Accumbens cholinergic interneurons dynamically promote dopamine release and enable motivation
Figures

Cholinergic interneuron (CIN) stimulation drives dopamine (DA) release in freely moving rats.
(a) Rat brain atlas section (Paxinos and Watson, 2006) showing the approximate location of fiber tips. (b) Representative traces of DA release (565 nm excitation, red) and isosbestic control (405 nm, purple), recorded from the location marked by a triangle in (a). Laser stimulation was delivered as two trains of pulses (470 nm, 10 mW, 4 ms, 16 Hz). Scale bars: 1 s, 1% dF/F. (c) Top: example session showing DA release in response to 16 Hz optogenetic stimulation of nucleus accumbens (NAc) CINs, with 1, 4, 16, 32, or 64 pulses, in pseudo-random order. Dashed lines demarcate trials with similar stimulation parameters. Bottom: normalized dF/F from RdLight aligned to the first pulse in a train of laser stimulation of NAc CINs (Stim parameters: 4 ms pulse, 16 Hz, 10 mW; n = 4 rats). Band shows ± SEM. dF/F traces for each recording were normalized to the maximum response evoked by 64 stimulation pulses. (d) DA release in response to a single laser pulse of varying duration (1, 2, 4, 6 ms) at 10 mW. Responses are normalized to 1 ms width evoked response for each subject and averaged. Band shows ± SEM. The magnitude of DA release depends on the pulse width (ANOVA: F(3,12) = 5.52, p=0.013). (e) DA release in response to four laser pulses (4 ms) of varying frequency (4, 8, 16 Hz; all 10 mW). Release patterns are normalized to the single pulse response of the same width and power for each subject, and averaged. Band shows ± SEM. The magnitude of DA release depends on the frequency of stimulation (bottom, ANOVA: F(3,12) = 9.17, p=0.002). (f) Paired-pulse ratio test: DA release patterns in response to a pair of 4 ms, 10 mW pulses. A two-way ANOVA revealed that there was no statistically significant interaction between the pulse order (first or second) and the delay between two pulses (F(2,6) = 1.05, p=0.35), and there were no main effects of inter-pulse interval (p=0.47) or pulse order (p=0.06).
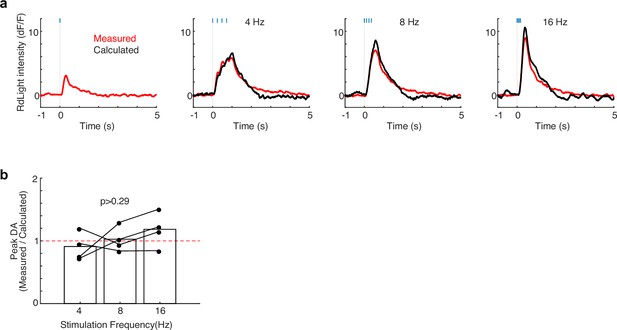
No evidence for short-term depression of cholinergic interneuron (CIN)-evoked dopamine (DA) release even at high stimulation frequencies.
(a) Representative evoked DA release following a single 4 ms pulse of optogenetic CIN stimulation on the left. This response is then summated four times at different frequencies (4, 8, 16 Hz) to calculate the predicted amplitude of DA response at those stimulation frequencies (black) and compare it to the measured DA response (red). (b) Quantitative comparison of measured and calculated DA response to repeated CIN stimulation. Two-way ANOVA finds no interaction between stimulation frequency and the ratio of measured to predicted peak DA response (F(2,9) = 1.41, p=0.293).

Cholinergic interneuron (CIN) activity and dopamine (DA) release similarly respond to reward delivery and ramp up with the reward approach.
(a) Dual-color photometry of CIN activity and DA fluctuations measured simultaneously through the same fiber. The locations of recording fiber tips are shown projected onto the nearest rat brain atlas section (Paxinos and Watson, 2006). (b) Example of simultaneous recording of spontaneous activity, from the triangle location in (a). Green: CIN GCaMP6f signal (excitation: 470 nm); red: RdLight1 (565 nm); purple: isosbestic control (405 nm). Dashed lines: unexpected food hopper clicks, delivering a sucrose pellet. Inset shows a zoomed-in epoch around a reward click. Note that CIN GCaMP and DA signals are distinct: transients in one signal are not always accompanied by transients in the other. Scale: 1% dF/F, 5 s. (c) A representative session with simultaneous CIN GCaMP6f and RdLight1 recording, aligned to hopper clicks. Trials are sorted by the reward collection time (the black dot indicates food-port entry). Both CIN activity and DA fluctuations show a rapid response to the click, and a separate ramping increase during the food-port approach is apparent for longer collection times. Color scale indicates z-scored signal range. (d) Top: average traces showing CIN activity and DA fluctuations aligned to the hopper click, peak responses (within 1 s from click) significantly differed from peak values aligned to random time points in the task (n = 1000 shuffles). Bottom: the slope of the signal ramps was significantly different from ramp slopes aligned to random time points throughout the session. The slope was determined by fitting a line that connects the maximum and minimum signal, 0.5 s before the food-port entry. Filled gray rectangles show chance levels (95% confidence ranges, from 1000 shuffles).
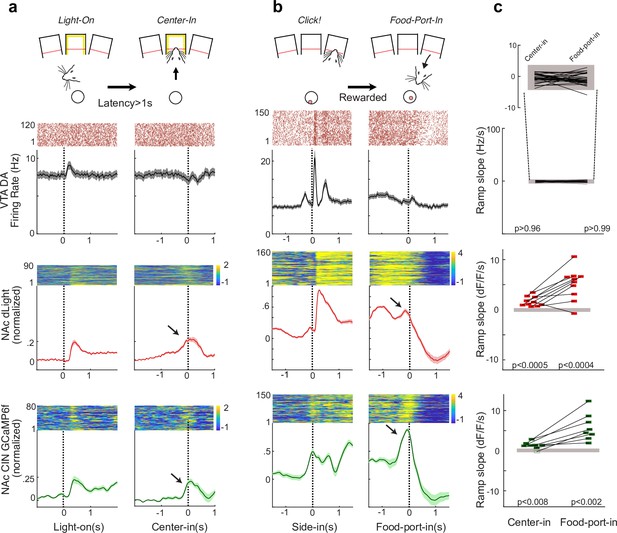
In an operant task, cholinergic interneuron (CIN) activity and dopamine (DA) release ramp up without corresponding increases in ventral tegmental area (VTA) DA cell firing.
(a) Illustration at top: trial initiation involves an approach to an illuminated center port. Data panels: upper and middle data rows: previously reported (Mohebi et al., 2019) spiking of identified lateral VTA DA cells (n = 29) and dLight DA signals (n = 10). Lower row shows CIN GCaMP signals (n = 8). Each panel shows a representative single example on top and the population average on the bottom. Shading indicates ± SEM. (b) Same as (a), but for the approach to the food port following the food delivery Click! sound, on rewarded trials. (c) Quantification of slopes during approach. Data points indicate individual DA neurons (top) or fiber placements (middle, bottom). Filled gray rectangles show chance levels (95% confidence ranges, from 1000 shuffles).
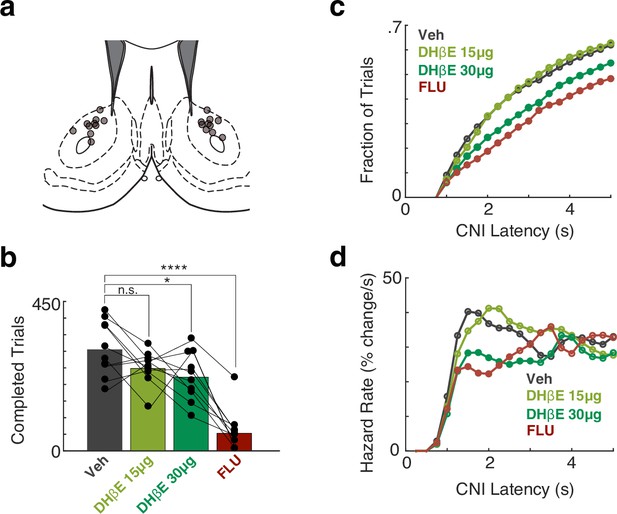
Blocking nucleus accumbens (NAc) dopamine or β2* nicotinic receptors diminishes motivation to work in the operant task.
(a) Placement of infusion cannula tips (circles) in NAc Core, based on postmortem histology. (b). The number of trials completed during a 2 hr Bandit session compared to the veh session. F(3,36) = 24.64, p<7.6 × 10–9. Rats treated with DHβE-30 µg and FLU completed fewer trials compared to the vehicle session (p<0.013, p<1 × 10–9, respectively). DHβE-15µg treatment caused a trend toward fewer completed trials (p=0.074) (c). Cumulative distribution of long latencies (latency >1 s) for different treatment conditions, binned at 250 ms. Filled circles denote statistical differences from the Vehicle condition (p<0.01). (d) The hazard rate of long latencies for different condition treatments demonstrates that blocking nicotinic transmission decreases the likelihood of a self-initiated approach (between 1 and 2 s from light-on) in a dose-dependent manner. Filled circles denote statistical differences from the veh condition (p<0.01).
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (LE rats, both male and female) | ChAT-Cre, Long-Evans (male and female rats) | RRRC | Long-Evans-Tg(ChAT-Cre)5.1Deis | |
| Recombinant DNA reagent | AAV5-EF1a-DIO- ChR2-eYFP | Addgene | Cat# 20298-AAV5 | |
| Recombinant DNA reagent | pAAV.Syn.Flex.GCaMP6f.WPRE.SV40 | Addgene | Cat# 100833-AAV5 | |
| Recombinant DNA reagent | AAV-CAG-RdLight1 | Patriarchi et al., 2020 | ||
| Chemical compound, drug | DHβE | Tocris Bioscience | Cat# 2349 | |
| Chemical compound, drug | Flupenthixol | Sigma-Aldrich | CAS# 51529-01-2 | |
| Software, algorithm | MATLAB | MathWorks, Inc. | ||
| Software, algorithm | LabView | National Instruments |





