Competition between myosin II and βH-spectrin regulates cytoskeletal tension
eLife assessment
The manuscript provides valuable insights into the regulatory role and mechanisms of the spectrin cytoskeleton in mechanotransduction in Drosophila. The data are compelling in establishing that alpha and beta spectrin regulate the Hippo signaling pathway independently via their effect on cytoskeletal tension. The work will be of interest to cell and developmental biologists, particularly those who focus on mechanotransduction and the cytoskeleton.
https://doi.org/10.7554/eLife.84918.3.sa0Valuable: Findings that have theoretical or practical implications for a subfield
- Landmark
- Fundamental
- Important
- Valuable
- Useful
Compelling: Evidence that features methods, data and analyses more rigorous than the current state-of-the-art
- Exceptional
- Compelling
- Convincing
- Solid
- Incomplete
- Inadequate
During the peer-review process the editor and reviewers write an eLife Assessment that summarises the significance of the findings reported in the article (on a scale ranging from landmark to useful) and the strength of the evidence (on a scale ranging from exceptional to inadequate). Learn more about eLife Assessments
Abstract
Spectrins are membrane cytoskeletal proteins generally thought to function as heterotetramers comprising two α-spectrins and two β-spectrins. They influence cell shape and Hippo signaling, but the mechanism by which they influence Hippo signaling has remained unclear. We have investigated the role and regulation of the Drosophila β-heavy spectrin (βH-spectrin, encoded by the karst gene) in wing imaginal discs. Our results establish that βH-spectrin regulates Hippo signaling through the Jub biomechanical pathway due to its influence on cytoskeletal tension. While we find that α-spectrin also regulates Hippo signaling through Jub, unexpectedly, we find that βH-spectrin localizes and functions independently of α-spectrin. Instead, βH-spectrin co-localizes with and reciprocally regulates and is regulated by myosin. In vivo and in vitro experiments support a model in which βH-spectrin and myosin directly compete for binding to apical F-actin. This competition can explain the influence of βH-spectrin on cytoskeletal tension and myosin accumulation. It also provides new insight into how βH-spectrin participates in ratcheting mechanisms associated with cell shape change.
Introduction
The spectrin cytoskeleton has been described as a lattice of cross-linked, spring-like proteins that provide structural support to cells (Liem, 2016; Machnicka et al., 2014). Spectrins were first discovered and characterized in red blood cells but are expressed in many cell types. Spectrins can bind to cell membranes and F-actin, linking them together. They are generally thought to act as heterotetramers, composed of two α subunits and two β subunits. Drosophila has one α-spectrin (α-Spec) and two β-spectrins: β-spectrin (β-Spec) and β-heavy spectrin (βH-Spec, encoded by karst [kst]), which could potentially generate two distinct spectrin heterotetramers: (αβ)2 and (αβH)2. β-Spec and βH-Spec interact with F-actin through their N-terminal domains, which contain two actin-binding calponin-homology (CH) domains, connecting the spectrin cytoskeleton to the actin cytoskeleton (Liem, 2016). In Drosophila epithelia, it has been reported that β-Spec localizes to the lateral sides of cells, βH-Spec localizes to the apical sides of cells, and α-Spec localizes both laterally and apically, leading to inferences that spectrin exists as lateral (αβ)2 tetramers and apical (αβH)2 tetramers (Dubreuil et al., 1997; Lee et al., 1997; Thomas et al., 1998; Zarnescu and Thomas, 1999). Despite common assumptions that spectrins act as tetramers, there is some evidence that alternative arrangements may exist. In Drosophila ovarian follicle cells, the absence of α-Spec diminishes the recruitment of βH-Spec to the apical domain, but it does not affect the recruitment of β-Spec to the lateral domain (Lee et al., 1997). Examination in Drosophila of a mutant form of α-Spec that, based on in vitro studies, is unable to bind β-Spec and compromised in its ability to bind βH-Spec revealed that it could nonetheless rescue the lethality of an α-Spec mutant (Khanna et al., 2015). Experiments done with a mammalian homolog of βH-Spec, βV-Spec, showed that it can homodimerize through its C-terminal region, raising the possibility that βV-Spec might be able to cross-link F-actin by itself (Papal et al., 2013).
Several studies have reported that spectrins also regulate Hippo signaling, with effects on readouts of Hippo signaling reported in Drosophila imaginal discs and ovarian follicles, as well as in cultured mammalian cells (Deng et al., 2015; Deng et al., 2020; Fletcher et al., 2015; Wong et al., 2015). Hippo signaling is a signal transduction network that responds to diverse upstream inputs, including the cytoskeleton and cells’ physical environment (Misra and Irvine, 2018; Zheng and Pan, 2019). Hippo signaling modulates cell proliferation and fate, largely through the regulation of Yap family transcriptional co-activator proteins (Yorkie, Yki, in Drosophila, YAP1 and TAZ in humans). Yki is primarily regulated through phosphorylation by the kinase Warts (Wts), which promotes the cytoplasmic localization of Yki. Various potential mechanisms for biomechanical regulation of Yki/Yap activity have been described, but the best-characterized mechanism in Drosophila is the Jub biomechanical pathway. This involves tension-dependent recruitment of an Ajuba LIM protein (Jub in Drosophila, LIMD1 in mammals) to α-catenin at adherens junctions (AJs) (Alégot et al., 2019; Ibar et al., 2018; Rauskolb et al., 2022; Rauskolb et al., 2014; Sarpal et al., 2019; Sun et al., 2015). Jub then recruits and inhibits Wts, resulting in increased Yki activity.
Studies of the influence of spectrins on Hippo signaling have suggested different mechanisms by which this might occur (Deng et al., 2015; Deng et al., 2020; Fletcher et al., 2015; Wong et al., 2015). Fletcher et al., 2015, focusing on βH-Spec, suggested that the spectrin cytoskeleton influences the membrane density, and thereby the activation state, of upstream regulators of Hippo signaling. Wong et al., 2015, focusing on β-Spec, suggested that spectrins might influence Hippo signaling by modulating levels of F-actin; increased levels of F-actin have been reported in other studies to be associated with increased Yki/Yap activity (Aragona et al., 2013; Fernández et al., 2011; Sansores-Garcia et al., 2011). Deng et al., 2015, focusing on α-Spec, reported that spectrins regulate levels of phosphorylated myosin light chain (required for myosin activation) but surprisingly did not affect levels of myosin or recruitment of Jub, leading them to infer that spectrins can act through a novel tension-dependent but Jub-independent pathway. These same authors later reported that in the pupal eye, spectrins can act through Jub, while suggesting that the action of spectrins in the pupal eye differs from their action in the wing disc (Deng et al., 2015; Deng et al., 2020).
The disparate models for how spectrins influence Hippo signaling have led to confusion over whether a distinct spectrin-based mechanism exists for the mechanical regulation of Hippo signaling. We were particularly interested in investigating claims that spectrins could alter cytoskeletal tension in wing discs without affecting Jub localization or levels of myosin. Our results reveal that both βH-Spec and α-Spec influence Jub recruitment to AJ, and their effects on Yki activity depend upon Jub. Together, these observations argue that spectrins influence Hippo signaling through the Jub biomechanical pathway. Unexpectedly, our investigations also reveal that βH-Spec and α-Spec act separately in wing discs - they do not co-localize, nor do they influence each other’s localization. These observations argue that βH-Spec does not act as part of an (αβH)2 tetramer in the wing disc but rather exerts its functions independently of α-Spec. Finally, we establish that βH-Spec and myosin reciprocally antagonize each other’s apical localization in wing discs - myosin inhibits βH-Spec, and βH-Spec inhibits myosin. We further show that myosin can compete with βH-Spec for binding to F-actin in vitro. Together with structural modeling, our observations argue that βH-Spec and myosin compete with each other for binding to F-actin in vivo. This competition could explain how βH-Spec influences myosin activity and further suggests a simple mechanism for the contribution of βH-Spec to ratcheting processes that alter cell shape via actomyosin contractility.
Results
βH-Spec regulates myosin activity and levels in wing imaginal discs
Prior studies have reported that mutation or RNAi-mediated knockdown of spectrins in wing and eye imaginal discs increased myosin activity, as visualized by staining for myosin light chain phosphorylated at activation sites (pMLC) (Deng et al., 2015; Forest et al., 2018). Surprisingly, it was also reported that levels of GFP-tagged myosin light chain (encoded in Drosophila by spaghetti squash, Sqh:GFP) or F-actin were nonetheless unaffected (Deng et al., 2015), whereas changes in myosin activity generally correlate with changes in myosin accumulation (Fernandez-Gonzalez et al., 2009; Noll et al., 2017). To re-examine this, we analyzed wing imaginal discs in which the apical, βH-Spec kst was knocked down in posterior wing cells by expressing UAS-RNAi lines under en-Gal4 control (RNAi validation is presented in Figure 1—figure supplement 1). This approach leaves unaffected anterior wing disc cells as an internal control. These experiments typically also include a neutral transgene expressed under en-Gal4 control to mark posterior cells (e.g. UAS-RFP) and a transgene expressing Dicer2 (Dcr2) to increase the efficacy of RNAi (Dietzl et al., 2007). This increased apical pMLC in posterior cells, where levels of βH-Spec were reduced (Figure 1—figure supplement 2B and D), consistent with previous reports (Deng et al., 2015; Forest et al., 2018). A difference in junctional tension between anterior (control) cells and posterior (βH-Spec RNAi) cells in our experiments was confirmed by measuring the recoil velocity of cell junctions after laser cutting, which demonstrated an increased tension in the βH-Spec depleted sides (Figure 1G). To examine myosin protein levels, we employed the myosin light chain GFP fusion Sqh:GFP. We found that depletion of βH-Spec led to increased levels of junctional myosin (Figure 1B). To quantify these effects on Sqh:GFP levels, we made maps of Sqh:GFP intensity normalized against E-cad intensity. These are displayed on a red (high) to blue (low) heat map (Pan et al., 2018) and we also used this analysis to calculate the ratio of intensities of the anterior (control) versus posterior (experimental) compartments (Alégot et al., 2019; Figure 1D–E, H–I). This was further confirmed by using a distinct βH-Spec RNAi line (UAS-kstRNAiHMS00882), which similarly increased myosin levels (Figure 1—figure supplement 2E).
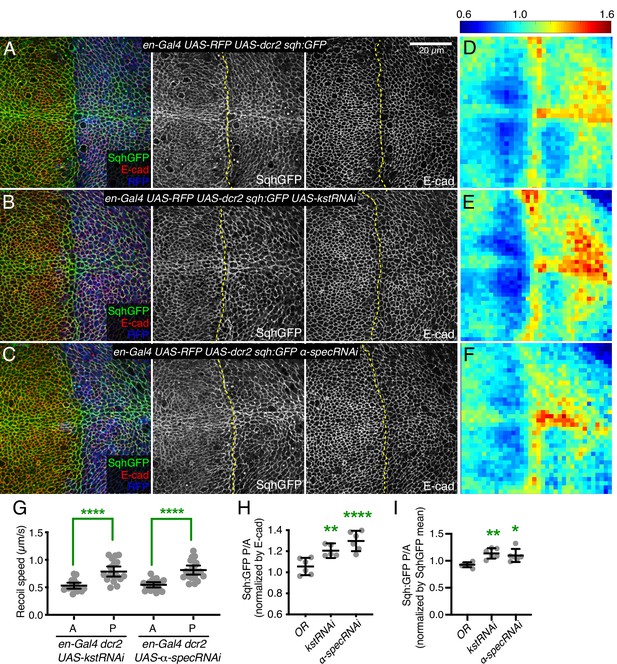
Knockdown of β-heavy spectrin (βH-Spec) or α-spectrin (α-Spec) increases junctional myosin levels in wing discs.
(A–C) Apical sections of wing discs stained for E-cad (red/gray) expressing en-Gal4 UAS-RFP UAS-dcr2 sqh:GFP crossed to control (Oregon-R, OR) (A), UAS-kstRNAi(v37075) (B), or UAS-α-specRNAi (C) showing the effect on Sqh:GFP (green/gray) levels and localization in the posterior compartment (marked by RFP, blue). Dashed yellow line marks A/P compartment boundary. Scale bar = 20 µm; all images are at the same magnification. Panels to the right, in gray, show single channels, as indicated. (D–F) Heat maps of relative junctional Sqh:GFP intensity of wing discs. Average levels of Sqh:GFP relative to E-cad levels are shown for the different genotypes analyzed in A–C. Heat map scale is indicated on the top. Number of wing discs used for analysis: Control (OR), n=6; UAS-kstRNAi, n=5; UAS-α-specRNAi, n=6. (G) Average recoil velocities after laser cutting of cell junctions in anterior (A) or posterior (P) compartments of wing discs expressing UAS-kstRNAi or UAS-α-specRNAi in posterior cells under en-Gal4 control (n=20). (H–I) Quantification of Sqh:GFP normalized to E-cadherin in posterior cells (P) compared to anterior cells (A) in wing disc expressing the indicated constructs, displayed as individual values, normalized by E-cad (H) or normalized by the mean intensity of Sqh:GFP (I). Data are shown as mean±95% CI. Statistical significance in (G) was determined by Student’s t-test between A and P. For (H) and (I), statistical significance was determined by a one-way analysis of variance (ANOVA) with Dunnett’s multiple comparison test relative to the control (Oregon-R): ns: not significant, *p<0.05; **p≤0.01; ***p≤0.001; ****p≤0.0001.
As earlier studies reporting no effect on myosin levels focused on mutation or knockdown of α-Spec (Deng et al., 2015), we considered the possibility that knockdown of α- and βH-Spec might differ in their effects on apical myosin accumulation. However, we found that knockdown of α-Spec also caused an increased accumulation of apical myosin, as well as pMLC and junctional tension, similar to the effects of βH-Spec knockdown (Figure 1C, F, H, I; Figure 1—figure supplement 2C and D). α-Spec and βH-Spec knockdown differed though in that the thickness of the wing disc epithelium was reduced by α-Spec knockdown, but not by βH-Spec knockdown (Figure 1—figure supplement 3), indicating that even though α-Spec and βH-Spec have similar effects on junctional myosin, their roles in wing disc cells differ. Studies in pupal eyes identified a role for α-Spec in attaching F-actin to membranes that was associated with maintaining proper cortical tension and cell shape (Deng et al., 2020); we think the reduced thickness of the epithelium is a reflection of this role in wing discs. That is, reduced stiffness of the lateral sides of cells when α-Spec is removed may allow cells to expand laterally, and through conservation of volume, simultaneously shorten along the apical-basal axis.
βH-Spec regulates Hippo signaling through Jub
It was previously argued that spectrins do not influence Hippo signaling through the Jub biomechanical pathway in wing discs, in part based on an apparent lack of effect of α-Spec mutation or depletion on Jub localization (Deng et al., 2015). However, multiple studies have consistently observed that Jub localization increases when tension at AJ is increased (Forest et al., 2018; Pan et al., 2018; Rauskolb et al., 2019; Rauskolb et al., 2014; Razzell et al., 2018). Thus, we examined Jub localization under conditions of βH-Spec depletion. In wing imaginal discs, Jub accumulates in puncta that often occur near intercellular vertices, together with a lower level, more even accumulation along the cell-cell junctions (Figure 2A and Figure 2—figure supplement 1B). Jub is recruited by a tension-dependent conformational change of α-catenin, and Jub puncta are increased when cytoskeletal tension is higher. Consistent with this, examination of Jub:GFP confirmed that the increased junctional tension and myosin activity caused by βH-Spec depletion is associated with increased junctional recruitment of Jub (Figure 2B, E and G–H and Figure 2—figure supplement 1C).
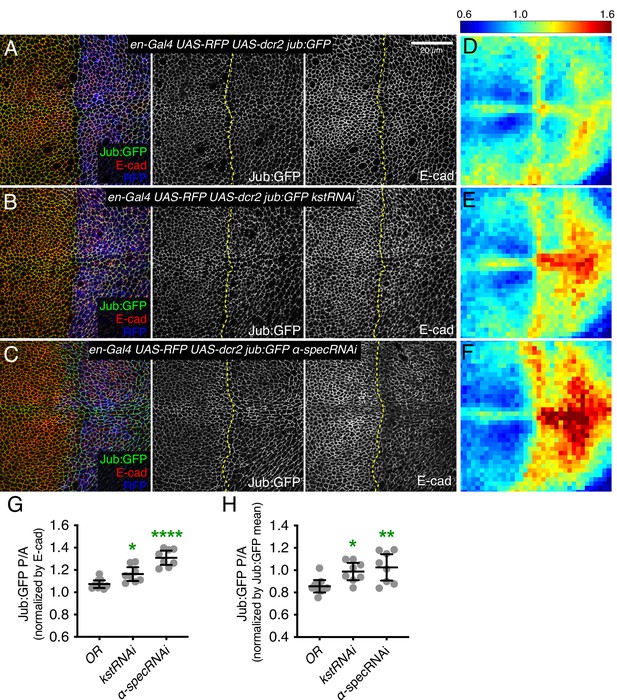
Knockdown of β-heavy spectrin (βH-Spec) or α-spectrin (α-Spec) increases junctional Jub levels in wing discs.
(A–C) Apical sections of wing discs expressing en-Gal4 UAS-RFP UAS-dcr2 Jub:GFP crossed to control (Oregon-R, OR) (A), UAS-kstRNAi(v37075) (B), or UAS-α-specRNAi (C) stained for E-cad (red/gray) showing the effect on Jub:GFP (green/gray) in the posterior compartment (marked by RFP, blue). Dashed yellow line marks the A/P compartment boundary. Panels to the right, in gray, show single channels, as indicated. Scale bar = 20 μm. (D–F) Heat maps of relative junctional Jub:GFP intensity of wing discs. Levels of Jub:GFP relative to E-cad levels are shown for the different genotypes analyzed. Heat map scale is indicated on the top. Number of wing discs used for analysis: Control (OR), n=9; UAS-kstRNAi, n=8; UAS-α-specRNAi, n=8. (G–H) Quantification of Jub:GFP normalized to E-cadherin (G) or to Jub:GFP mean intensity (H) in posterior cells compared to anterior cells (P/A) in wing discs expressing the indicated constructs, displayed as individual values. Data are shown as mean±95% CI. Statistical significance was determined by one-way analysis of variance (ANOVA) with Dunnett’s multiple comparison test relative to the control (Oregon-R): ns: not significant, *p<0.05; **p≤0.01; ***p≤0.001; ****p≤0.0001.
To assess the functional significance of increased Jub localization to AJ under βH-Spec knockdown conditions, we assayed the ability of RNAi-mediated jub knockdown to suppress βH-Spec (kst) RNAi phenotypes. Knockdown of βH-Spec throughout the developing wing under nub-Gal4 control increases wing size (wings were 116% and 119% of nub-Gal4 UAS-dcr2 control size for the two different UAS-RNAi lines used) (Figure 3A, C–D, and K; Deng et al., 2015; Fletcher et al., 2015). Knockdown of jub leads to smaller wings (Das Thakur et al., 2010; Rauskolb et al., 2011). In animals with simultaneous RNAi knockdown of jub and kst, wing size is similar to that of jub RNAi wings (Figure 3B, E–F, and K). The epistasis of jub to kst is consistent with the hypothesis that βH-Spec regulates wing size through its tension-dependent regulation of Jub. To further illustrate this, we reduced tension in wing discs with βH-Spec knockdown by simultaneous RNAi knockdown of Rho kinase (Rok) (Rauskolb et al., 2014; Winter et al., 2001). This reduced wing size and junctional Jub localization similar to that observed in control wing discs with Rok knockdown (Figure 3—figure supplement 1; Rauskolb et al., 2014).
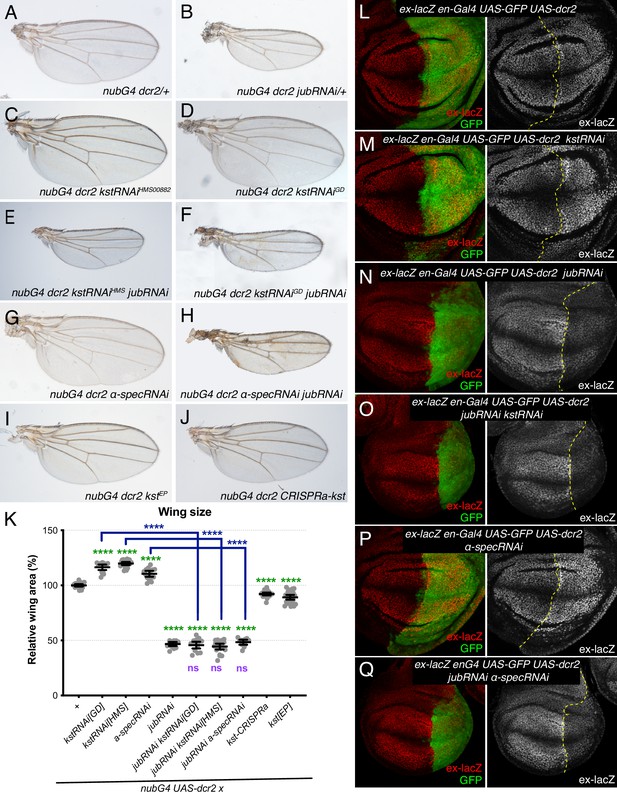
Jub is required for β-heavy spectrin (βH-Spec) and α-spectrin (α-Spec) regulation of wing size and ex-lacZ.
(A–J) Representative adult wings from flies cultured at 29°C and expressing UAS transgenes altering spectrin and/or Jub expression under control of a nub-Gal4 (nubG4) driver. (K) Quantification of wing area (mean±95% CI). Number of wing discs used for analysis: Control (OR), n=20; UAS-kstRNAi[GD], n=20; UAS-kstRNAi[HMS], n=21; UAS-α-specRNAi, n=20; UAS-jubRNAi, n=20, UAS-jubRNAi UAS-kstRNAi[GD], n=20; UAS-jubRNAi UAS-kstRNAi[HMS], n=20; UAS-jubRNAi UAS-α-specRNAi, n=20; UAS-kstCRISPRa, n=22; UAS-kst[EP], n=26. Statistical significance was determined by a one-way analysis of variance (ANOVA) with Tukey’s multiple comparison test. Statistical comparisons are shown relative to nub-Gal4 UAS-dcr2/+in green, relative to nub-Gal4 UAS-dcr2 UAS-jubRNAi in purple and relative to UAS-kstRNAi in blue. (L–Q) Third-instar wing discs expressing ex-lacZ en-Gal4 UAS-dcr2 UAS-GFP (green) crossed to OR (L), UAS-kstRNAi (M), UAS-jubRNAi (N), UAS-jubRNAi UAS-kstRNAi (O), UAS-α-specRNAi (P), UAS-jubRNAi UAS-α-specRNAi (Q), stained for expression of ex-lacZ (red/white). Dashed yellow line indicates the A/P compartment boundary.
To confirm that the relationship between Jub and βH-Spec revealed by analysis of adult wing size corresponds to changes in Hippo pathway activity, we analyzed the expression of a transcriptional reporter of expanded (ex), ex-lacZ, which is a direct target of Yki (Hamaratoglu et al., 2006). Knockdown of βH-Spec in posterior compartments through the expression of UAS-kst RNAi under en-Gal4 control caused a mild increase in ex-lacZ expression compared to the anterior compartment and to control posterior compartments (Figure 3L and M; Fletcher et al., 2015). Knockdown of jub reduces ex-lacZ expression (Das Thakur et al., 2010; Rauskolb et al., 2011). Simultaneous RNAi knockdown of jub and kst reduced ex-lacZ expression, similar to that in jub RNAi cells (Figure 3N and O). The suppression of the influence of kst on Hippo signaling is again consistent with the inference that βH-Spec regulates Hippo signaling through the Jub biomechanical pathway.
As claims that spectrins act independently of Jub in wing discs were based primarily on analysis of α-Spec, we also examined the effect of α-Spec knockdown on Jub levels. When α-Spec was knocked down in posterior cells by en-Gal4-driven RNAi, we observed that recruitment of Jub to cell junctions was increased (Figure 2C and F–H). Moreover, as for βH-Spec, we found that the increased wing size and ex-lacZ expression caused by knockdown of α-Spec were suppressed by knockdown of jub (Figure 3G, H, K, P and Q). Thus, as for βH-Spec, and as suggested for pupal eyes (Deng et al., 2020), our observations imply that α-Spec also regulates Hippo signaling through Jub in wing discs.
βH-Spec localizes independently from α-Spec in wing disc cells
Prior studies of spectrin function in imaginal discs have assumed that they function as heterotetramers, with an apical complex composed of (αβH)2 subunits and a lateral complex composed of (αβ)2 subunits (Deng et al., 2015; Deng et al., 2020; Fletcher et al., 2015; Thomas et al., 1998), as was originally suggested for ovarian follicle cells (Lee et al., 1997). However, our examination of spectrin localization in wing imaginal discs suggested that the apical-most distributions of α-Spec and βH-Spec differ. To directly compare them, we used an antibody against α-Spec (Dubreuil et al., 1987) and a fully functional genomic YFP-trap of βH-Spec (Kst:YFP) (Lye et al., 2014). βH-Spec is localized, as reported previously, at the apicobasal level of the AJ in wing discs (Fletcher et al., 2015; Forest et al., 2018; Figure 4A and C). Conversely, α-Spec is enriched in a sub-apical region just below this, with slightly lower levels extending all along the lateral membrane (Figure 4B and C). Only very low levels of α-Spec are detected in the apical plane where βH-Spec is detected, and their distributions in this plane appear to differ (Figure 4A).
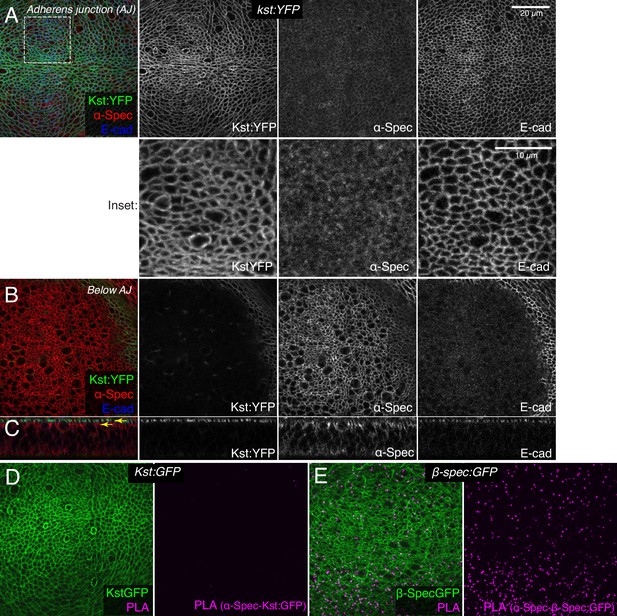
β-Heavy spectrin (βH-Spec) and α-spectrin (α-Spec) do not co-localize in wing discs.
(A–C) Wing discs expressing Kst:YFP immunostained with α-Spec (red/gray) and E-cad (blue/gray) antibodies showing the localization of βH-Spec (Kst:YFP, green/gray) and α-Spec at the adherens junction (AJ) (A), below the AJ (B) and in cross sections (C). Upper yellow arrow in cross section indicates AJ layer, lower yellow arrow indicates ‘Below AJ’ layer. Inset shows higher magnification of single channels from the boxed region in A. (D, E) Wing discs expressing Kst:GFP (D) or β-Spec:GFP (E), with GFP in green and signal from proximity ligation assays (PLA) using rabbit anti-GFP and mouse α-Spec antibodies in magenta.
To confirm that βH-Spec and α-Spec localize independently of each other in wing discs, we examined the consequences of depleting α-Spec, β-Spec, or βH-Spec on each other’s localization. For this, we used en-Gal4-driven UAS-RNAi lines to knock down one of the spectrin proteins, and then examined whether the localization of the others was affected. These experiments revealed, as expected, that β-Spec is not required for βH-Spec localization (Figure 5B), but it does strongly reduce α-Spec localization (Figure 5E). α-Spec is not required for βH-Spec localization (Figure 5A) but its knockdown slightly reduces β-Spec localization (Figure 5D). Finally, βH-Spec depletion does not affect α-Spec localization (Figure 5C). These observations suggest that while α-Spec needs β-Spec to localize to lateral membranes, the localization of βH-Spec and α-Spec are functionally independent. To further examine the relationship between spectrins, we performed proximity ligation assays (PLA) using antibodies against α-Spec and GFP. A PLA signal consistent with close association of α-Spec and β-Spec was observed in wing discs expressing GFP-tagged β-Spec (Figure 4E). Conversely, no significant PLA signal was detected in wing discs expressing GFP-tagged βH-Spec, implying that α-Spec and βH-Spec are not closely associated in wing discs (Figure 4D). Together with the distinct localization of these proteins revealed by imaging, these observations argue against the existence of (αβH)2 complexes in wing imaginal discs.
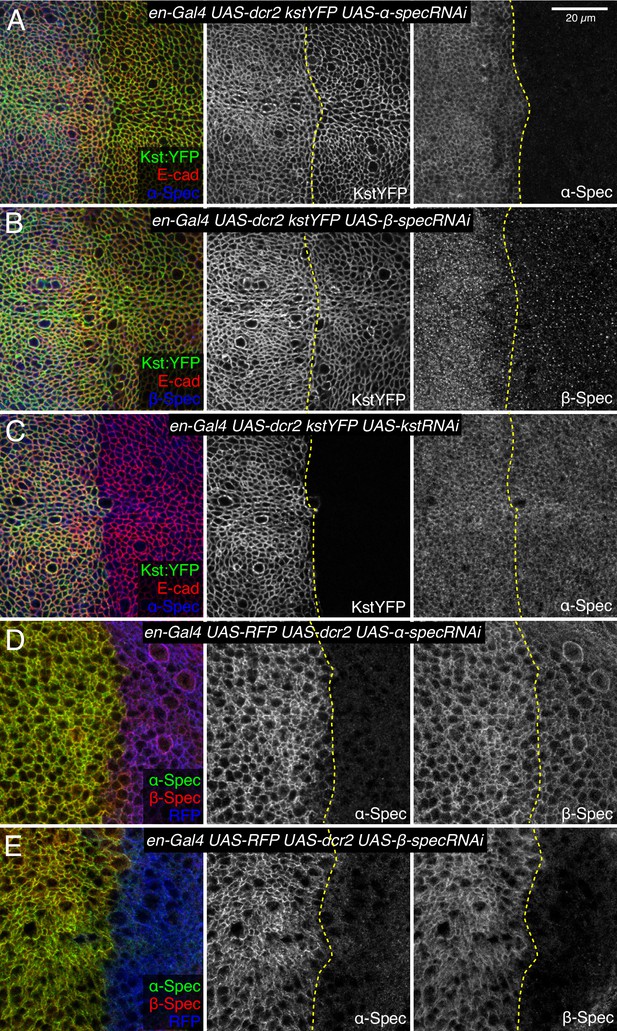
Influence of spectrin knockdowns on spectrin subunit localization in wing discs.
(A–C) Apical sections of wing discs expressing en-Gal4 UAS-dcr2 Kst:YFP (green/gray) along with UAS-α-specRNAi (A), UAS-β-specRNAi (B), and UAS-kstRNAi (C) immunostained for α-spectrin (α-Spec) (A and C, blue/gray) or β-spectrin (β-Spec) antibodies (B, blue/gray). The adherens junction (AJ) layers were obtained by ImSAnE, using as a reference the E-cad channel and seven layers were projected (2.1 μm). (D,E) Sections of wing discs expressing en-Gal4 UAS-RFP (blue) UAS-dcr2 crossed with UAS-α-specRNAi (D) or UAS-β-specRNAi (E) stained with mouse α-Spec (green/gray) and rabbit β-Spec antibody (red/gray). Dashed yellow lines indicate the A/P compartment boundary. Scale bar = 20 μm.
βH-Spec and apical myosin antagonize each other’s localization to apical F-actin
The discovery that βH-Spec and α-Spec localize independently led us to consider what factors might influence the apical localization of βH-Spec. Intriguingly, the distribution of βH-Spec in wing disc cells appears very similar to the apical distribution of F-actin and myosin, and similarities between the localization of βH-Spec and myosin in Drosophila embryos have been noted previously (Krueger et al., 2020; Thomas and Kiehart, 1994). To determine whether myosin and βH-Spec co-localize in wing discs, we imaged discs expressing GFP-tagged βH-Spec (Kst:GFP) and mCherry-tagged myosin light chain (sqh-Sqh:mCherry). This revealed extensive co-localization between these proteins in the apical region of wing disc epithelial cells (Figure 6A, B). Quantitation of these images yielded a Pearson’s correlation coefficient score of 0.636. For comparison, the Pearson’s correlation coefficient score between Kst:YFP and α-Spec (Figure 4A) was 0.18. Both Kst:GFP and Sqh:mCherry also co-localized with F-actin, with Pearson’s correlation coefficient scores of 0.641 and 0.612, respectively.
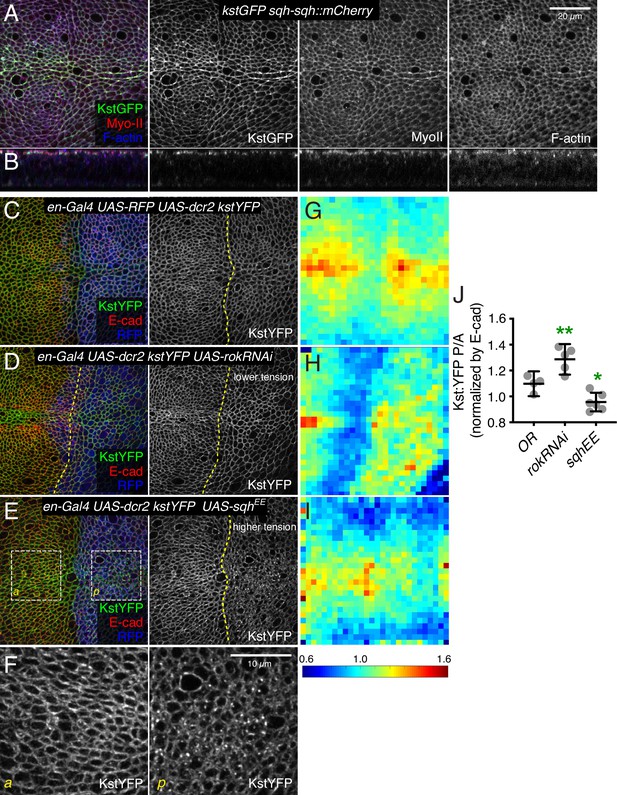
β-Heavy spectrin (βH-Spec) co-localizes with myosin and is regulated by myosin activity.
(A,B) Wing disc co-expressing Kst:GFP (green/gray) and the labeled myosin II subunit sqh:mCherry (red/gray) in apical horizontal sections (A) and lateral sections (B), co-stained with phalloidin for F-actin (blue/gray). (C–E) Apical sections of wing discs expressing en-Gal4 UAS-RFP UAS-dcr2 kst:YFP crossed to (Oregon R, OR) (C), UAS-rokRNAi (D), or UAS-sqhEE (E) in the posterior compartment (marked by RFP, blue), showing the effect of altering myosin activity on Kst:YFP. Dashed yellow lines mark the A/P compartment boundary. Scale bar = 20 μm. (F) Higher magnification of the boxed regions indicated in E. Scale bar = 10 μm. (G–I) Heat maps of junctional Kst:YFP intensity relative to E-cad in en-Gal4 UAS-RFP UAS-dcr2 kst:YFP crossed to (Oregon R, OR) (C), UAS-rokRNAi (D), or UAS-sqhEE (E) wing discs (n=5 for each genotype), as in the representative examples shown in C–E. Heat map scale is at bottom. (J) Quantification of Kst:YFP intensity normalized to E-cadherin in posterior cells (P) compared to anterior cells (A) in wing discs expressing the indicated constructs under en-Gal4 control (n=5). Data are shown as mean±95% CI, error bars indicate CI. Statistical significance was determined by a one-way analysis of variance (ANOVA) with Dunnett’s multiple comparison test relative to the control (Oregon-R): ns: not significant, *p<0.05; **p≤0.01.
The extensive co-localization of βH-Spec and myosin prompted us to investigate the functional relationship between them further. As noted above, reduction of βH-Spec leads to increased apical myosin levels and activity. To investigate whether myosin reciprocally regulates βH-Spec, we expressed transgenes that modulate actomyosin contractility. RNAi-mediated knockdown of Rok reduces myosin activity by reducing phosphorylation of myosin light chain (Winter et al., 2001). In wing discs, knockdown of Rok is also associated with reduced recruitment of myosin to apical junctions and reduced junctional tension (Rauskolb et al., 2014; Figure 6—figure supplement 1C), consistent with the generally positive correlation between myosin activity and co-localization with F-actin (Fernandez-Gonzalez et al., 2009; Noll et al., 2017). Conversely, examination of βH-Spec in wing disc cells expressing Rok RNAi revealed increased levels of βH-Spec along apical junctions (Figure 6D). To increase myosin activity, we expressed a constitutively activated, phosphomimetic form of myosin light chain, SqhEE (Winter et al., 2001). This increases the recruitment of myosin to AJ and increases junctional tension (Rauskolb et al., 2014; Figure 6—figure supplement 1D), but decreases the recruitment of βH-Spec to AJ, and a portion of βH-Spec instead appears in apical vesicles (Figure 6E, F). To quantify these effects on βH-Spec levels, we made maps of βH-Spec intensity normalized against E-cad intensity, displayed on a red (high) to blue (low) heat map. Calculation of the ratio of intensities of the anterior (control) versus posterior (experimental) compartments further confirmed our observation that myosin antagonizes localization of βH-Spec to AJ (Figure 6G–J). Thus, βH-Spec and myosin II localization to AJ are affected in opposite ways by changes in cytoskeletal tension.
The opposing effects of changes in myosin activity on myosin and βH-Spec localization, together with the observation that loss of βH-Spec leads to increased myosin activity and apical localization, raised the possibility that myosin and βH-Spec compete for localization to apical F-actin. To further investigate this possibility, we overexpressed βH-Spec using a CRISPR-activator (CRISPRa) approach. This involves the expression of a transcriptional activator fused to dCas9 under UAS control, which can then be recruited to a gene of interest using a single-guide RNA (sgRNA) targeted upstream of the transcription start site (TSS) (Jia et al., 2019; Jia et al., 2018). To verify the overexpression of βH-Spec, we employed it in flies with GFP-tagged βH-Spec at the endogenous locus (Kst:GFP). To avoid excessive cell death caused by Kst overexpression, we expressed this construct under inducible conditions, using the temperature-sensitive Gal4 repressor Gal80ts. Both imaging and western blotting of wing discs confirmed that this CRISPRa approach effectively increased expression of Kst:GFP (Figure 7—figure supplement 1A–B). Examination of myosin under βH-Spec overexpression conditions, using Sqh:GFP, revealed a substantial reduction of myosin localization to AJs (Figure 7A, C and E–F), and a reduction in tension along cell junctions as assayed by the recoil speed after laser cutting (Figure 7I). Apical cell areas were also increased, consistent with a reduction of junctional tension (Figure 7B and Figure 7—figure supplement 1). As an independent method to overexpress βH-Spec, we used a previously described EP-element insertion near kst, P[EPgy2]EY01010 (UAS-kstEP) (Pogodalla et al., 2021). Overexpression of βH-Spec using UAS-kstEP under en-Gal4 control also reduced myosin levels at AJ (Figure 7—figure supplement 1C), although the effect appeared weaker than that induced by the CRISPRa approach. These results provide further evidence that βH-Spec antagonizes myosin recruitment to AJs and establish that this effect can be observed under both increased and decreased βH-Spec expression conditions.
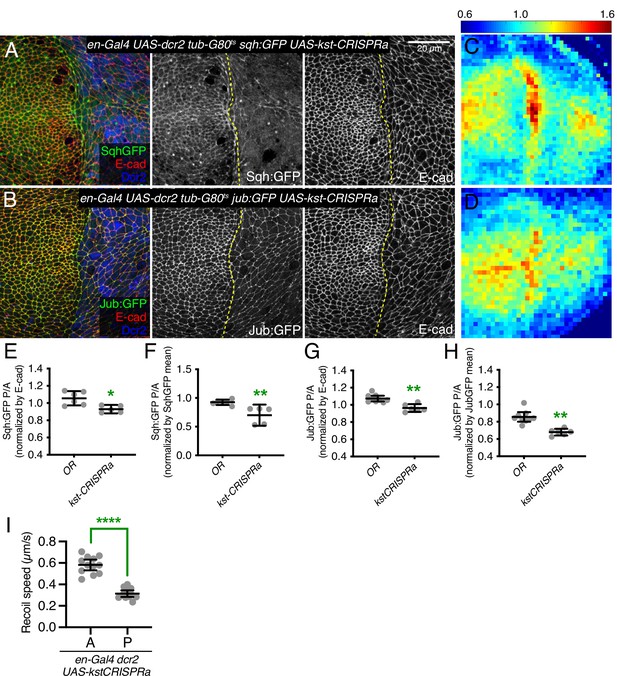
β-Heavy spectrin (βH-Spec) overexpression reduces in junctional tension in wing discs.
(A–B) Apical sections of wing imaginal discs expressing en-Gal4 UAS-RFP UAS-dcr2 sqh:GFP (A) or en-Gal4 UAS-RFP UAS-dcr2 jub:GFP (B) crossed to UAS-kst-CRISPRa showing the effect on Sqh:GFP or Jub:GFP levels and localization in the posterior compartment (marked by RFP, blue). Yellow dashed line indicates the A/P compartment boundary. Scale bar = 20 μm; all images are at the same magnification. (C–D) Heat maps of relative junctional Sqh:GFP (C) or Jub:GFP (D) intensity of wing discs. Levels of Sqh:GFP relative to E-cad levels are shown for the different genotypes analyzed in A–B. Heat map scale is indicated on the top. Number of wing discs used for analysis: UAS-kst-CRISPRa with Sqh:GFP, n=5; UAS-kst-CRISPRa with Jub:GFP, n=6. (E–F) Quantification of Sqh:GFP overlapping E-cad in posterior cells (P) compared to anterior cells (A) in wing disc expressing the indicated constructs, displayed as individual values, normalized by E-cad (E) or normalized by the mean intensity of Sqh:GFP (F). (G–H) Quantification of Jub:GFP overlapping E-cad in posterior cells (P) compared to anterior cells (A) in wing disc expressing the indicated constructs, displayed as individual values, normalized by E-cad (G) or normalized by the mean intensity of Jub:GFP (H). (I) Average recoil velocities after laser cutting of cell junctions in anterior (A) or posterior (P) compartments of wing discs expressing UAS-kstCRISPRa in posterior cells (n=20). Data are shown as mean±95% CI. Statistical significance for (E-I) was determined by Student’s t-test, *p<0.05; **p≤0.01; ***p≤0.001; ****p≤0.0001.
Consistent with these reductions in apical myosin accumulation, βH-Spec overexpression also reduced junctional recruitment of Jub (Figure 7B, D, G, and H and Figure 7—figure supplement 1D) and decreased wing size (wings were 92% and 89% of nub-Gal4 UAS-dcr2 control size, for the two different overexpression constructs) (Figure 3I–K).
βH-Spec and myosin compete for binding to F-actin
The reciprocal antagonism between myosin and βH-Spec suggested that they could compete for binding to F-actin. βH-Spec contains two N-terminal actin-binding domains, CH1 and CH2 (Liem, 2016), and myosin contains an actin-binding region in its motor domain (Duan et al., 2018). While spectrins and myosin have been purified and characterized in vitro, we lack a mechanistic understanding of the interaction between βH-Spec and F-actin and how it might affect myosin binding. To address this, we conducted in vitro co-sedimentation assays with purified protein domains and F-actin. We found that the isolated Drosophila βH-Spec actin-binding region binds F-actin weakly (Figure 8A), in agreement with previous reports of F-actin binding by other spectrin CH1-CH2 domains (Avery et al., 2017; Duan et al., 2018). Constitutively active Drosophila myosin II (subfragment-1-like protein) binding to F-actin appears stronger than βH-Spec binding, as at the same concentration a greater fraction of myosin II is bound to F-actin (Figure 8A, B). To investigate the antagonism between these proteins observed in vivo, we performed biochemical competition assays between the βH-Spec CH domains and myosin for binding to F-actin. F-actin was preincubated with an excess of βH-Spec CH domains to maximize binding to F-actin, and then increasing concentrations of myosin were added. We found that myosin can displace the actin-binding region of βH-Spec from F-actin by ~50% at the highest myosin concentration that we could use (Figure 8C, bottom right). Myosin binding to F-actin was unaffected by preincubation with βH-Spec CH domains (Figure 8—figure supplement 1A), likely due to active myosin’s higher binding affinity for F-actin.
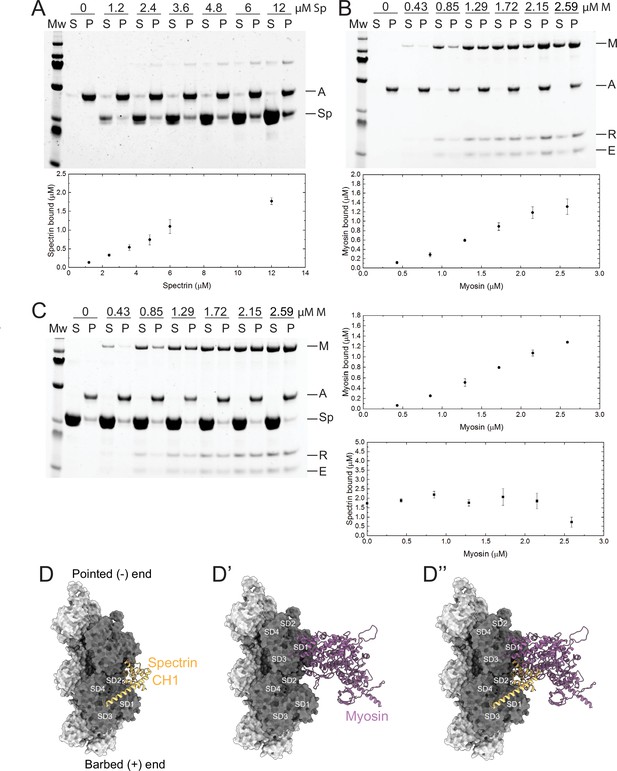
β-Heavy spectrin (βH-Spec) and myosin have overlapping binding sites on F-actin.
(A–C) Co-sedimentation assays with βH-Spec CH domains (spectrin), F-actin, and myosin II subfragment-1-like protein (myosin). For all sedimentation assays, A, M, Sp, E, and R refer to F-actin, myosin heavy chain, spectrin, myosin essential light chain, and myosin regulatory light chain, respectively. Mw indicates marker and S and P refer to supernatant and pellet, respectively. Quantification shows mean ± SD of the sedimentation behavior of spectrin and myosin with F-actin (n=3). (A) Co-sedimentation assay between spectrin (0–12 μM) and F-actin (2 μM). (B) Co-sedimentation assay between myosin (0–2.59 μM) and F-actin (2 μM). (C) Co-sedimentation assay between spectrin (12 μM), myosin (0–2.59 μM), and F-actin (2 μM). Quantification shows that myosin reduces spectrin binding by ~50% (bottom panel). (D–D’’) Model of the Drosophila CH1 domain (yellow) bound to F-actin (gray). (D) Model of the Drosophila myosin motor domain (purple) bound to F-actin (gray). (D’) Superimposition of the CH1 and myosin motor domain on F-actin. The two strands of the actin filament are shown in light and dark gray and subdomains are indicated.
To gain a structural understanding of the antagonism between βH-Spec and myosin, we built homology models of the Drosophila CH1-CH2 domain of βH-Spec and the myosin II motor domain and compared them with previous cryo-EM structures of the human actin-bound β-III spectrin actin binding CH1 domain and actin-bound myosin (Avery et al., 2017; von der Ecken et al., 2016). The superimposition of the isolated Drosophila βH-Spec CH1 domain model on the human β-III spectrin CH1 domain in a complex with F-actin structure revealed a binding site between actin subdomains (SD) SD1 and SD2 along the filament (Figure 8D). The binding region of myosin on F-actin includes SD1, SD2, and SD3 (Figure 8D’). The superimposition of both models suggests that the binding position of the CH1 domain on F-actin sterically interferes with the formation of a strong actin-myosin interface (Figure 8D’’). Modeling of the βH-Spec CH1-CH2 domain indicates that the CH2 domain presents additional steric hindrance for myosin binding to F-actin (Figure 8—figure supplement 1B–B’’). Thus, structural modeling suggests that the binding of spectrin or myosin to individual binding sites on F-actin is mutually exclusive, which could explain why they compete for F-actin association in vivo and in vitro.
Discussion
Multiple models for how spectrins regulate Hippo signaling have been proposed (Deng et al., 2015; Deng et al., 2020; Fletcher et al., 2015; Wong et al., 2015). We focused on investigating claims that spectrins could alter cytoskeletal tension in wing discs by regulating pMLC levels without affecting localization of myosin or Jub (Deng et al., 2015). In contrast to prior studies, we observed that when βH-Spec or α-Spec levels are decreased by RNAi, levels of junctional myosin are increased. This increase in junctional myosin levels is associated with increased junctional tension and with increased recruitment of Jub to AJ. We are not certain why these effects were missed in prior studies, but we note that our observations are consistent with studies linking recruitment of both myosin and Jub to AJ under tension (Alégot et al., 2019; Fernandez-Gonzalez et al., 2009; Ibar et al., 2018; Noll et al., 2017; Rauskolb et al., 2019; Rauskolb et al., 2014; Razzell et al., 2018; Sarpal et al., 2019). Moreover, our results are further supported by the observation that jub is genetically required for the influence of spectrin knockdown on Yki activity and wing growth. Taken together, these observations imply that βH-Spec and α-Spec regulate Hippo signaling in wing discs through the Jub biomechanical pathway, rather than through hypothesized alternate mechanisms.
Spectrin has been suggested to form two distinct complexes in Drosophila epithelial cells, (αβ)2 and (αβH)2 heterotetramers, which localize to the lateral and apical sides of cells, respectively (Deng et al., 2015; Dubreuil et al., 1997; Fletcher et al., 2015; Lee et al., 1997; Thomas et al., 1998; Zarnescu and Thomas, 1999). However, our observations indicate that βH-Spec functions independently of α-Spec in wing imaginal discs. α-Spec and βH-Spec do not exhibit significant co-localization. Moreover, α-Spec and βH-Spec are not required for each other’s localization to apical cell junctions. This contrasts with the requirement for β-Spec for recruitment of α-Spec to lateral membranes in wing discs. The requirement is not entirely reciprocal, as α-Spec knockdown only partially reduces β-Spec recruitment, but this likely reflects mechanisms that recruit spectrins to cell membranes: β-Spec subunits, but not α-Spec subunits, have a pleckstrin homology domain that can mediate membrane association, as well as possessing the CH domains that mediate F-actin association. Thus β-Spec can associate with lateral membranes without α-Spec, but α-Spec does not have a way to associate with lateral membranes without β-Spec.
An independent role for βH-Spec has also been suggested in mammalian photoreceptors, where it was reported that that mammalian βV-Spec does not co-localize with αII-spectrin, and that βV-Spec could form homodimers, potentially allowing it to cross-link actin by itself, enabling α-Spec-independent functions (Papal et al., 2013). The observation that a mutation in Drosophila α-Spec that disrupts binding to β-Spec in vitro has only mild phenotypes also suggests that spectrin functions do not depend entirely on αβ interactions (Khanna et al., 2015). Collectively, our results together with these earlier studies emphasize that the dogma that “spectrin comprises α- and β-subunits that interact in an antiparallel manner to form an αβ dimer” (Liem, 2016) should be revised. Nonetheless, our results do not exclude the possibility that βH-Spec and α-Spec might act together in a physical complex in other tissues, or under distinct physiological conditions.
The conclusion that βH-Spec and α-Spec act independently in wing discs implies that they influence tension at apical junctions through distinct mechanisms. We suggest that α-Spec could influence AJ tension in wing discs through the mechanism proposed to explain the influence of α- and β-Spec in pupal eyes (Deng et al., 2020). It was inferred that α- and β-Spec maintain cell rigidity by linking F-actin to membranes. In the absence of α- or β-Spec, it was proposed that dissociation between F-actin and the membrane leads to an expansion of the apical regions. This expansion increases cytoskeletal tension at AJs, which bind F-actin independently of spectrins. Consistent with this suggested mechanism, we observed a decrease in cell height in α-Spec knockdown cells in wing discs, in conjunction with increased tension at apical junctions. The alteration in cell shape was not observed in βH-Spec knockdown cells, further supporting the conclusion that βH-Spec and α-Spec act in different ways to regulate junctional tension.
Instead, our experiments analyzing the relationship between βH-Spec and myosin revealed an entirely different mechanism by which βH-Spec influences tension at AJ. We observed a mutual antagonism between βH-Spec and myosin in vivo for localization to apical F-actin: decreasing βH-Spec increases junctional myosin, while increasing βH-Spec decreases junctional myosin. Reciprocally, increasing myosin activity decreases βH-Spec localization to apical F-actin, while decreasing myosin activity increases βH-Spec localization to apical F-actin. In vitro studies with purified protein domains revealed that myosin can compete with βH-Spec for binding to F-actin. Finally, computational modeling of protein structures revealed that myosin and βH-Spec would interfere with each other’s binding to F-actin. Together, these observations indicate that βH-Spec and myosin can directly compete with each other for localization to F-actin. We suggest therefore that the influence of βH-Spec on junctional tension is likely to be a direct consequence of its competition with myosin for overlapping binding sites on F-actin.
Despite βH-Spec and myosin sharing overlapping binding sites on F-actin, and competing reciprocally in vivo, in our co-sedimentation experiments we could only detect a partial ability of myosin to compete for βH-Spec binding, and we could not detect an ability of βH-Spec to compete for myosin binding. Several factors are likely to contribute to these observations. First, we could not use higher protein concentrations of myosin or βH-Spec due to the need to keep the salt concentration constant and close to physiological levels, and to prevent protein precipitation. Second, it has been suggested for β-Spec that the CH2 domain regulates the actin binding function of the CH1 domain through steric hindrance when the two domains are associated (Avery et al., 2017). A specific mutation in β-Spec CH2 (L253P) has been shown to lower the energetic barrier between closed and open structural states, increasing the affinity of β-Spec for F-actin around 1000-fold (Avery et al., 2016), but it is unknown how β-Spec or βH-Spec conformational changes are normally regulated in vivo and whether both proteins share this regulatory feature. Additionally, phosphorylation of myosin regulatory light chain shifts myosin from a compact, autoinhibited conformation to a filamentous, active conformation (Kiehart and Feghali, 1986; Vasquez et al., 2016). The autoinhibited conformation binds F-actin very weakly (KD>100 µM) (Heissler and Manstein, 2013; Sellers et al., 1982) compared to the active conformation, suggesting that spectrin could outcompete autoinhibited myosin more effectively than active myosin for binding to F-actin. Our in vitro experiments used an active form of myosin. In addition, other factors including other actin-binding proteins and cytoskeletal tension are likely to influence the dynamic localization and actin-binding properties of both proteins (Duan et al., 2018; Greenberg et al., 2016).
The competition between βH-Spec and myosin also provides key insights into how βH-Spec contributes to ratcheting of apical constriction. The apical constriction of cells in the ventral furrow that initiates Drosophila mesoderm invagination occurs through fast constriction pulses interrupted by pauses during which cells must stabilize their constricted state before reinitiating constriction (Martin et al., 2009; Xie and Martin, 2015). This ratcheting-like behavior is thought to be a consequence of the finite length of actin filaments. Myosin contracts the cytoskeleton by driving filaments past each other, and extensive contractions require release and reassociation with new pairs of filaments. βH-Spec participates in ratcheting of apical constriction (Krueger et al., 2020). When βH-Spec is knocked down, cells can undergo cycles of unratcheted apical constriction during which they alternately constrict and then expand. Consequently, most βH-Spec-depleted embryos fail to complete normal mesoderm invagination. It was proposed that the actin cross-linking function of βH-Spec could hold F-actin in place for the next cycle of myosin-mediated contraction, but this raises the question of how myosin and βH-Spec association with F-actin are coordinated so that βH-Spec prevents relaxation without interfering with constriction. Our results suggest a simple solution: since they compete for the same binding site, the release of myosin from F-actin at the end of a cycle of contraction would naturally be coupled to the accessibility of F-actin for binding by βH-Spec. Thus, the competition between myosin and βH-Spec for binding to F-actin enables myosin-mediated cell contraction to effectively alternate with βH-Spec-mediated stabilization.
Materials and methods
Drosophila genetics
Request a detailed protocolUnless otherwise indicated, crosses were performed at 29°C. Protein localization and expression levels were monitored using previously characterized transgenes: ex-lacZ (Hamaratoglu et al., 2006), kst:YFP (Lye et al., 2014), kst:GFP (Nagarkar-Jaiswal et al., 2015), jub:GFP (Sabino et al., 2011), sqh:GFP (Royou et al., 2004), sqh:mCherry (Martin et al., 2009), and β-Spec:GFP (II) (this paper).
To manipulate gene expression in the posterior compartment, en-Gal4 UAS-RFP; UAS-dcr2 flies were crossed with to UAS-RNAi or overexpression lines. RNAi transgenes used were UAS-kstRNAi (v37075), UAS-kstRNAi (HMS00882), UAS-α-specRNAi (v25387), UAS-β-specRNAi (GL01174), UAS-rokRNAi (v104675), and UAS-jubRNAi (v38442). To increase myosin activity, we used UAS-sqhEE (Winter et al., 2001), and to increase βH-Spec levels, we used UAS-kstCRISPRa (II) (this paper) and UAS-kstP[EPgy2]EY01010 (Pogodalla et al., 2021).
DNA cloning
Request a detailed protocolTo overexpress βH-Spec (UAS-kstCRISPRa), we used a second-generation CRISPR/Cas9-transcriptional activation approach (Jia et al., 2018), allowing us to recruit the transcriptional machinery near the TSS of kst under UAS control. For this, we made the following primers to generate a gRNA located less than 400 nt from the TSS: 5’-TTCGGATAAGCCGACAGGGTCTAT and 5’-AAACATAGACCCTGTCGGCTTATC-3’. These primers were duplexed and cloned in the FlySAM 2.0 vector using the BbsI site (Jia et al., 2019). Transgenic flies were made by injection, inserting the construct into the attP40 site (BestGene).
The actin binding domain (aa 1–278) of kst was cloned into pGEX-3X at the EcoRI site, using the following primers: 5’-gatctgatcgaaggtcgtggaATGACCCAGCGGGACGGC-3’ and 5’-atcgtcagtcagtcacgatgTTACTTCTTGCGATCTGCGTCCATTAGC-3’, and assembled using NEBuilder HiFi DNA Assembly (New England Biolabs, E2621) to generate plasmid pGEX-3X-ABDkst.
Recombineering was used to generate the β-Spec:GFP construct. A left homology arm (LHA, 1 kb before the stop codon of β-Spec) and a right homology arm (RHA, 1 kb after the stop codon of β-Spec) were cloned in pL452-cEGFP (Venken et al., 2008) by Gibson assembly into EcoRI (for LHA) and NotI (for RHA) sites, respectively, using the following primers: LHA_b-spec_FWD 5’ gacctgcagccaagctatcgATACATGGCTGCCAAGGC 3’; LHA_b-spec_REV 5’ gatcggaattgggctgcaggCTTTTTCTTTAAAGTAAAAAACGATCTGC 3’; RHA_b-spec_FWD 5’ caagtaactagttctagagcAGTAACAGCCGTAACGCAAC 3’ and RHA_b-spec_FWD 5’ tggagctccaccgcggtggcCGGCAATTGGTGTACTTTAAAG 3’. The regions of the primers in lowercase indicate homology with the vector.To activate the recombination machinery, SW106 Escherichia coli containing the P[acman] clone CH322-20K3 (chloramphenicol resistant) (Venken et al., 2009) were incubated at 42°C for 17 min before making electrocompetent cells. A fragment containing LHA-loxP-NeoR/KanR-loxP-EGFP-RHA was amplified and electroporated into the cells. Recombinant clones were selected by chloramphenicol/kanamycin resistance and then floxed by inducible Cre expression by adding 0.1% L-arabinose. The final construct (LHA-loxP-EGFP-RHA) was confirmed by Sanger sequencing and injected for insertion in flies into the attP40 site (BestGene).
Histology and imaging
Request a detailed protocolFor most experiments wing discs were fixed in 4% paraformaldehyde for 15 min at room temperature. Sqh:GFP discs were fixed for 12 min. Primary antibodies used were mouse anti-α-Spec (1:50, Developmental Studies Hybridoma Bank [DSHB], 3A9, deposited to the DSHB by Branton, D/Dubreuil, R, RRID:AB_528473), rabbit anti-β-Spec (1:100, a gift from Christian Klämbt) (Hülsmeier et al., 2007), rabbit anti-Dcr2 (1:800, Abcam, ab4732, RRID:AB_449344), rabbit anti-pMLC (T18/S19) (1:50, Cell Signaling Technologies, #3671, RRID:AB_330248), rat anti-E-cad (1:200, DSHB, DCAD2-c, deposited to the DSHB by Uemura, T, RRID:AB_528120), mouse β-galactosidase (1:200, DSHB, JIE7, deposited to the DSHB by Mason, TL/Partaledis, JA, RRID:AB_528101). Secondary antibodies were used at a 1:100 dilution, and included anti-rat Alexa Fluor 647 (Jackson ImmunoResearch, 712-605-153, RRID:AB_2340694), anti-rabbit Alexa Flour 647 (Jackson ImmunoResearch, 711-605-152, RRID:AB_2492288), anti-mouse Alexa Fluor 647 (Jackson ImmunoResearch, 715-605-151, RRID:AB_2340863), anti-mouse Cy3 (Jackson ImmunoResearch, 715-165-151, RRID:AB_2315777), anti-rabbit Cy3 (Jackson ImmunoResearch, 711-165-152, RRID:AB_2307443), anti-rat Cy3 (Jackson ImmunoResearch, 712-165-153, RRID:AB_2340667), anti-mouse Alexa Fluor 488 (Thermo Fisher Scientific, A-21202, RRID:AB_141607) and anti-rabbit Alexa Flour 488 (Thermo Fisher Scientific, A-21206, RRID:AB_2535792). DNA was stained using Hoechst (Invitrogen, H3570). Wing discs were removed and mounted on a slide in Vectashield (Vector Laboratories, H-1000). Confocal images were captured on a Leica SP8 microscope.
Proximity ligation assay
Request a detailed protocolPLA was performed with the Duolink Proximity Ligation Assay kit according to the manufacturer’s instructions (Sigma). Fixation was performed as in the normal immunostaining procedure. For the permeabilization step, 0.5% Triton X-100 in PBS was used in two washes of 20 min each. Antibodies used for PLA include rabbit anti-GFP (1:200; ChromoTek # PABG1-20, RRID:AB_2749857) and mouse α-Spec (1:50, DSHB, 3A9, RRID:AB_528473) antibodies, and secondary anti-mouse MINUS (Sigma-Aldrich Cat# DUO92004, RRID:AB_2713942) and anti-rabbit PLUS (Sigma-Aldrich Cat# DUO92002, RRID:AB_2810940) probes were used.
Immunoblotting
Request a detailed protocolWing discs (20 discs per lane) were lysed in 2× Laemmli Sample Buffer (Bio-Rad, #1610737) supplemented with protease inhibitor cocktail (Roche) and phosphatase inhibitor cocktail (Calbiochem). Protein samples were loaded in 4–15% gradient gels (Bio-Rad). Antibodies used for immunoblotting include mouse anti-GFP (1:1000; Cell Signaling Technology, #2955, RRID:AB_1196614) and as a loading control rabbit anti-GAPDH (1:5000; Santa Cruz Biotechnology, sc-25778, RRID:AB_10167668). Blots were visualized with fluorescent-conjugated secondary antibodies (LI-COR Biosciences) and the Odyssey Imaging System (LI-COR Biosciences).
Laser ablation
Request a detailed protocolLive imaging and laser ablation experiments were performed as previously described (Rauskolb et al., 2014). en-Gal4 UAS-RFP/CyO; UAS-dcr2/TM6B flies were crossed with UAS-kstRNAi; Ubi-E-cad:GFP/TM6B, UAS-a-specRNAi; Ubi-E-cad:GFP/TM6B or UAS-kstCRISPRa; Ubi-E-cad:GFP/TM6B flies. Eggs were collected at 25°C for 4 hr and then shifted to 29°C for 88 hr. Wing disc culture was based on the procedure of Dye et al., 2017. A stock medium was prepared using Grace’s medium (Sigma, G9771) without sodium bicarbonate but with the addition of 5 mM Bis-Tris and the pH was adjusted to 6.6–6.7 at room temperature. This was stored at 4°C for less than a month. Before every experiment, we added 5% fetal bovine serum (Thermo Fisher, 10082147), penicillin-streptomycin (Thermo Fisher, #15070063, 100× stock solution), and 10 nM 20-hydroxy-ecdysone (Sigma, H5142) to the medium. Larvae were floated on 25% sucrose and transferred into glass dishes with culture medium. Larvae of the desired genotype were selected and sterilized in 70% ethanol for 1 min. Then, we drew a circle on the glass bottom of a 35 mm glass-bottomed Petri dish (MatTek, P35G-0-14C) using glue made by mixing heptane with tape (Tesa, 5388). Wing discs were dissected out of larvae, transferred into this Petri dish, and oriented using tungsten needles. Then, we covered the discs with a Cyclopore Polycarbonate membrane (GE Health, 7060-2513) and glued it to the glass bottom to immobilize discs. Discs were imaged every 0.2 s on a Perkin Elmer Ultraview spinning disc confocal microscope, and ablation of junctions was achieved using a Micropoint pulsed laser (Andor Technology) tuned to 365 nm. Paired cutting of junctions, one in the anterior compartment and another in the posterior compartment at a similar location, were performed and compared. The displacement of vertices for the first second after ablation was used to calculate the velocities.
Quantification and statistical analysis
Request a detailed protocolTo obtain the surface of the wing disc and remove signals from the peripodial epithelium, we used the MATLAB toolbox ImSAnE (Heemskerk and Streichan, 2015) to detect and isolate a slice of the disc epithelium that surrounds the AJs, using E-cad as a reference, as described previously (Pan et al., 2016). The KstGFP, KstYFP, SqhGFP, and JubGFP images were created using ImSAnE.
For the fluorescence intensity heat maps, a custom MATLAB script was used (Alégot et al., 2017; Alégot et al., 2019; Pan et al., 2018). The script generates a 3D mask with the normalization channel (E-cad) keeping only the relevant pixels. The wing disc center (intersection between AP/DV boundaries) is picked for each image manually. Then, the picture is split into blocks of a given xy size (3×3 µm2), starting from the center, and the average intensity per pixel of each channel is measured. The intensity of the reference channel and the channel of interest are normalized over their respective average intensity. The ratio of the channel of interest over the reference channel is then determined. To average several discs, only matrices of the same xy size blocks were used. The center of the disc serves as a reference point; smaller matrices were expanded to correspond to the size of the biggest matrix and filled with NaN (Not-a-Number). We determined the minimum number of values required (usually three) to average the ratio for a given position. This means that the edges of the average disk are composed of the same minimum number of values, which corresponds to the n given for each experiment. Finally, signals from several wing discs were averaged and represented by the heat map, and a posterior versus anterior ratio was calculated.
Pearson’s correlation coefficient was calculated to establish co-localization between different proteins by using the Coloc 2 Plugin for ImageJ (https://imagej.net/plugins/coloc-2).
Statistical significance was determined with GraphPad Prism software by performing Student’s t-test (for comparison between two observations) or analysis of variance (ANOVA) with p<0.05 set as the criteria for significance. The Dunnett test was used to derive adjusted p-values for comparisons against the control experimental value, and the Tukey test was used to derive adjusted p-values for multiple comparisons.
Protein production and purification
Request a detailed protocolTo purify the actin binding domain of βH-Spec fused with GST, we transformed BL21-DE3 cells (NEB) with pGEX-3X-ABDkst. Protein expression was induced with 0.2 mM isopropyl-β-D-thiogalactoside at room temperature for 12–15 hr. Cells were harvested and lysed by sonication in lysis buffer: PBS (pH 7.4), 1% Triton X-100, 5 mM dithiothreitol, 1 mM phenylmethyl sulfonyl fluoride, and complete Mini Protease Inhibitor Cocktail (Roche). After centrifugation, the supernatant was collected and passed through a 1 mL GST-Trap column (Cytiva) at 4°C. Then, the column was washed with PBS (pH 7.4) until the absorbance reached a steady baseline. To remove the GST-tag, the column was loaded with 80 units of Factor Xa (New England Biolabs, P8010) in cleavage buffer: 50 mM Tris-HCl, 150 mM NaCl, 1 mM CaCl2, pH 7.5, and incubated for 16 hr at room temperature. To elute the ABD of βH-Spec while removing the protease simultaneously, we used a HiTrap Benzamidine FF (Cytiva) column in tandem with the GST-trap column and eluted the ABD of βH-Spec with cleavage buffer. To switch buffers, we dialyzed the protein against 25 mM HEPES pH 7.4, 150 mM NaCl, 1 mM TCP overnight. Then, the purified protein was concentrated by ultrafiltration to 30 µM and stored at –80°C until used in experiments.
G-actin was prepared from rabbit muscle acetone powder as reported (Lehrer and Kerwar, 1972) and further purified with size exclusion chromatography on a Superdex 75 pg column (Cytiva, # 28989333) in buffer containing 5 mM Tris/HCl pH = 8, 0.2 mM CaCl2, 0.5 mM ATP, 1 mM DTT. G-actin was polymerized to F-actin by the addition of 10× polymerization buffer (100 mM HEPES pH 7.0, 500 mM KCl, 20 mM MgCl2, 10 mM EDTA). Drosophila nonmuscle myosin-2 subfragment-1-like protein (Zip, amino acids 1–813) was recombinantly overproduced together with the myosin regulatory (Sqh) and essential light chain (Mlc-c) in the baculovirus/Sf9 insect cell system (Thermo Fisher Scientific) and prepared as described (Heissler et al., 2015).
Co-sedimentation assays
Request a detailed protocolFor co-sedimentation assays, βH-Spec CH domains or myosin were incubated with F-actin for 15 min at room temperature in an assay buffer containing 10 mM HEPES pH 7.4, 100 mM NaCl, 0.1 mM EGTA, 20 µM ATP, and 1 mM DTT and subsequently sedimented (100,000 × g, 15 min, 4°C, TLA-100 rotor) in an Ultima MAX-XP ultracentrifuge (Beckman). For competition assays, βH-Spec and actin were preincubated for 15 min at room temperature before the addition of myosin. Supernatant and pellet fractions were separated and the pellet fraction was resuspended in an equal volume of assay buffer. Samples were supplemented with NuPAGE LDS sample buffer (Invitrogen, #NP0007) and heated for 10 min at 90°C. Supernatant and pellet fractions were resolved on 4–12% NuPAGE Bis-Tris polyacrylamide gels (Invitrogen, NP0323BOX). Gels were incubated with PageBlue protein staining solution (Thermo Scientific, #24620) and destained with water. Gels were documented with a ChemiDoc MP (Bio-Rad) and densitometric analysis was performed with Fiji (Schindelin et al., 2012). Data plots and secondary analysis were performed in Origin 2019.
Model building
Request a detailed protocolA homology model of the Drosophila myosin motor domain (Zip, amino acids 1–813) in the actin-bound state was modeled using the cryo-EM structure of the human nonmuscle myosin-2C motor domain in the rigor state (PDB entry: 5JLH) as a template. Both motor domains share ~72% sequence identity at the amino acid level. The motor domain model was built using Modeler (Sali and Blundell, 1993). The model of the Drosophila βH-Spec calponin homology domain tandem (CH1-CH2, amino acids 1–278) was modeled using ColabFold (Mirdita et al., 2022). This model was superimposed onto the cryo-EM structure of the human β-III spectrin CH1 domain bound to F-actin (PDB entry: 6ANU) for binding site analysis.
Data availability
All data generated or analyzed during this study are included in the manuscript and supporting file.
References
-
Recruitment of Jub by alpha-catenin promotes Yki activity and Drosophila wing growthJournal of Cell Science 132:jcs222018.https://doi.org/10.1242/jcs.222018
-
Spectrin couples cell shape, cortical tension, and Hippo signaling in retinal epithelial morphogenesisThe Journal of Cell Biology 219:e201907018.https://doi.org/10.1083/jcb.201907018
-
Drosophilia spectrin. I. Characterization of the purified proteinThe Journal of Cell Biology 105:2095–2102.https://doi.org/10.1083/jcb.105.5.2095
-
Segregation of two spectrin isoforms: polarized membrane-binding sites direct polarized membrane skeleton assemblyMolecular Biology of the Cell 8:1933–1942.https://doi.org/10.1091/mbc.8.10.1933
-
Myosin II dynamics are regulated by tension in intercalating cellsDevelopmental Cell 17:736–743.https://doi.org/10.1016/j.devcel.2009.09.003
-
The Spectrin cytoskeleton regulates the Hippo signalling pathwayThe EMBO Journal 34:940–954.https://doi.org/10.15252/embj.201489642
-
The apical scaffold big bang binds to spectrins and regulates the growth of Drosophila melanogaster wing discsThe Journal of Cell Biology 217:1047–1062.https://doi.org/10.1083/jcb.201705107
-
A perspective on the role of myosins as mechanosensorsBiophysical Journal 110:2568–2576.https://doi.org/10.1016/j.bpj.2016.05.021
-
Tissue cartography: compressing bio-image data by dimensional reductionNature Methods 12:1139–1142.https://doi.org/10.1038/nmeth.3648
-
Nonmuscle myosin-2: mix and matchCellular and Molecular Life Sciences 70:1–21.https://doi.org/10.1007/s00018-012-1002-9
-
Tension-dependent regulation of mammalian Hippo signaling through LIMD1Journal of Cell Science 131:jcs214700.https://doi.org/10.1242/jcs.214700
-
flySAM transgenic CRISPRa system manualBio-Protocol 9:e3147.https://doi.org/10.21769/BioProtoc.3147
-
Spectrin tetramer formation is not required for viable development in DrosophilaThe Journal of Biological Chemistry 290:706–715.https://doi.org/10.1074/jbc.M114.615427
-
Cytoplasmic myosin from Drosophila melanogasterThe Journal of Cell Biology 103:1517–1525.https://doi.org/10.1083/jcb.103.4.1517
-
Cytoskeletal integrators: the spectrin superfamilyCold Spring Harbor Perspectives in Biology 8:a018259.https://doi.org/10.1101/cshperspect.a018259
-
Spectrins: a structural platform for Stabilization and activation of membrane channels, receptors and transportersBiochimica et Biophysica Acta (BBA) - Biomembranes 1838:620–634.https://doi.org/10.1016/j.bbamem.2013.05.002
-
Colabfold: making protein folding accessible to allNature Methods 19:679–682.https://doi.org/10.1038/s41592-022-01488-1
-
The Hippo signaling network and its biological functionsAnnual Review of Genetics 52:65–87.https://doi.org/10.1146/annurev-genet-120417-031621
-
The dynamics of Hippo signaling during Drosophila wing developmentDevelopment 145:dev165712.https://doi.org/10.1242/dev.165712
-
Zyxin links fat signaling to the Hippo pathwayPLOS Biology 9:e1000624.https://doi.org/10.1371/journal.pbio.1000624
-
Organization and function of tension-dependent complexes at adherens junctionsJournal of Cell Science 132:jcs224063.https://doi.org/10.1242/jcs.224063
-
The force-sensitive protein Ajuba regulates cell adhesion during epithelial morphogenesisThe Journal of Cell Biology 217:3715–3730.https://doi.org/10.1083/jcb.201801171
-
Reassessing the role and dynamics of nonmuscle myosin II during furrow formation in early Drosophila embryosMolecular Biology of the Cell 15:838–850.https://doi.org/10.1091/mbc.e03-06-0440
-
Drosophila Ajuba is not an Aurora-A activator but is required to maintain Aurora-A at the CentrosomeJournal of Cell Science 124:1156–1166.https://doi.org/10.1242/jcs.076711
-
Comparative protein Modelling by satisfaction of spatial restraintsJournal of Molecular Biology 234:779–815.https://doi.org/10.1006/jmbi.1993.1626
-
Fiji: an open-source platform for biological-image analysisNature Methods 9:676–682.https://doi.org/10.1038/nmeth.2019
-
The binding of smooth muscle heavy meromyosin to actin in the presence of ATPThe Journal of Biological Chemistry 257:13880–13883.
-
Localization of Hippo signalling complexes and warts activation in vivoNature Communications 6:8402.https://doi.org/10.1038/ncomms9402
-
Beta-Spectrin regulates the Hippo signaling pathway and modulates the basal actin networkThe Journal of Biological Chemistry 290:6397–6407.https://doi.org/10.1074/jbc.M114.629493
-
Apical Spectrin is essential for epithelial morphogenesis but not apicobasal polarity in DrosophilaThe Journal of Cell Biology 146:1075–1086.https://doi.org/10.1083/jcb.146.5.1075
-
The Hippo signaling pathway in development and diseaseDevelopmental Cell 50:264–282.https://doi.org/10.1016/j.devcel.2019.06.003
Article and author information
Author details
Funding
National Institute of General Medical Sciences (131748)
- Kenneth D Irvine
National Institute of General Medical Sciences (143539)
- Krishna Chinthalapudi
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
We thank Christian Klämbt for the β-Spec antibody, and Richard Ebright and Bryce Nickels for sharing equipment for protein purification. This research was supported by National Institutes of Health grants GM131748 (KDI) and R01GM143539 (KC).
Version history
- Received:
- Preprint posted:
- Sent for peer review:
- Reviewed Preprint version 1:
- Reviewed Preprint version 2:
- Version of Record published:
Cite all versions
You can cite all versions using the DOI https://doi.org/10.7554/eLife.84918. This DOI represents all versions, and will always resolve to the latest one.
Copyright
© 2023, Ibar et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,807
- views
-
- 209
- downloads
-
- 6
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Citations by DOI
-
- 3
- citations for umbrella DOI https://doi.org/10.7554/eLife.84918
-
- 3
- citations for Version of Record https://doi.org/10.7554/eLife.84918.3






