Room-temperature crystallography reveals altered binding of small-molecule fragments to PTP1B
Figures
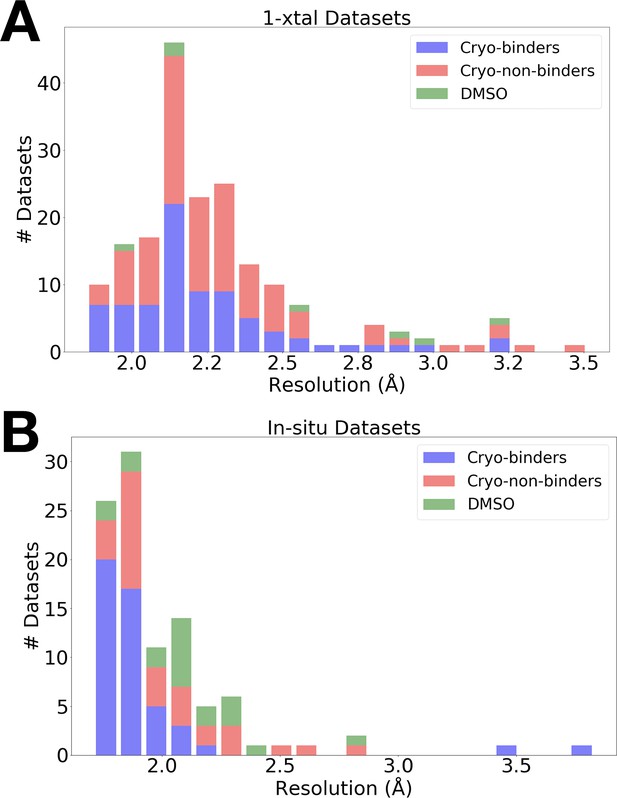
Resolution distributions from room-temperature (RT) crystallographic screens.
Histogram of X-ray resolutions of datasets soaked with DMSO (green), cryo-hits compounds (blue), or cryo-non-hits (red), collected at RT via (A) 1-xtal or (B) in situ data collection techniques.
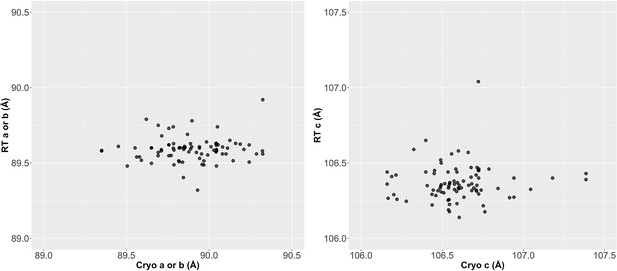
Unit cell is more variable at cryogenic (cryo) than at room temperature (RT).
A comparison of RT vs. cryo unit cells (left: cryo a or b length vs. RT a or b length, right: cryo c length vs. RT c length) reveals the cryo datasets’ unit cell size has more variability than those at RT.
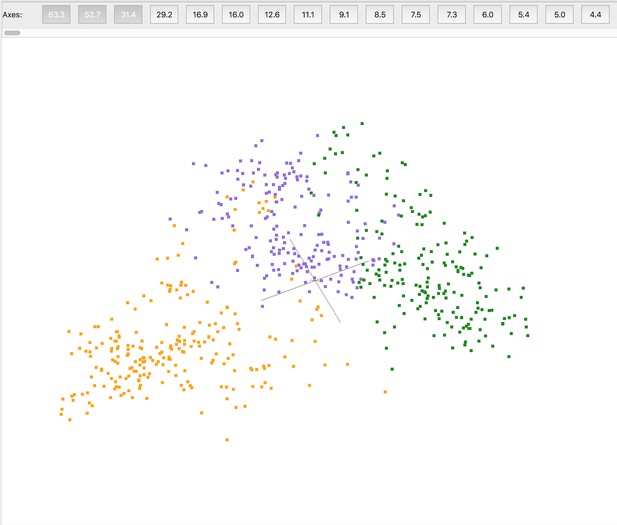
Pre-clustering partial datasets from in situ crystallography.
Partial datasets or ‘wedges’ are represented in a reduced dimensionality space based upon structure factor amplitude differences. These wedges can be approximately divided into three clusters (orange, purple, green). Some wedges at the cluster interfaces were used in multiple clusters. Wedges within a cluster were subsequently merged to generate complete datasets for input to PanDDA. Image from cluster4× (Ginn, 2020).
-
Figure 1—figure supplement 2—source data 1
Statistics for clusters of individual in situ wedges, prior to merging into complete datasets.
Values shown are averages across all wedges within each cluster. See Figure 1—figure supplement 2 for visualization of clusters using cluster4x.
- https://cdn.elifesciences.org/articles/84632/elife-84632-fig1-figsupp2-data1-v1.csv
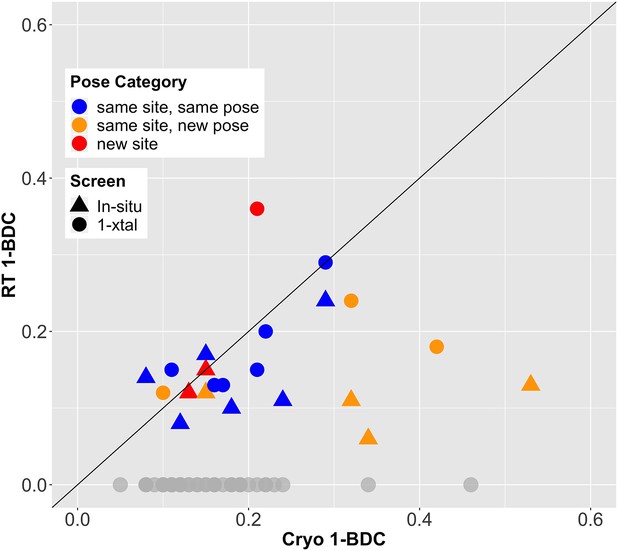
Fragment-binding occupancies are different and often lower at room temperature (RT).
1-BDC (a proxy for occupancy) is plotted for each binding event observed in either of two RT screens vs. in the previous cryogenic (cryo) screen. For two datasets, two binding events for the same fragment in the same structure are included as separate points. See Table 2 for definitions of pose categories. Those that did not show binding at RT are in gray along the x-axis. In some additional cases, RT event maps were calculated using the cryo 1-BDC to identify bound ligands at RT; these cases would sit artificially on the diagonal, and are not shown here.
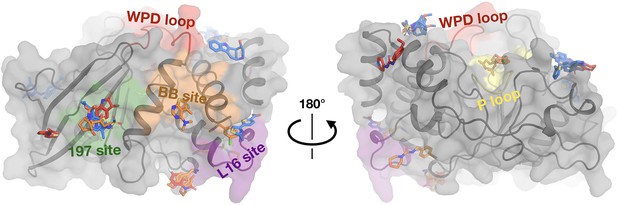
Fragments have a similar distribution across protein sites but different binding modes at room temperature (RT).
Overview of fragments bound across PTP1B at RT, colored by RT pose compared to cryogenic (cryo) pose: same site, same pose (blue); same site, new pose (orange); new site (red). See Table 2 for more details on the definitions of these classifications. Also highlighted are the active-site WPD loop (red), P loop (yellow), 197 allosteric site (green), BB allosteric site (orange), and L16 allosteric site (purple) (Keedy et al., 2018). The protein is shown in its open conformation with the WPD loop and L16 in the open state. The α7 helix is not shown since it is disordered when the protein is in the open state, which is favored at higher temperatures (Keedy et al., 2018). α7 does become ordered in one RT fragment-bound structure, but is not shown here.
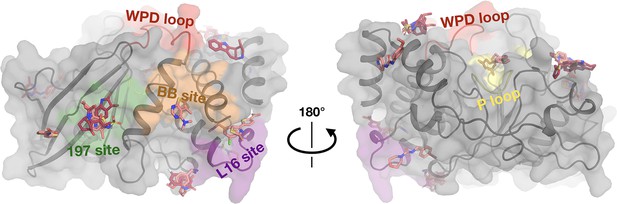
Fragments bound at room temperature (RT) colored by RT screen.
Same views and fragments as Figure 3, but with fragments colored based on the RT screen: 1-xtal (dark pink) or in situ (light pink).
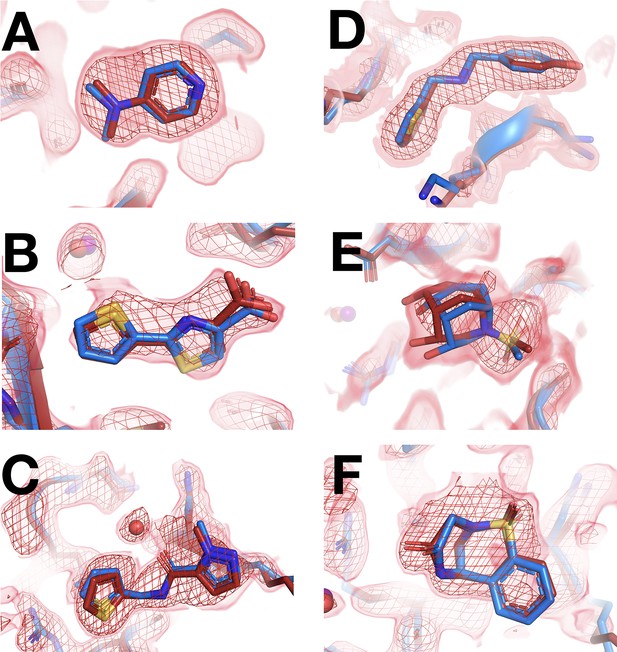
Fragments that bind similarly at room (RT) vs. cryogenic (cryo) temperatures.
For each dataset, the RT PanDDA event map is in red (contour levels below), the RT model is in red (waters in red), and the corresponding cryo model is in blue (waters in purple). Datasets are named as follows: x####=RT in situ, z####=RT 1-xtal, y####=cryo. (A–C) in situ. (D–F) 1-xtal. (A) RT: x0224 (2.0 σ), cryo: y0118. (B) RT: x0285 (1.5 σ), cryo: y0772. (C) RT: x0262 (1.5 σ), cryo: y1656. (D) RT: z0007 (2.0 σ), cryo: y1710. (E) RT: z0015 (1.8 σ), cryo: y1554. (F) RT: z0025 (1.5 σ), cryo: y1294. This figure contains selected examples of fragments that bind similarly at RT vs. cryo; for all examples, see Figure 4—figure supplement 1 for 1-xtal and Figure 4—figure supplement 2 for in situ. For examples with no RT density for the cryo ligand using the cryo 1-BDC, see Figure 4—figure supplement 3.
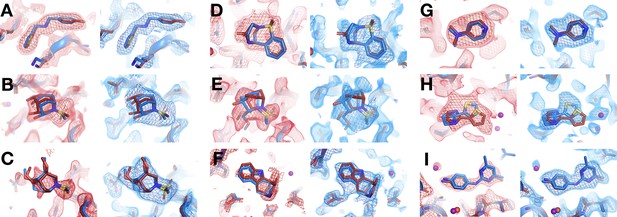
All fragments that bind similarly at room (RT) vs. cryogenic (cryo) temperatures from 1-xtal screen.
For each panel: RT event map density on left, cryo event map density on right (blue). Same coloring as main Figure 4. (A) RT: z0007 (2 σ), cryo: y1710 (1.2 σ). (B) RT: z0015 (1.8 σ), cryo: y1554 (1.5 σ). (C) RT: z0021 (1.1 σ), cryo: y1312 (1.5 σ). (D) RT: z0025 (1.5 σ), cryo: y1294 (1.5 σ). (E) RT: z0028 (1.2 σ), cryo: y1819 (1.2 σ). (F) RT: z0033 (0.8 σ), cryo: y1842 (1.2 σ). (G) RT: z0089 (2 σ), cryo: y0118 (1.5 σ). (H) RT: x0267 (1.5 σ), cryo: y0112 (1.5 σ). (I) RT: z0115 (1.5 σ), cryo: y0660 (1.5 σ).
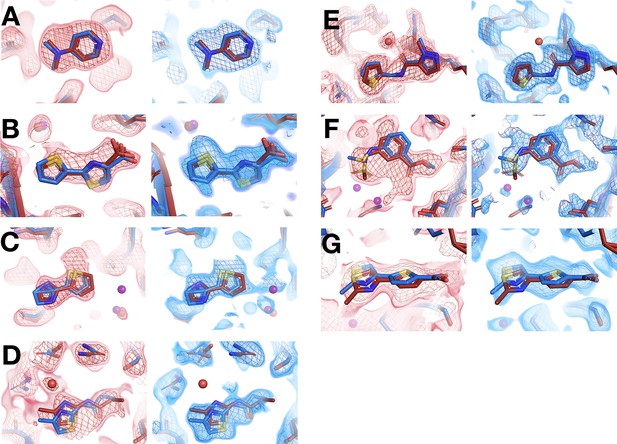
All fragments that bind similarly at room (RT) vs. cryogenic (cryo) temperatures from in situ screen.
For each panel: RT density on left, cryo on right. Same coloring as main Figure 4. (A) RT: x0224 (2 σ), cryo: y0118 (1.5 σ). (B) RT: x0285 (1.5 σ), cryo: y0772 (0.9 σ). (C) RT: x0267 (1.5 σ), cryo: y0112 (0.9 σ). (D) RT: x0228 (1.9 σ), cryo: y0076 (1.2 σ). (E) RT: x0262 (1.5 σ), cryo: y1656 (1.2 σ). (F) RT: x0158 (2 σ), cryo: y0175 (1 σ). (G) RT: x0283 (2 σ), cryo: y0829 (1.5 σ).
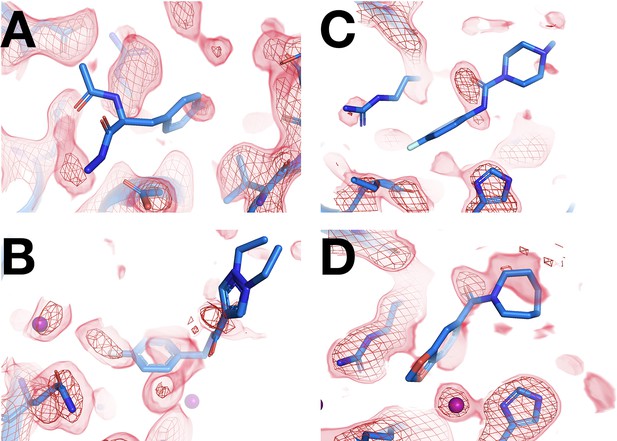
Fragments that bind at cryogenic (cryo) but not at room temperature (RT).
Using the same 1-BDC as cryo, there is no RT density for the cryo ligand. (A) RT: z0011 (1.5 σ), cryo: y0363. (B) RT: z0023 (2 σ), cryo: y0522. (C) RT: x0199 (1.8 σ), cryo: y0049. (D) RT: x0219 (1.5 σ), cryo: y0426.
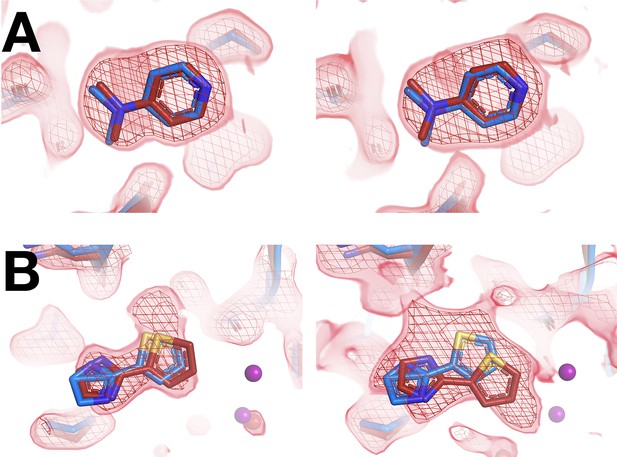
Selected matching ligands across both screens have consistent binding poses.
For each panel: room temperature (RT) in situ density on left, RT 1-xtal density on right. Same coloring as main Figure 4. (A) RT: x0224 (2 σ), cryogenic (cryo): y0118; RT: z0089 (2 σ), cryo: y0118. (B) RT: x0267 (1.5 σ), cryo: y0112; RT: z0102 (1.5 σ), cryo: y0112.
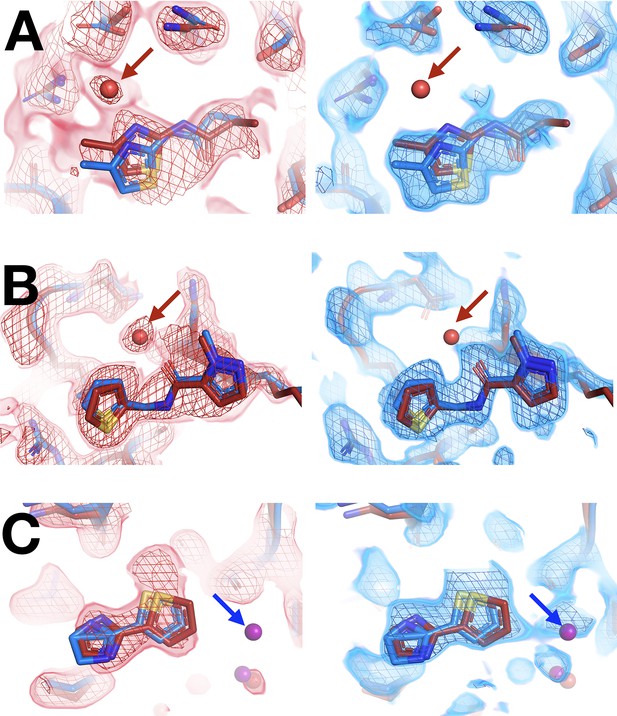
Differences in solvation around fragments at room (RT) vs. cryogenic (cryo) temperature.
Red arrows indicate an RT water with only RT event map density. Blue arrows indicate a cryo water with only cryo event map density. Typical map contour levels used when modeling are shown, but conclusions were similar when visualizing at different map contour levels. For each panel: RT density on left, cryo on right. Same coloring as main Figure 4. (A) RT: x0228 (1.9 σ), cryo: y0076 (1.2 σ). (B) RT: x0262 (1.5 σ), cryo: y1656 (1.2 σ). (C) RT: x0267 (1.5 σ), cryo: y0112 (0.9 σ). This figure contains selected examples; see also Figure 6.
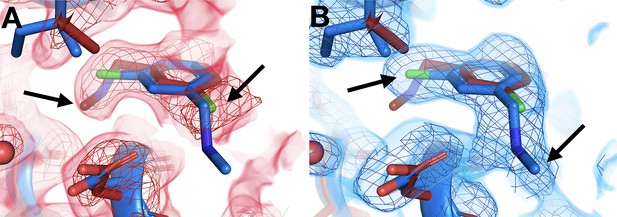
Temperature-dependent ligand conformational heterogeneity.
(A) In the room temperature (RT) dataset (x0227), the RT event map contoured at 2 σ (red) matches the RT model (red) rather than the cryogenic (cryo) model (blue) for both substituent groups of the ring. (B) In the cryo dataset (y0071), the cryo event map contoured at 1.2 σ (blue) matches the cryo model rather than the RT model.
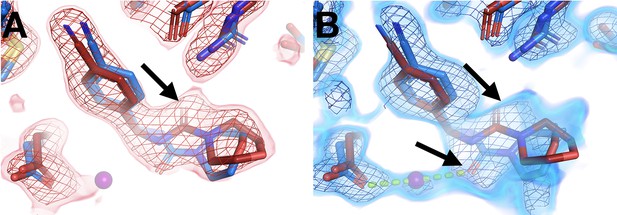
Temperature modulates fragment pose and solvation within the same site.
(A) In the room temperature (RT) dataset (x0260), the RT event map contoured at 1.6 σ (red) matches the RT model (red), but shows little evidence for the cryogenic (cryo) model (y0180, blue). (B) In the corresponding cryo dataset, the cryo event map contoured at 1.6 σ (blue) matches both the cryo model (blue) and the RT model. Notably, only at cryo does the event map include density for a water molecule (purple ball) next to the fragment carbonyl group and well positioned for a hydrogen bond (pale green dashed line) with the cryo fragment pose.
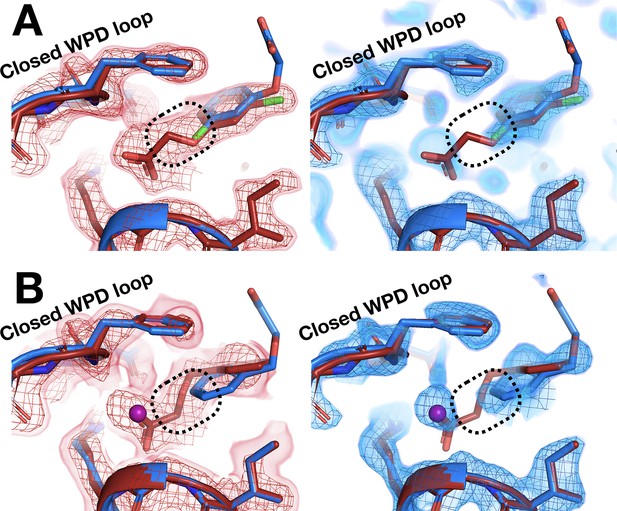
Room-temperature (RT) fragment pose is flipped compared to the cryogenic (cryo) pose.
(A) Left: RT density (red) 1.5 σ, z0055 (red); y0884 (blue). Right: cryo density (blue) 1 σ, z0055 (red); y0884 (blue). (B) Left: RT density (red) 2 σ, x0256 (red); y0650 (blue). Right: cryo density (blue) 0.8 σ, x0256 (red); y0650 (blue) (not previously deposited to the PDB). Density is linked at RT (dashed box), consistent with the fragment pose, but is cut off at cryo, even at lower contour. There is little to no density for the open state of the WPD loop (not shown).
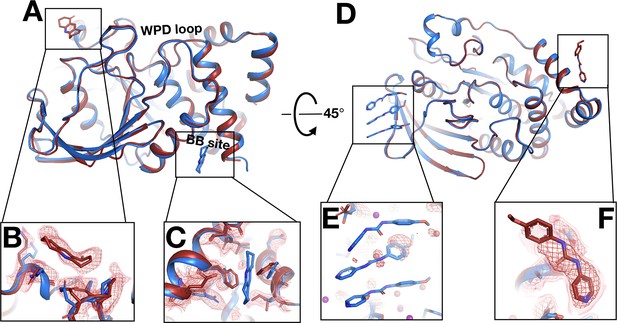
Fragments bind at new sites only at room temperature (RT).
(A–C) First example. (A) The two sites are ~38 Å away from one another. (B) In the RT dataset (z0042), the RT event map, calculated with 1-BDC of 0.36 and contoured at 1.5 σ (red), supports a bound fragment in the RT model (red) at a new site while the cryogenic (cryo) model (y1525) (blue) has no bound fragment. (C) By contrast, the RT event map (same contour) does not show any density for the cryo model (blue) from the previous cryo dataset (y1525). (D–F) Second example. (D) The two sites are ~46 Å away from one another. (E) The RT event map contoured at 1.75 σ (red) (same contour) does not support the cryo model (blue) from the previous cryo dataset (y0572). (F) By contrast, at a new site the RT event map (same contour) supports a bound fragment in the RT model (x0225) (red). The cryo model has no bound fragment.
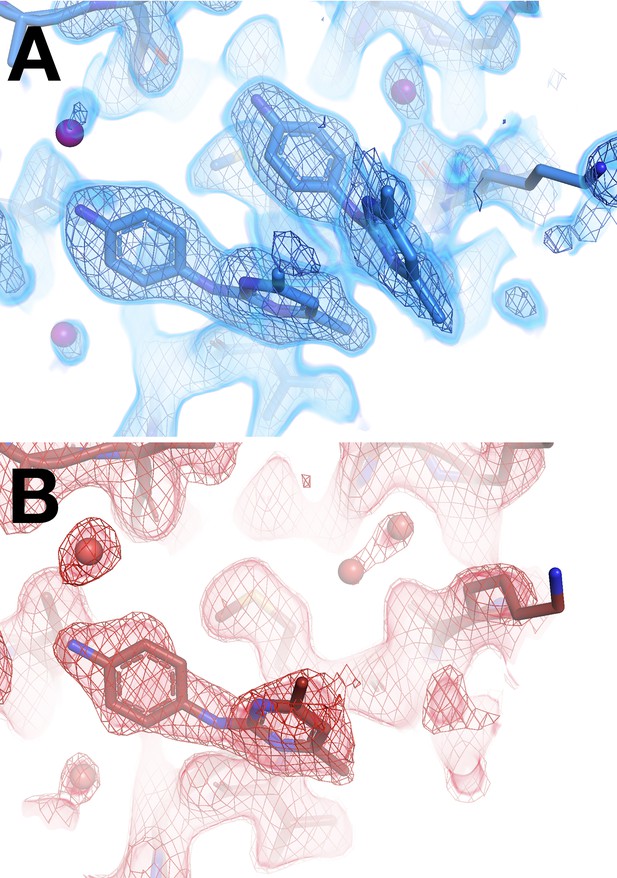
Only half of a cryogenic (cryo) stacking artifact disappears at room temperature (RT).
(A) Cryo y0660 (blue); cryo density (blue) 1.5 σ. (B) RT z0115 (red); RT density (red) 0.7 σ.
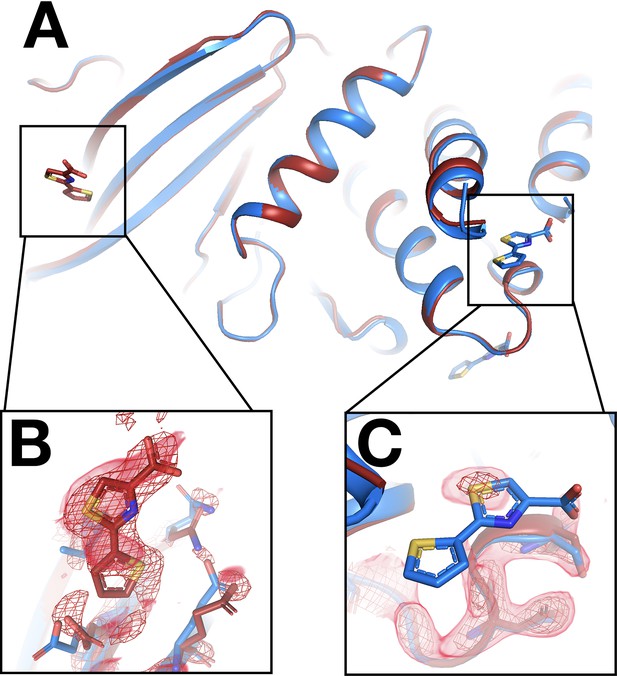
Fragment that binds at a new site only at room temperature (RT).
(A) The two sites are ~40 Å away from one another. (B) In the RT event map contoured at 2.5 σ (red), the RT dataset (x0285) supports a bound fragment at a new site. The cryogenic (cryo) model (blue) from the previous cryo dataset (y0772) has no bound fragment. (C) By contrast, the RT event map, calculated with 1-BDC of 0.15 (same contour), does not support the cryo model. (The site where the RT map matches the cryo site is not shown.)
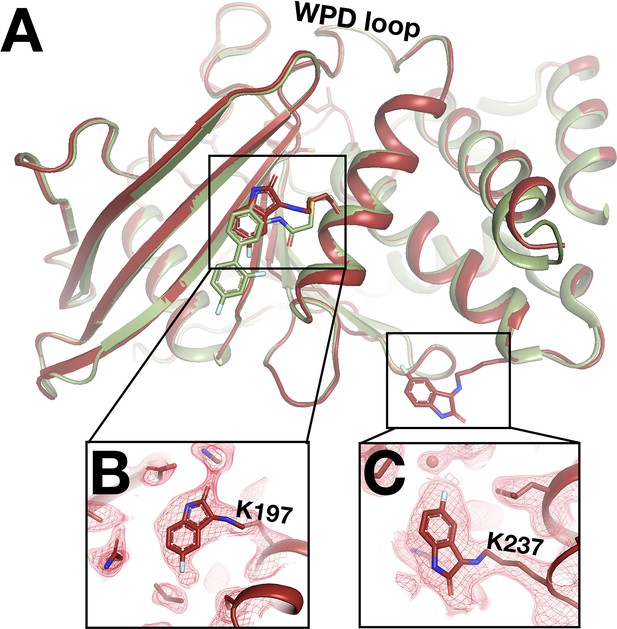
Unanticipated covalent adducts at previously reported allosteric sites only at room temperature (RT).
(A) RT structure with the fragment covalently bound to both K197 and K237 (z0048, red), aligned with cryogenic (cryo) structure with a previously reported allosteric inhibitor covalently bound to K197C (6b95, green). (B) Fragment bound to K197 at the 197 allosteric site, with RT event density at 1.5 σ. (C) Fragment bound to K237 at the L16 allosteric site, with RT event density at 1.5 σ.
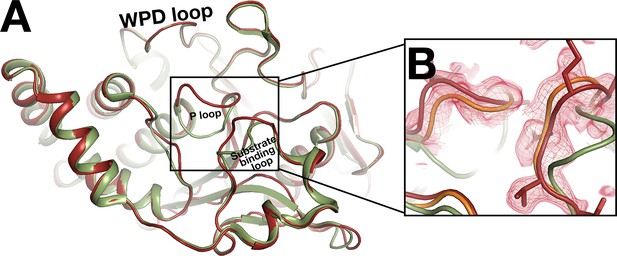
Conformational difference for the active-site P loop.
(A) 180° rotation around the vertical axis of main Figure 9A, to view the backside of z0048 (red) aligned with 6b95 (green). The WPD loop remains open, but the P loop and the nearby substrate-binding loop adopt different conformations. (B) z0048 aligned with 6b95 and a structure with Cys215 in the P loop oxidized, PDB ID 1oes (orange). Room temperature (RT) event density is shown at 1.5 σ (red mesh). The new loop conformation at RT matches 1oes, not 6b95.
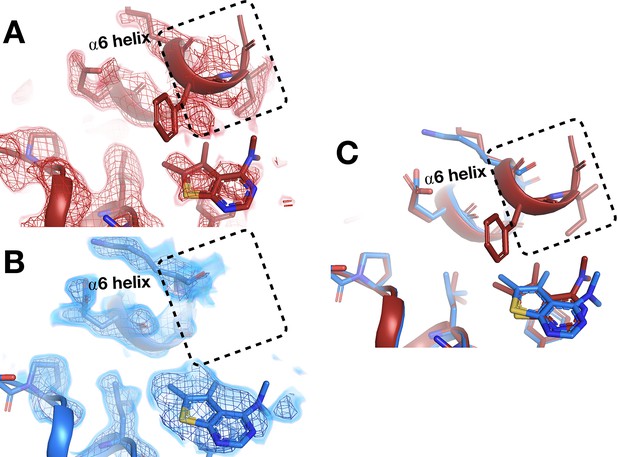
Temperature-dependent ordering of an α-helix augmenting a fragment-binding site.
(A) In the BB allosteric site, the room temperature (RT) density, x0222 (red); contoured at 1.25 σ, is consistent with an extended and more ordered α6 helix (dashed box). (B) In contrast, the cryogenic (cryo) density, y0205 (blue); contoured at 1.75 σ, becomes disordered and therefore the α6 helix is not modeled as extended as in the RT model (dashed box). (C) Overlay of the two models showing the fragment pose is extremely similar whereas the RT helix is extended and more ordered (dashed box).
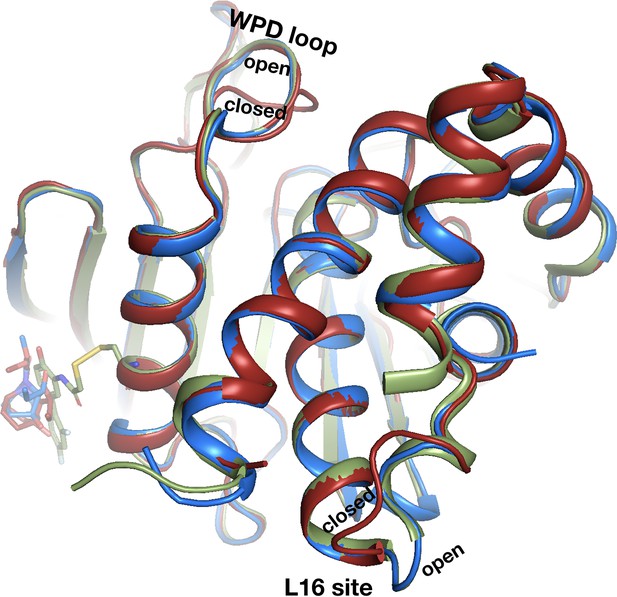
Fragments in allosteric L16 site shift the α6 helix to different extents.
Fragments are shown in order of the extent to which they push the α6 helix toward the active site (up in this view) at cryogenic (cryo), calculated based on RMSD to the closed conformation (purple, 1sug). As the helix is pushed up, the fragments tend to bind closer to and intercalate below it, indicating a correlation between the fragment position under the helix and how much the helix is perturbed. Two room-temperature (RT) fragments are shown, each after their corresponding cryo fragment; the effect on the helix is similar for each fragment at cryo vs. RT.
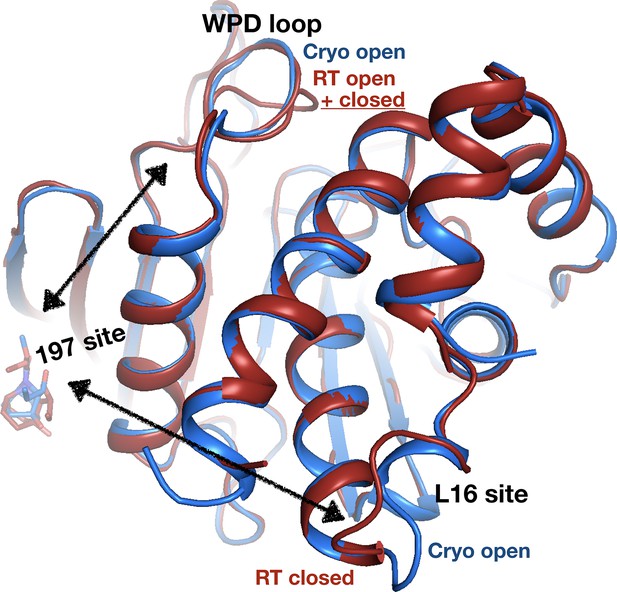
Allosteric protein responses at key sites seen only at room temperature (RT).
Although the fragment binds in a similar manner and in the same allosteric site (the 197 site) in both the RT model (z0032) (red) and the cryogenic (cryo) model (y1763) (blue), the protein response is different between the two temperatures. At cryo, the protein retains the default open conformation, with loop 16 in the L16 site open and the WPD loop also open. Alternatively, at RT, the L16 site is fully closed, while the WPD loop exhibits alternate conformations with the loop both open and closed. The α7 helix (not shown) remains disordered in both temperatures.
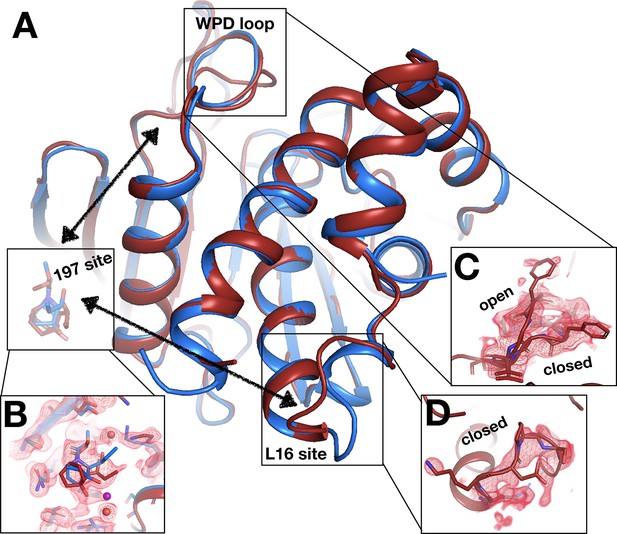
Electron density evidence for allosteric protein responses at key sites seen only at room temperature (RT).
(A) Same as main Figure 11. (B) z0032 (red), y1763 (blue), RT density, 1.5 σ, indicate the fragment binds in the 197 site. (C) RT density, 1.5 σ, showing the WPD loop adopts alternative conformations with the WPD loop open and closed. (D) RT density, 1.5 σ, is consistent with loop 16 modeled in the closed conformation.
Covalent allosteric inhibitor matches fragment with allosteric response at room temperature (RT).
Overlay of z0032 with PDB ID 6b95, which includes a covalent inhibitor targeting the K197C mutation in the allosteric 197 site. Same coloring as in Figure 9.
Tables
X-ray datasets collected for both room-temperature crystallographic screens.
The total datasets tally for the in situ screen derives from a larger number of partial datasets or ‘wedges’ that were merged (see Materials and methods). The cryo-hit and cryo-non-hit categories are defined in Materials and methods. Datasets from crystals soaked with DMSO only are included in the total datasets tally, but not in the unique fragments tally.
| 1-xtal | In situ | |
|---|---|---|
| Total raw X-ray datasets collected | 269 | 111 |
| Processed datasets with unique fragments | 86 | 80 |
| Cryo-hit | 38 | 48 |
| Cryo-non-hit | 48 | 32 |
| DMSO (negative control) | 7 | 20 |
Characteristics of fragment hits for room-temperature (RT) screens.
| RT pose category | 1-xtal | In situ | Total |
|---|---|---|---|
| Same site, same pose | 9 | 7 | 16 |
| Same site, new pose* | 4 | 5 | 9 |
| New site | 3 | 2 | 5 |
| Protein change | 2 | 3 | 5 |
| Not hit | 71 | 66 | 137 |
-
*
The ‘same site, new pose’ category includes cases in which the fragment pose is the same but the protein conformation is notably altered at RT vs. cryo. See Table 2—source data 1 for more information on which individual datasets fit into which categories.
-
Table 2—source data 1
List of all fragment hits for room-temperature (RT) screens.
* The fragment pose is the same but the protein conformation is altered at RT vs. cryo. ** We assume the cryo pose is different but the cryo model was not published since the ligand density was poorly defined.
- https://cdn.elifesciences.org/articles/84632/elife-84632-table2-data1-v1.csv
-
Table 2—source data 2
Chemical properties of fragments and their binding sites.
H-bonds and non-H-bond interactions were calculated using Probe (Word et al., 1999) H-bonds are from ligand atoms to protein or water atoms. Other parameters were calculated using SwissADME (Daina et al., 2017). To test the significance of the difference in each parameter between the two fragment categories, Student’s t-tests were performed (*p<0.05, **p=0.054).
- https://cdn.elifesciences.org/articles/84632/elife-84632-table2-data2-v1.csv
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Peptide, recombinant protein | Human PTP1B recombinant protein | This paper | Purified from Escherichia coli BL21 cells | |
| Software, algorithm | PanDDA software | PanDDA (https://pandda.bitbucket.io/) | Version 0.2.14 |





