, head-fixed mouse from –0.2 to +0.8 s following the electrical stimulus (vertical green line marks the onset time). Traces are arranged in the approximate orientation of the EEG array over the skull surface. Traces in black and gray represent signals that did and did not pass a quality control step, respectively. The red star and blue circles mark the approximate insertion point of the bipolar stimulating electrode and the Neuropixels probes, respectively. Inset: Single current pulses were biphasic (200 μs/phase), charge-balanced, and cathodic-first, with a current amplitude between 10 and 100 μA. (D) Event-related potential (ERP; –0.3 to +1.1 s around stimulus onset) with all EEG electrode traces superimposed (butterfly plots). Each of the four panels represents data from a different stimulated area and depth: top and bottom left – superficial and deep layer (same as in panel C) MOs stimulation in the same subject; top and bottom right – superficial and deep layer primary somatosensory area (SSp) stimulation in a different subject. The dashed vertical line indicates the duration of the evoked signal; the marker above matches with the marker representing the value in panels E and F. The ‘baseline σ’ indicates the SD (in μV) over all electrodes during the 2 s preceding the stimulus. (E) Duration of the ERPs for all subjects based on the stimulation depth: superficial (N=12) vs. deep (N=18). (F) Normalized magnitude of the ERPs for all subjects based on the stimulation depth: superficial (N=12) vs. deep (N=18). For further details, see method ‘ERP duration and magnitude’ and Figure 1—figure supplement 1. Boxplots show median (orange line), 25th, and 75th percentiles; whiskers extend from the box by 1.5× the inter-quartile range (IQR). Student’s two-tailed t-test; * weak evidence to reject null hypothesis (0.05>p>0.01), ** strong evidence to reject null hypothesis (0.01>p>0.001), and *** very strong evidence to reject null hypothesis (0.001>p).
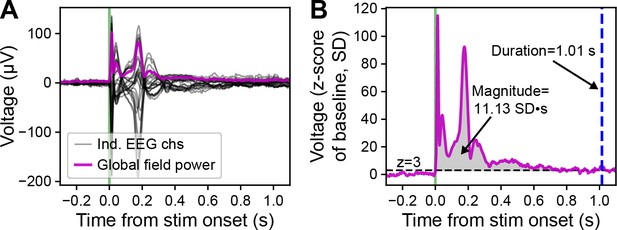
Demonstration of duration and magnitude calculation for evoked responses.
(A) Butterfly plot of the event-related potential (ERP; –0.3 to +1.1 s around stimulus onset, green vertical line) from deep stimulation in MOs (black lines). The global field power (magenta line) is calculated as the standard deviation of voltage values across all electrodes at each time sample. Same example as shown in Figure 1C and D, bottom left. (B) The global field power (baseline z-score; –0.3 to +1.1 s around stimulus onset) from panel A. The duration of the evoked response is measured by the length of time the global field power remains above a threshold of three SDs above the baseline mean (z=3 SD, represented by the horizontal, black, dashed line) in the response window from stimulus onset to +2 s after. The duration of this example is 1.01 s, represented by the vertical, dashed, blue line. The magnitude of the evoked response is measured by the AUC of the z-scored global field power above the threshold (z=3 SD) in the response window from stimulus onset to +2 s after. The magnitude of this example is 11.13 SD∙s, represented by the gray shaded region.
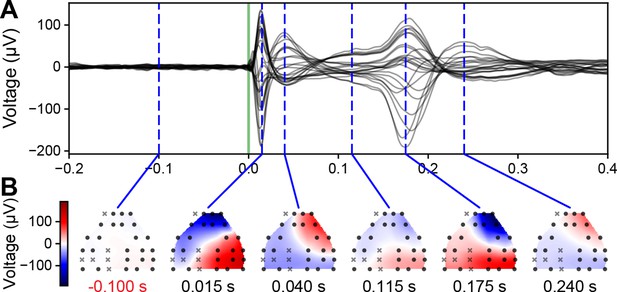
Spatial extent of evoked EEG responses in awake, head-fixed mice.
(A) Butterfly plot of the event-related potential (ERP; –0.2 to +0.4 s around stimulus onset, green vertical line) from deep stimulation in MOs. Dashed vertical blue lines indicate the time of the spatial interpolation below. Same example as shown in Figure 1C and D bottom left. (B) The spatial interpolation of the instantaneous voltage across the surface of the mouse brain sampled by the EEG electrodes. Black circles and gray x-marks represent electrodes that did and did not pass a quality control step, respectively. The white band running across cortex represents the transition between the two polarities of the EEG signals.
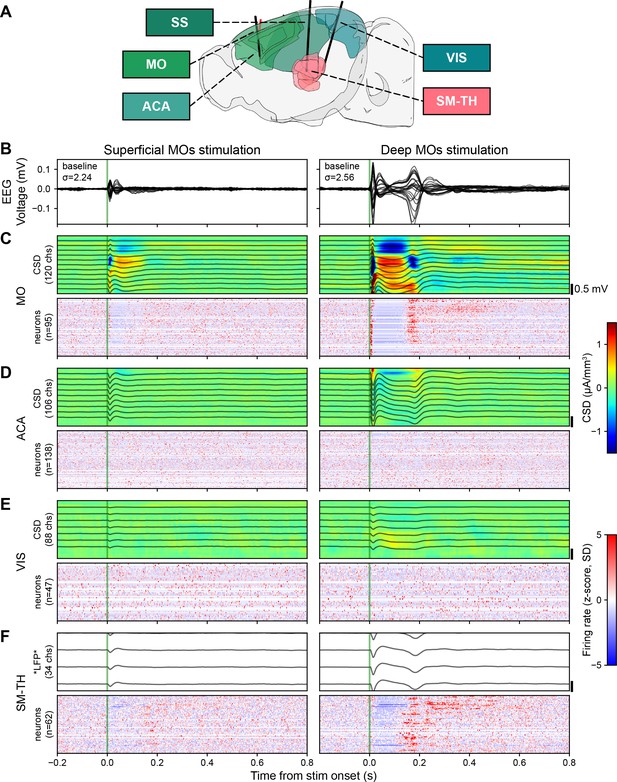
Electrical stimulation evokes strong responses in the EEG, LFP, CSD, and in some populations of neurons (locally in MO and in SM-TH) when deep layers of MOs are directly activated.
(A) Sagittal schema of the mouse brain, highlighting motor (MO), anterior cingulate (ACA), somatosensory (SS), visual (VIS), and somatomotor-related thalamic (SM-TH) areas (created using brainrender; Claudi et al., 2021). Solid black lines show the approximate locations of three acutely inserted Neuropixels probes; the red line indicates the stimulating electrode in the deep layers of MOs. (B) Butterfly plots of the event-related potential (ERP; –0.2 to +0.8 s around stimulus onset) evoked during the awake state. Each column represents data from a different stimulated depth (superficial and deep MOs) in the same subject (same subject shown in Figure 1D left top and bottom). (C) Evoked responses from the Neuropixels electrodes in MO. (Top) From the measured LFP band (black traces representing 1 out of every 10 channels), the CSD response was computationally inferred (heat map, red and blue represent sources and sinks, respectively). The number of Neuropixels channels used to compute CSD is indicated along the y-axis. Bottom: Normalized firing rate, reported as a z-score of the average, pre-stimulus firing rate, of all neurons (only regular spiking [RS] neurons in cortical regions) recorded by the Neuropixels probes targeting the area of interest. The number of neurons (n) in each area is included along the y-axis. (D) Evoked responses from ACA, same as panel C. (E) Evoked responses from VIS, same as panel C. (F) Evoked responses from SM-TH, CSD was not computed for SM-TH because thalamic structures, unlike cortex, do not contain oriented neural elements; therefore, it would not be interpretable.
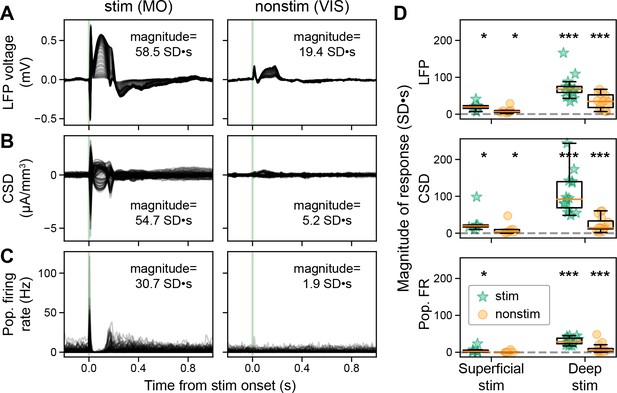
Direct stimulation of deep, but not superficial, cortical layers evokes widespread responses.
(A) Evoked local field potential (LFP; –0.2 to +1.2 s around stimulus onset) from deep MOs stimulation (same subject as Figure 1D bottom left), all recorded cortical sites along the Neuropixels shaft are superimposed. The two columns represent data from stimulated (left) and a non-stimulated cortex (right). The magnitude is the integrated area of the response (0 to +0.5 s from stimulus onset), z-scored relative to the integrated area of the background activity calculated by shuffling the stimulation onset 1000 times. (B) Evoked current source density (CSD) from deep MOs stimulation, all recorded cortical sites along the Neuropixels shaft are superimposed. (C) Evoked neuronal firing rates from deep MOs stimulation, each trace represents a single RS neuron. (D) Magnitude of the evoked LFP (top), CSD (middle), and population spiking (bottom) to superficial (left) and deep stimulation (right) for stimulated and non-stimulated cortical regions (green stars and orange circles, respectively), dashed gray line at zero. Superficial stimulation: n=6 stimulated regions in N=6 mice and n=17 non-stimulated cortical regions in N=7 mice. Deep stimulation: n=15 stimulated regions in N=15 mice and n=35 non-stimulated cortical regions in N=16 mice. Boxplots show median (orange line), 25th, and 75th percentiles; whiskers extend from the box by 1.5× the IQR. Wilcoxon signed-rank test, corrected for multiple comparisons using Banjamini-Hochberg false discovery rate; * weak evidence to reject null hypothesis (0.05>p>0.01), ** strong evidence to reject null hypothesis (0.01>p>0.001), and *** very strong evidence to reject null hypothesis (0.001>p).
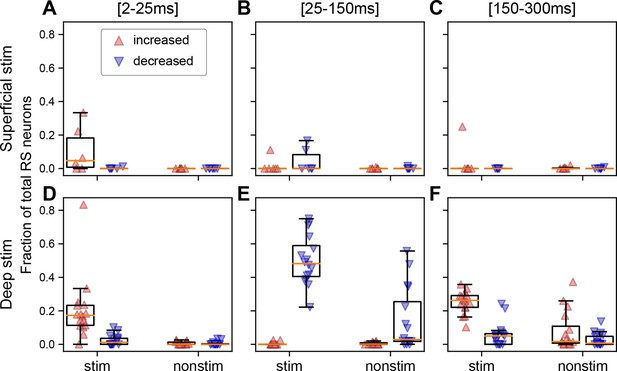
The fraction of the neuron population per ipsilateral cortical area in each subject that is significantly modulated depends on the depth of stimulation and the temporal window.
(A) Fraction of regular spiking (RS) neurons per cortical area in each subject that exhibit a significantly increased (red upward triangle) or decreased (blue downward triangle) response in the first 25 ms for superficial stimulation. Fraction of RS neurons that exhibit a significant increase or decrease (B) 25–150 ms or (C) 150–300 ms following the stimulus. (D–F) Same as panels A–C but for deep stimulation. Panels A–C are derived from 870 RS neurons from stimulated cortex and 4110 RS neurons from non-stimulated cortical regions in N=7 mice and panels D–F from 2559 RS neurons from stimulated cortex and 9363 RS neurons from non-stimulated cortical regions in N=17 mice. Boxplots show median (orange line), 25th, and 75th percentiles; whiskers extend from the box by 1.5× the IQR.
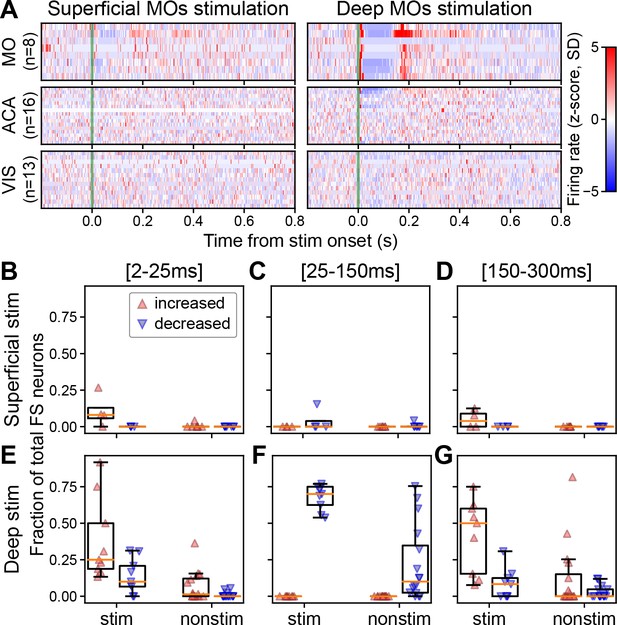
The fraction of the FS neuron population that is significantly modulated depends on the depth of stimulation and the temporal window.
(A) Normalized firing rate, reported as a z-score of the average, pre-stimulus firing rate, of fast spiking (FS) neurons recorded by the Neuropixels probes targeting cortical areas of interest (left: superficial MOs stimulation; right: deep MOs stimulation). The number of neurons (n) in each area is included along the y-axis. (B) Fraction of FS neurons that exhibit a significantly increased (red upward triangle) or decreased (blue downward triangle) response in the first 25 ms for mice that received superficial stimulation. (C) Fraction of FS neurons that exhibit a significantly increased or decreased response 25–150 ms following the stimulus. (D) Fraction of FS neurons that exhibit a significantly increased or decreased response 150–300 ms following the stimulus. (E–G) Same as panels B–D but for subjects that received deep stimulation. Data from experiments with superficial cortical stimulation (180 FS neurons from stimulated cortex and 585 FS neurons from non-stimulated cortical regions in N=7 mice) and with deep cortical stimulation (387 FS neurons from stimulated cortex and 1422 FS neurons from non-stimulated cortical regions in N=16 mice). Boxplots show median (orange line), 25th, and 75th percentiles; whiskers extend from the box by 1.5× the IQR.
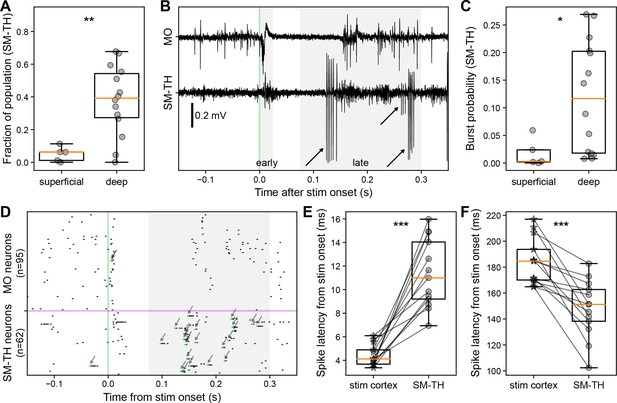
Cortical and thalamic neural dynamics evoked by cortical electrical stimulation.
(A) Fraction of somatomotor-related thalamic (SM-TH) neurons that exhibit a significant increase or decrease in firing rate compared to baseline between 2 ms and 300 ms following stimulus onset for superficial (N=5) and deep stimulation (N=14). (B) Raw traces from the Neuropixels spike band data for motor (MO; top) and SM-TH (bottom; –0.15 to +0.35 s) for one exemplar trial with deep MOs stimulation. Action potential bursts in the SM-TH are flagged with arrows. (C) Probability (fraction of total trials) that the SM-TH produces bursts within 75–300 ms from stimulus onset for superficial (N=5) and deep stimulation (N=14). (D) Single trial raster plot showing spiking of MO (top) and SM-TH neurons (bottom) in response to a single deep electrical pulse (at the green vertical line). Action potential bursts are flagged with arrows. (E) Latency to first-spike (2–25 ms) for responsive RS neurons in stimulated cortex (stars) and in associated SM-TH (circles). Populations are recorded simultaneously in each subject, represented by the connecting black lines (N=13). (F) Latency to spike in the late window (75–300 ms) for responsive RS neurons in stimulated cortex (stars) and in SM-TH (circles), as in panel E. Boxplots show median (orange line), 25th, and 75th percentiles; whiskers extend from the box by 1.5× the IQR. Student’s two-tailed t-test or paired t-test (for normally distributed data), or Mann-Whitney U test (non-parametric); * weak evidence to reject null hypothesis (0.05>p>0.01), ** strong evidence to reject null hypothesis (0.01>p>0.001), and *** very strong evidence to reject null hypothesis (0.001>p).
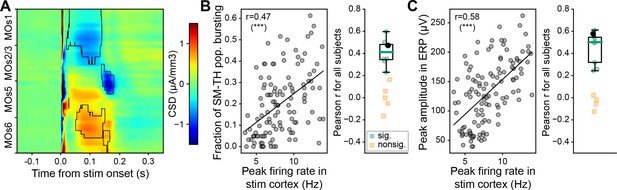
Thalamic origins of the late ERP component.
(A) Population level (N=9) current source density (CSD) analysis of MOs after deep MOs stimulation (sinks blue and sources red). The black traces outline the areas with significant and consistent responses across subjects (Wilcoxon signed-rank test, p<0.05). (B) Left: Pearson correlation between fraction of somatomotor-related thalamic (SM-TH) neurons that burst and peak cortical population firing rate on a trial-by-trial basis for one mouse (same subject as in Figure 1D bottom left). Right: Same as left, computed for all (N=13) mice with deep stimulation; the black circle represents the subject from the left panel, green circles represent subjects with a significant correlation (p<0.05), and yellow squares represent subjects with a non-significant correlation. (C) Left: Pearson correlation between peak amplitude of the second, late component in the event-related potential (ERP) and peak cortical population firing rate on a trial-by-trial basis for one example mouse (as in panel B). Right: Same as left, computed for all subjects with deep stimulation (N=13), represented as in panel B. Boxplots show median (green line), 25th, and 75th percentiles; whiskers extend from the box by 1.5× the IQR. * Weak evidence to reject null hypothesis (0.05>p>0.01), ** strong evidence to reject null hypothesis (0.01>p>0.001), and *** very strong evidence to reject null hypothesis (0.001>p).
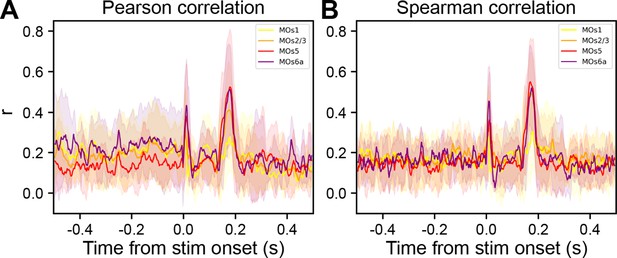
ERP is more correlated to CSD of deep, rather than superficial, cortical layers.
Time-resolved correlation of EEG with layer-specific CSD power (traces color coded by layers) at single trial level across all subjects (N=9 mice): (A) Pearson correlation and (B) Spearman correlation.
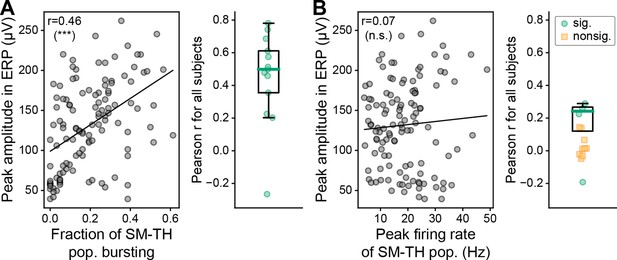
The amplitude of the second, late component in the ERP is correlated with SM-TH bursting, but not the population firing rate.
(A) Left: Pearson correlation between the peak amplitude of the second, late component in the ERP and the fraction of SM-TH neurons that burst on a trial-by-trial basis for one example mouse (same subject as in Figure 1D bottom left). Right: Same as left, computed for all subjects with deep stimulation (N=12 mice), green circles represent subjects with a significant correlation (p<0.05). (B) Left: Pearson correlation between the peak amplitude of the second, late component in the ERP and the peak SM-TH population firing rate on a trial-by-trial basis for one example mouse. Right: The correlation value (Pearson r) for all subjects with deep stimulation (N=12 mice), green circles represent subjects with a significant correlation (p<0.05) and yellow squares represent subjects with a non-significant correlation. Boxplots show median (green line), 25th, and 75th percentiles; whiskers extend from the box by 1.5× the IQR. * Weak evidence to reject null hypothesis (0.05>p>0.01), ** strong evidence to reject null hypothesis (0.01>p>0.001), and *** very strong evidence to reject null hypothesis (0.001>p).
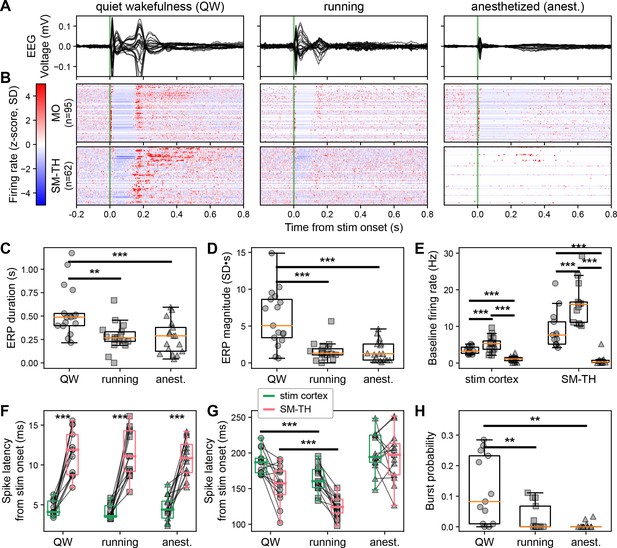
Brain state modulates the ERP via cortico-thalamo-cortical interactions.
(A) Butterfly plot of ERPs during non-running (quiet wakefulness), running (active wakefulness), and isoflurane-anesthetized states (same subject as in Figure 1D bottom left). (B) Normalized firing rate, reported as a z-score of the average, pre-stimulus firing rate, of all RS neurons recorded by the Neuropixels probes targeting the stimulated cortex (MO) and SM-TH. (C) Duration and (D) magnitude of the ERPs for all states (see also Figure 1E and F): quiet wakefulness, running, and anesthetized (N=17). (E) Baseline rates of cortical (stim cortex) and SM-TH neurons across all states (stim cortex: N=16; SM-TH: N=13). Latency to first-spike in (F) early (2–25 ms) and (G) late (100–300 ms) windows for responsive RS neurons in the stimulated cortex (green boxes) and in the SM-TH (pink boxes) across all states. Populations were recorded simultaneously in each subject, represented by the connecting black lines (N=12). (H) Probability (fraction of total trials) of SM-TH spiking bursts within 75–300 ms from the stimulus onset for the three states (N=13). Boxplots show median, 25th, and 75th percentiles; whiskers extend from the box by 1.5× the IQR. One-way or two-way RM ANOVA (for normally distributed data), or Friedman test (non-parametric); * weak evidence to reject null hypothesis (0.05>p>0.01), ** strong evidence to reject null hypothesis (0.01>p>0.001), and *** very strong evidence to reject null hypothesis (0.001>p).
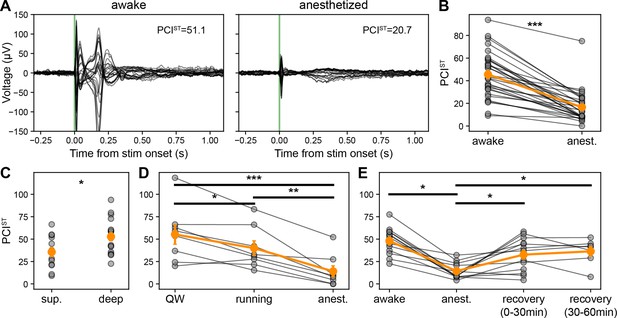
Perturbational complexity is modulated by cortico-thalamo-cortical interactions.
(A) Butterfly plot of ERPs (–0.3 to +1.1 s) from awake (left) and anesthetized states (right). Same subject as in Figure 1D bottom left, annotated with PCI, state-transition (PCIST) values. (B) PCIST calculated using EEG evoked responses (baseline –0.8 to –0.002 s and response 0.002–0.8 s) for awake and anesthetized states. Individual values represented with gray connected circles (N=31 sessions across 24 mice). (C) PCIST for superficial vs. deep cortical stimulation while mice are awake (includes quiet wakefulness and running trials). (D) PCIST for quiet wakefulness, running, and anesthetized states. Individual values represented with gray connected circles (N=8). (E) PCIST for quiet wakefulness, anesthetized, and two subsequent recovery states. Individual values represented with gray connected circles (N=13). Orange circles and error bars represent mean ± SEM. Student’s two-tailed t-test, paired t-test, or one-way RM ANOVA; * weak evidence to reject null hypothesis (0.05>p>0.01), ** strong evidence to reject null hypothesis (0.01>p>0.001), and *** very strong evidence to reject null hypothesis (0.001>p).

ERP and evoked population activity during isoflurane anesthesia do not show evidence of global responses.
(Top). ERP (-0.2 to +0.8 s around stimulus onset) with all EEG electrode traces superimposed. Data represented is the same: red traces have been processed with the average reference montage, black traces have not. (Bottom) Population mean firing rates from the areas of interest from the same experiment as above.

Evoked firing rates for neurons in the areas of interest in response to deep stimulation in MO during the awake state.
(Left) Firing rates of all neurons normalized by the average, pre-stimulus firing rate. (Right) Firing rates of all neurons normalized by the maximum post-stimulus firing rate.





